Abstract
It was well documented that macro/trace elements were associated with the neurodevelopment. We aimed to investigate the relationship between copper (Cu)/zinc (Zn)/iron/calcium (Ca)/magnesium (Mg) levels and cerebral palsy (CP) by performing a meta-analysis. We searched the PubMed, Embase, Cochrane and Chinese WanFang databases from January 1985 to June 2022 to yield studies that met our predefined criteria. Standard mean differences (SMDs) of Cu/Zn/Iron/Ca/Mg levels between CP cases and healthy controls were calculated using the fixed-effects model or the random-effects model, in the presence of heterogeneity. 95% confidence intervals (CI) were also computed. Sensitivity analysis was performed by omitting each study in turn. A total of 19 studies were involved in our investigation. CP cases showed markedly lower Cu, Zn, iron and Ca levels than those in controls among overall populations (SMD = − 2.156, 95% CI − 3.013 to − 1.299, P < 10−4; SMD = − 2.223, 95% CI − 2.966 to − 1.480, P < 10−4; SMD = − 1.092, 95% CI − 1.513 to − 0.672, P < 10−4; SMD = − 0.757, 95% CI − 1.475 to − 0.040, P = 0.038) and Asians (SMD = − 2.893, 95% CI − 3.977 to − 1.809, P < 10−4; SMD = − 2.559, 95% CI − 3.436 to − 1.683, P < 10−4; SMD = − 1.336, 95% CI − 1.807 to − 0.865, P < 10−4; SMD = − 1.000, 95% CI − 1.950 to − 0.051, P = 0.039). CP cases showed markedly lower Zn level than that in controls among Caucasians (SMD = − 0.462, 95% CI − 0.650 to − 0.274, P < 10−4). No significant differences of Cu, iron and Ca levels between CP cases and controls among Caucasians (SMD = − 0.188, 95% CI − 0.412 to 0.037, P = 0.101; SMD = − 0.004, 95% CI − 0.190 to 0.182, P = 0.968; SMD = 0.070, 95% CI − 0.116 to 0.257, P = 0.459) were observed. No marked difference of Mg level between CP cases and controls was noted among overall populations (SMD = − 0.139, 95% CI − 0.504 to 0.226, P = 0.455), Asians (SMD = − 0.131, 95% CI − 0.663 to 0.401, P = 0.629), and Caucasians (SMD = − 0.074, 95% CI − 0.361 to 0.213, P = 0.614). Sensitivity analysis did not change the overall results significantly for Cu, Zn, iron and Mg. CP cases demonstrated significantly lower levels of Cu/Zn/iron/Ca than those in healthy controls, particularly in Asians. Decreasing trend of Cu/Zn/iron/Ca levels merit attention, particularly in the population with high susceptibility to CP. Frequent monitoring and early intervention may be needed.
Similar content being viewed by others
Introduction
Cerebral palsy (CP), a group of motor disorders and cognitive disturbances, is an important health concern in the newborns, particularly in the premature or low-birthweight neonates1. CP is likely to lead to the musculoskeletal problems, even epilepsy2. Many children with CP have limitations during their daily activities, including feeding, dressing, and balance. Some CP cases are complicated with malnutrition. CP Progression results in an increased morbidity and mortality, which indicates that early prevention and intervention for CP is of great importance. Strenuous efforts have been made to identify the risk factors for CP susceptibility. Birth asphyxia and genetic factors were involved in the development of CP3. In spite of the progress of the prenatal diagnosis and interventions, the prevalence of CP did not decline obviously, which indicated that CP may be a multi-factor disease, an in-depth investigation of other potential risk factors for CP is imperative.
Trace elements status is closely associated with the immune system function via their effects on many biological Processes, while the well worked immune function required the micronutrients participating in cell metabolism and replication. For instance, leukocytes proliferation induced by acute infection was impaired by insufficient supply of trace elements, including iron, zinc, magnesium and manganese4. Trace elements also exerted effects on the cellular transfer and the levels of other important nutrients5. For example, iron was an important constituent of hemoglobin, which carried the oxygen and participated in the energy metabolism. It is also proved that certain trace elements affect the chemical synaptic transmission in the brain and peripheral central nervous system6. Cu and Zn play an important role in the activation of enzymes that are involved in catecholamine transmission. On the other hand, macro-elements, such as Ca and Mg play an important role in the physical development. Ca and Mg exert effects in the transmission of neural stimuli.
