Abstract
Monitoring the presence of commensal and pathogenic respiratory microorganisms is of critical global importance. However, community-based surveillance is difficult because nasopharyngeal swabs are uncomfortable and painful for a wide age range of participants. We designed a methodology for minimally invasive self-sampling at home and assessed its use for longitudinal monitoring of the oral, nasal and hand microbiota of adults and children within families. Healthy families with two adults and up to three children, living in and near Liverpool, United Kingdom, self-collected saliva, nasal lining fluid using synthetic absorptive matrices and hand swabs at home every two weeks for six months. Questionnaires were used to collect demographic and epidemiological data and assess feasibility and acceptability. Participants were invited to take part in an exit interview. Thirty-three families completed the study. Sampling using our approach was acceptable to 25/33 (76%) families, as sampling was fast (76%), easy (76%) and painless (60%). Saliva and hand sampling was acceptable to all participants of any age, whereas nasal sampling was accepted mostly by adults and children older than 5 years. Multi-niche self-sampling at home can be used by adults and children for longitudinal surveillance of respiratory microorganisms, providing key data for design of future studies.
Similar content being viewed by others

Introduction
Respiratory tract infections (RTI) affect the sinuses, throat, airways, and lungs1. Lower RTIs (including pneumonia and bronchitis) are the leading cause of death in children under five worldwide2,3 and the third leading cause of death in all ages in the United Kingdom (UK)4,5. A quarter of the population of England and Wales visit their GP because of an RTI each year6. RTIs are responsible for 60% of all antibiotic prescriptions in primary care, which constitutes a significant cost to the national health system7 and contributes to antimicrobial resistance8. Common causes of RTIs are the bacterium Streptococcus pneumoniae3,4 (pneumococcus) and a plethora of viruses which include respiratory syncytial virus9, coronavirus SARS-CoV-2, and seasonal influenza. RTI occurrence changes with host characteristics, geographical region, environmental factors, antibiotic use, and lifestyle habits10,11,12,13.
Healthy individuals carry commensal bacteria but can also asymptomatically carry pathogenic bacteria in their upper respiratory tract (URT). Together, the microbiota, is likely to influence respiratory health14 and is constantly shared through person to person contact especially within a household15. Interactions with viruses, antibiotics and other factors can alter bacterial composition and are likely to influence transmission of bacterial pathogens between household members. Although the presence and abundance of pathogenic bacteria are strongly associated with susceptibility and severity of RTIs in children11,12,13, it is unclear why some people get mild or severe disease, while others remain asymptomatic when infected with the same pathogen.
Research so far has focused on the relationship between the respiratory microbiome, including viruses, and RTI occurrence and severity by comparing healthy to RTI-infected individuals (single measurement case–control studies). Typically, these involve mothers and children up to 5 years10,11,12,13. This is because large community-based surveillance studies involving repeated sampling of individuals across a wide age range are difficult to conduct due to the nature of the currently recommended URT sampling methods. To detect pneumococcal carriage in the URT, a pre-requisite for disease occurrence, the World Health Organisation (WHO) recommends the use of nasopharyngeal swabs (NPS) in children and both NPS and oropharyngeal swabs in adults16. However, sampling, especially in children, is challenging as nasopharyngeal swabs can cause significant discomfort and pain. Saliva and nasal lining