Based on the fact that CP is essentially a neurological disorder, we speculated that macro/trace elements levels may be associated with CP. Some available evidence showed that certain trace elements, such as copper (Cu) deficiency were correlated with learning and behavior disorders7. Meanwhile, markedly lower level of zinc (Zn) was observed in severe CP compared with that in controls8. Previous review showed a high rate of malnutrition in the children with CP, while hypocalcemia, reduced serum levels of Zn, Cu and vitamin D being reported the most9. Mg sulfate given antenatally in threatened preterm labor has a reduction in the risk of CP at 2 years of age10. The administration of vitamin D and Ca produced a large, nonsignificant effect on bone mineral density in the lumbar spine11. To have an in-depth understanding of the relationship between alterations of macro/trace elements and CP is helpful for CP prevention and intervention.
Meta-analysis is a good way to pool the available evidence from single study to produce a more comparatively robust result, which increases the statistical power significantly. Therefore, we conducted a meta-analysis with the aim of clarifying the differences of Cu/Zn/Iron/Ca/Mg levels between CP and healthy controls in children.
Materials and methods
Search strategy
We performed the literature search in terms of the preferred reporting items for systematic reviews and meta-analysis guidelines12, we searched the papers that reported the levels of Cu/Zn/Iron/ Ca/Mg, both in CP and healthy controls from January 1985 to June 2022 by using PubMed, Embase, Cochrane and Chinese WanFang databases. We used the searched terms as follows: (1) macro/trace element, micronutrient, magnesium, Mg, calcium, Ca, iron, zinc, Zn, copper and Cu; (2) urine, serum and plasma; and (3) cerebral palsy, CP. We also reviewed the references of extracted literature. The paper with the larger number of participants was enrolled if the same subjects were recruited in more than one study. Our preprint of “Association between Cu/Zn/Iron/Ca/Mg levels and Chinese children with cerebral palsy” (https://doi.org/10.21203/rs.3.rs-703495/v1) was stored in websites (researchgate.net/publication/353611059_Association_between_CuZnIronCaMglevels_and_Chinese_children_with_cerebral_palsy), (doc.taixueshu.com/search?sourceTye = all&keywordTyPe = 1&keyword = Association + between + Cu/Zn/Iron/Ca/Mg + levels + and + cerebral + palsy&resultSearch = 0).We cited this preprint. This preprint has not been published in whole or in part in any formal journal elsewhere.
Study selection criteria
-
1)
Study design: case–control study
-
2)
Case: cerebral Palsy, control: healthy participants
-
3)
Outcome of interest: Cu/Zn/Iron/Ca/Mg levels in cases and controls
Exclusion criteria
-
1)
Study design: case report, comment, editorials and reviews
-
2)
Case and control: lack of detailed number of cases and controls, multiple publications of the same data
-
3)
Outcome of interest: lack of detailed data of Cu/Zn/Iron/Ca/Mg levels
Data extraction
We collected the data of mean and standard deviation (SD) of Cu/Zn/Iron/Ca/Mg levels. We also extracted the study characteristics from enrolled investigations. Data were recorded as the followings: first author’s last name; year of publication; ethnicity; number of cases and controls; confounding factors and testing method of Cu/Zn/ Iron/Ca/Mg levels.
Statistical analyses
We used the standard mean difference (SMD) to test the differences of Cu/Zn/ Iron/Ca/Mg levels between CP cases and controls across studies. Heterogeneity of SMDs across studies was tested by using the Q statistic (significance level at P < 0.05). The I2 statistic, a quantitative measure of inconsistency across studies, was also calculated. The SMDs were calculated using either fixed-effects models or, in the presence of heterogeneity, random-effects models (Q test, P < 0.05). Sensitivity analysis was performed by omitting each study in turn. Potential publication bias was assessed by Egger’s test at the P < 0.05 level of significance if the number of recruited studies were more than 10. Trim and fill analysis was used to identify the funnel plot asymmetry caused by publication bias and test the solidity of the results. All analyses were Performed using STATA version 12.0 (Stata Corp, College Station, TX).
Results
Literature search
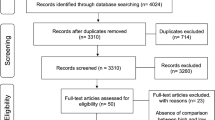
We firstly extracted 236 papers from the PubMed, Embase and Cochrane and Chinese Wan Fang databases. Most of these papers were removed due to that they were not associated with CP or Cu/Zn/Iron/Ca/Mg levels. After full-text review of the remaining studies, three studies were excluded due to the lack of detailed data. One study was excluded because it did not include control group. Finally, nineteen studies8,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 were included in this meta-analysis. A flow diagram showing the study selection is presented in Fig. 1.
Study characteristics
The characteristics of the nineteen enrolled studies are shown in Table 1. They were published between 1989 and 2022. Fourteen studies were about Cu, sixteen for Zn, twelve for iron, nine for Ca, and nine for Mg. Seventeen studies adjusted for confounding factors. The participants were from Asians and Caucasians.
Cu level in CP and controls
A total of 1394 CP cases and 1133 controls were included for testing the Cu level. Atomic absorption spectroscopy (AAS) was used in testing the Ca level in five studies with anodic stripping (AS) in two studies, and inductively coupled plasma atomic emission spectrometry (ICP-AES) in two studies. CP cases showed markedly lower Cu level than that in controls among overall populations (SMD = − 2.156, 95% CI − 3.013 to − 1.299, P < 10−4, Table 2, Fig. 2) and Asians (SMD = − 2.893, 95% CI − 3.977 to − 1.809, P < 10−4, Table 2, Fig. 2). No significant difference of Cu status between CP and controls among Caucasians (SMD = − 0.188, 95% CI − 0.412 to 0.037, P = 0.101, Table 2, Fig. 2) was observed. Sensitivity analysis did not change the overall results significantly (95% CI − 3.610 to − 0.531). Publication bias was observed (P < 10−4, funnel plot in Supplemental Material 1). Trim and fill analysis showed that addition of 4 virtual studies still yielded significant heterogeneity without changing overall result markedly.
Zn level in CP and controls
A total of 1871 CP cases and 1784 controls were included for testing the Zn level. AAS was used in testing the Zn level in seven studies with AS in two studies, and ICP-AES in two studies. CP cases showed markedly lower Zn level than that in controls among overall populations (SMD = − 2.223, 95% CI − 2.966 to − 1.480, P < 10−4, Fig. 3), Asians (SMD = − 2.559, 95% CI − 3.436 to − 1.683, P < 10−4, Fig. 3), and Caucasians (SMD = − 0.462, 95% CI − 0.650 to − 0.274, P < 10−4, Fig. 3). Sensitivity analysis did not change the overall results significantly (95% CI − 3.240 to − 0.717). Publication bias was observed (P = 0.023, funnel plot in Supplemental Material 2). Trim and fill analysis showed that addition of 5 virtual studies still yielded significant heterogeneity without changing overall result markedly.
Iron level in CP and controls
A total of 1292 CP cases and 1071 controls were included for testing the iron level. AAS was used in testing the iron level in three studies with AS in two studies, ICP-AES in one study, turbidimetric method (TM) in one study. CP cases showed markedly lower iron level than that in controls among overall populations (SMD = − 1.092, 95% CI − 1.513 to − 0.672, P < 10 − 4, Fig. 4) and Asians (SMD = − 1.336, 95% CI − 1.807 to − 0.865, P < 10−4, Fig. 4). No significant difference of Iron status between CP and controls among Caucasians (SMD = − 0.004, 95% CI − 0.190 to 0.182, P = 0.968, Table 2, Fig. 4) was observed. Sensitivity analysis did not change the overall results significantly (95% CI − 1.666 to − 0.500). Publication bias was observed (P = 0.023, funnel plot in Supplemental Material 3). Trim and fill analysis showed that it did not need addition of virtual studies.
Ca level in CP and controls
A total of 1081 CP cases and 845 controls were included for testing the Ca level. AAS was used in testing the Ca level in three studies with AS in two studies. CP cases demonstrated significantly lower Ca level than that in controls among overall populations (SMD = − 0.757, 95% CI − 1.475 to − 0.040, P = 0.038, Fig. 5) and Asians (SMD = − 1.000, 95% CI − 1.950 to − 0.051, P = 0.039, Fig. 5). No significant difference of Ca status between CP and controls among Caucasians (SMD = 0.070, 95% CI − 0.116 to 0.257, P = 0.459, Table 2, Fig. 5) was observed. Sensitivity analysis changed the overall results a little (95% CI − 1.713 to 0.204). Publication bias was not observed (P = 0.346).
Mg level in CP and controls
A total of 1041 CP cases and 843 controls were included for testing the Mg level. AAS was used in testing the Mg level in two studies with AS in another two. No marked difference of Mg level between CP cases and controls was noted among overall populations (SMD = − 0.139, 95% CI − 0.504 to 0.226, P = 0.455, Fig. 6), Asians (SMD = − 0.131, 95% CI − 0.663 to 0.401, P = 0.629, Fig. 6), and Caucasians (SMD = − 0.074, 95% CI − 0.361 to 0.213, P = 0.614, Fig. 6). Sensitivity analysis did not change the overall results (95% CI − 0.595 to 0.310). Publication bias was not observed (P = 0.984).
Discussion
CP, one of the most common developmental disabilities during the childhood throughout the lifespan, is a clinical syndrome characterized by a motor disorder. CP has attracted much attention of doctors and parents of patients due to its harms to neurological and motor systems in children. Identification of the potential risk factors for CP susceptibility is helpful for the early prevention and treatment of CP. Adequate micronutrient supply in early postnatal period may be an important tool for neuroprotection. Cu, Iron, Zn are shown to play significant role in proper neurodevelopment and brain functioning. Our meta-analysis showed that CP cases demonstrated significantly lower levels of Cu, Zn, iron and Ca than those in controls among overall populations and Asians, which indicated that the deficiency of Cu/Zn/iron/Ca should be paid more attention in the population with higher susceptibility to CP. The homeostasis of Cu/Zn/iron/Ca may be very important for neuroprotection. Early monitoring and intervention may be helpful for CP prevention and treatment.
Several facts may account for our findings. CP is a neurological disorder usually induced by preterm birth or infection. Metal ions are closely associated with the normal functioning of human body31. Trace elements deficiency is likely to cause the immune dysfunction, resulting in the increased risk of infection. Cu is a key cofactor for various enzymes, such as Cu/Zn superoxide dismutase, which plays an important role for neurological development32. Cu is also involved in the redox reactions33. CP cases are prone to Cu deficiency34. Suboptimal Cu status was shown to be associated with poor motor performance35. Cu deficiency is also known to be associated with higher susceptibility to traumatic brain36. Cu deficiency also affects the role of other cellular constituents involved in antioxidant activities, such as iron, selenium, and also plays an important role in diseases in which oxidative stress is elevated. Oxidative stress was involved in the brain injury. Hence, the disorder of Cu may cause brain dysfunction. For example, higher level of Cu was associated with decreased risk of Parkinson’s disease, which is the second most common neurodegenerative disease37.
Zn is necessary for the survival of various types of cells. Lots of enzymes exert the effects by creating bonds with Zn ions38. Zn plays a role in cell proliferation as an element of transcription factors and enzymes of DNA replication, Zn deficiency leads to a decline of Th1 immunity and promotes inflammatory reactions. Zn is also present throughout the central nervous system, playing a role in synaptic transmission, neuroregulation and neuroprotection39. Zn also promotes spinal cord injury recovery through upregulating Zn transporter-1 and brain-derived neurotrophic factors40. Zn inhibits free radical by promoting metallothionein production. Meanwhile, Zn is crucial for retinol-binding protein synthesis and vitamin A mobilization. Zn plays a role in removing the heavy metals from the body, such as Pb, As and Hg, which are implicated in the pathophysiology of Parkinson’s disease41. Based on the comprehensive role of Zn in the body, Zn disorder may result in the unpredictable injuries including neurological lesions.
Iron is an important constituent of hemoglobin, which transfers oxygen. Iron deficiency leads to anemia. Thus, iron regulates and influences the activity of various organs, as well as the whole organism. Iron also exerts effects in the catalysis of enzymatic reactions42. Th cells maturation was impaired in children with iron-deficiency anemia and was regenerated by the supplementation of iron. Iron participates in the neurodevelopment43. Iron deficiency lowers the chances of recovery of the central nervous system and influences the children’s adaptation ability. Hence, iron homeostasis is important for neuroprotection.
Ca, an imPortant constituent of bones, Plays a vital role in the muscle contraction and relaxation, and also regulates the electrical conduction system of the heart44. Ca also regulates the function of enzymes and is associated with the metabolism of other trace elements45. Intracellular calcium concentration is an important regulator of several signaling mechanisms, which regulate various kinds of biological processes46. Alterations in calcium concentration play a vital role in muscle contraction and relaxation47. Dysregulated calcium levels have been observed in several muscular dystrophies, including Duchenne muscular dystrophies48. Metabolic bone disease is characterized by impaired Ca and P balance49. These previous evidence shows that Ca is closely associated with motor disorder and is a potential therapeutic target for CP.
Mg, another important constituent of bones, is an antagonist of Ca, prevents excessive acetylcholine release and stimulation at the neuromuscular junction. Notably, we observed null difference of Mg status between CP and controls, which may be due to that Mg was not directly associated with the neurodevelopment. Notably, Mg sulfate was commonly applied in obstetrics due to its prevention effect of eclamptic seizures50, which may also affect the Mg status in neonates. Further larger numbers of studies are needed to validate our findings.
Our findings supported the idea that nutritional status influences the neurodevelopment, neurocognitive performances, and later life health outcomes. Appropriate nutritional diet is important for lowering the adverse health consequences. Also, compared with the included previously published single studies, our study was a pooled investigation with robust significances. Although the positive association between Cu/Zn/iron/Ca and CP provided novel insight for CP prevention and therapy, several limitations should be considered. First, the between-study heterogeneity may distort the final results, the random-effects model decreased the influence of the heterogeneity. On the other hand, the sensitivity analysis did not change the overall results, which indicated that our conclusion was comparatively more robust. The participants were largely from Asians, which may limit the generalization of our results. More studies from different ethnicities may be recruited in the future for more robust results. Second, the publication bias was noted for the association between Cu/Zn/Iron and CP, trim and fill analysis did not change the overall results, indicating our results were comparatively solid. Finally, despite the significant differences of Cu/Zn/iron/Ca between CP and controls, the cause-effect relationship between trace elements levels and CP risk remains inconclusive. The enrolled participants were all children with a lower age, we speculated that trace elements deficiency may precede the CP onset. Due to the lack of specific age in our investigation, further larger number studies should be performed to make a meta-regression analysis regarding the age. Further exploring the influence of trace elements on CP occurrence will have greater clinical value. In terms of our findings, the following issues should be addressed: (1) time-series analysis of the alteration of trace elements in CP, (2) longitudinal observation of the association between trace elements levels and CP Progress, (3) clarification of the cause-effect relationship between trace elements status and CP risk in prospective studies.
In conclusion, our investigation indicates that CP cases demonstrated significantly lower levels of Cu/Zn/iron/Ca than those in healthy controls. Monitoring and intervention of Cu/Zn/iron/Ca disorder may be helpful for CP Prevention and therapy.
Data availability
The original extracted data can be obtained from the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com/) and Cochrane (https://www.cochranelibrary.com/) and Chinese Wan Fang databases (https://www.wanfangdata.com.cn/) (through searching the include article title for the full-text paper). The human data or humans were not directly involved in the study.
References
Spittle, A. J., Morgan, C., Olsen, J. E., Novak, I. & Cheong, J. L. Y. Early diagnosis and treatment of cerebral palsy in children with a history of preterm birth. Clin. Perinatol. 45(3), 409–420 (2018).
Hollung, S. J. et al. Comorbidities in cerebral palsy: A patient registry study. Dev. Med. Child Neurol. 62(1), 97–103 (2020).
Sun, L. et al. Variants of the OLIG2 Gene are associated with cerebral palsy in Chinese Han infants with hypoxic-ischemic encephalopathy. Neuromol. Med. 21(1), 75–84 (2019).
Elmadfa, I. & Meyer, A. L. The role of the status of selected micronutrients in shaping the immune function. Endocr. Metab Immune Disord. Drug Targets 19(8), 1100–1115 (2019).
Chatelain, M., GasParini, J., Haussy, C. & Frantz, A. Trace metals affect early maternal transfer of immune components in the feral pigeon. Physiol. Biochem. Zool. 89(3), 206–212 (2016).
Wendołowicz, A., Stefańska, E. & Ostrowska, L. Influence of selected dietary components on the functioning of the human nervous system. Rocz Panstw Zakl Hig. 69(1), 15–21 (2018).
Zheng, J. et al. Multi-copper ferroxidase-deficient mice have increased brain iron concentrations and learning and memory deficits. J. Nutr. 148(4), 643–649 (2018).
Schoendorfer, N. C. et al. Micronutrient, antioxidant, and oxidative stress status in children with severe cerebral palsy. JPEN 37(1), 97–101 (2013).
da Silva, D. C. G. et al. Malnutrition and nutritional deficiencies in children with cerebral palsy: A systematic review and meta-analysis. Public Health 205, 192–201 (2022).
Ingran, L. & Nicola, J. R. Magnesium as a neuroprotective agent: A review of its use in the fetus, term infant with neonatal encephalopathy, and the adult stroke patient. Dev Neurosci. 40(1), 1–12 (2018).
Hough, J. P., Boyd, R. N. & Keating, J. L. Systematic review of interventions for low bone mineral density in children with cerebral palsy. Pediatrics. 125(3), e670–e678 (2010).
Moher, D., Liberati, A., Tezlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 8(5), 336–341 (2010).
Li, Z. Study on serum trace elements levels in 168 cerebral palsy cases. Chin. J. Misdiagn. 3(11), 1674–1675 (2003) ((in Chinese)).
Yuan, H., Zhang, G., Long, Y., Chen, D. & Tao, Y. Study on elements (Ca, Zn, Fe, Cu, Mg) of blood in 128 CP. GuangDong Weiliang Yuansu Kexue. 14(5), 13–15 (2007) ((in Chinese)).
Liang, H. et al. Study on urine zinc and copper in children with cerebral palsy. Trace Elem. Health Res. 16(3), 26–27 (1999) ((in Chinese)).
Chen, X., Gao, Z., Sun, L., Dong, X. & Zhang, T. To research the microelement and immune function in children with cerebral palsy. Int. TCM Psychol. Syst. Bioinform. 3, 379–381 (2010) ((in Chinese)).
Wang, H. et al. Study on the change of serum zinc and copper levels in children with cerebral Palsy. Jia Mushi Med. Coll. Res. 12(3), 270–271 (1989) ((in Chinese)).
Hu, Y. Study on the serum trace elements in children with cerebral Palsy. Med. J. Chin. People’s Health 20(21), 2513–2515 (2008) ((in Chinese)).
Li, Q., Du, C. & Ren, X. Analysis of serum zinc level in children with cerebral Palsy. Chin. J. Pract. Nerv. Dis. 19(12), 95–96 (2016) ((in Chinese)).
Hao, Q., Gao, Y. & Li, F. Study on the change of serum iron in children with cerebral Palsy. China Clin. Rehabil. 8(21), 4276–4276 (2004) ((in Chinese)).
Li, M. et al. Nutritional status and intervention for children with cerebral palsy. Chin. J. Rehabil. Theory Pract. 20(12), 1150–1152 (2014) ((in Chinese)).
Li, L. Association between trace elements and growth/development in children with cerebral palsy. Chin. J. Pract. Nerv. Dis. 19(11), 79–80 (2016) ((in Chinese)).
Peng, G. Study on the calcium and bone density in children with cerebral palsy. Stud. Trace Elem. Health 22(6), 16–17 (2005) ((in Chinese)).
Yang, H. et al. Association between zinc and children with cerebral palsy. Chin. J. Rehabil. Theory Pract. 31(2), 166–167 (2009) ((in Chinese)).
Hao, Q., Guo, X., Wu, T. & Dong, J. Association between serum trace elements and cause of Children with cerebral palsy. Chin. J. Rehabil. Theory Pract. 8(4), 109–111 (2000) ((in Chinese)).
Tang, Y. Study on the change of serum trace elements and oxidative stress in children with severe cerebral palsy. Chin. J. Pract. Nerv. Dis. 19(18), 93–94 (2016) ((in Chinese)).
Tinkov, A. A., Skalnaya, M. G. & Skalny, A. V. Serum trace element and amino acid profile in children with cerebral palsy. J. Trace Elem. Med. Biol. 64, 126685 (2021).
Khalique, A. et al. Multivariate analysis of the selected metals in the hair of cerebral palsy patients versus controls. Biol. Trace Elem. Res. 111(1–3), 11–22 (2006).
Carman, K. B. et al. Evaluation of micronutrient levels in children with cerebral palsy. Pediatr. Int. 64(1), e15005 (2022).
Kalra, S., Aggarwal, A., Chillar, N. & Faridi, M. M. A. comparison of micronutrient levels in children with cerebral palsy and neurologically normal controls. Indian J. Pediatr. 82(2), 140–144 (2015).
Polyudova, T. V., Eroshenko, D. V. & Korobov, V. P. Plasma, serum, albumin, and divalent metal ions inhibit the adhesion and the biofilm formation of Cutibacterium (propionibacterium) acnes. AIMS Microbiol. 4(1), 165–172 (2018).
Lasiene, J. et al. Neuregulin 1 confers neuroprotection in SOD1-linked amyotrophic lateral sclerosis mice via restoration of C-boutons of spinal motor neurons. Acta NeuroPathol. Commun. 4, 15 (2016).
Ognik, K. et al. The effect of copper nanoparticles and copper (II) salt on redox reactions and epigenetic changes in a rat model. J. Anim. Physiol. Anim. Nutr. 103(2), 675–686 (2019).
Janet, Y. et al. Influence of copper on early development: Prenatal and postnatal considerations. Biofactors 36(2), 136–52 (2010).
Bumoko, G. M. M. et al. Lower serum levels of selenium, copper, and zinc are related to neuromotor impairments in children with konzo. J. Neurol. Sci. 349(1–2), 149–153 (2015).
Klevay, L. M. Myelin and traumatic brain injury: The copper deficiency hypothesis. Med. Med. Hypotheses 81(6), 995–998 (2013).
Kim, M. J. et al. Association of metals with the risk and clinical characteristics of Parkinson’s disease. Parkinsonism Relat. Disord. 55, 117–121 (2018).
Khan, M. F., Kundu, D., Hazra, C. & Patra, S. A strategic approach of enzyme engineering by attribute ranking and enzyme immobilization on zinc oxide nanoparticles to attain thermostability in mesophilic Bacillus subtilis lipase for detergent formulation. Int. J. Biol. Macromol. 136, 66–82 (2019).
Justice, J. A. et al. Molecular neuroprotection induced by zinc-dependent expression of hepatitis C-derived protein NS5A targeting Kv21 potassium channels. J. Pharmacol. Exp. Ther. 367(2), 348–355 (2018).
Li, X. et al. Zinc improves functional recovery by regulating the secretion of granulocyte colony stimulating factor from microglia/ macrophages after spinal cord injury. Front. Mol. Neurosci. 1(12), 18 (2019).
Tamegart, L. et al. Crocus sativus restores dopaminergic and noradrenergic damages induced by lead in Meriones Shawi: A possible link with Parkinson’s disease. Acta Histochem. 121(2), 171–181 (2019).
Stoyanovsky, D. A. et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction?. Free Radic. Biol. Med. 133, 153–161 (2019).
Wang, Y., Wu, Y., Li, T., Wang, X. & Zhu, C. Iron metabolism and brain development in premature infants. Front. Physiol. 10, 463 (2019).
Nishikawa, K. et al. Calcium-dependent titin- thin filament interactions in muscle: Observations and theory. J. Muscle Res. Cell Motil. 41(1), 125–139 (2020).
Gaglianone, R. B. et al. ComParative study of calcium and calcium-related enzymes with differentiation markers in different ages and muscle types in mdx mice. Histol. Histopathol. 35(2), 203–216 (2020).
Pingel, J., Vandenrijt, J., KamPmann, M. L. & Andersen, J. D. Altered gene expression levels of genes related to muscle function in adults with cerebral palsy. Tissue Cell 76, 101744 (2022).
Wahid, M. et al. Mechanistic insights of Cucumis melo L. seeds for gastrointestinal muscle spasms through calcium signaling pathway-related gene regulation networks in WGCNA and in vitro, in vivo studies. Comput. Biol. Med. 155, 106596 (2023).
Marks, A. R. Targeting ryanodine receptors to treat human diseases. J Clin Invest. 133(2), e162891 (2023).
Rayannavar, A. & Calabria, A. C. Screening for metabolic bone disease of prematurity. Semin. Fetal Neonatal Med. 25(1), 101086 (2020).
Brookfield, K. F. & Mbata, O. Magnesium sulfate use in pregnancy for preeclampsia prophylaxis and fetal neuroprotection: Regimens in high-income and low/middle-income countries. Obstet. Gynecol. Clin. North. Am. 50(1), 89–99 (2023).
Author information
Authors and Affiliations
Contributions
W.L. concepted and designed this study, H.Z. collected the data, S.M. analyzed the data and writed this paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Mao, S. & Li, W. Association between Cu/Zn/Iron/Ca/Mg levels and cerebral palsy: a pooled-analysis. Sci Rep 13, 18427 (2023). https://doi.org/10.1038/s41598-023-45697-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45697-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.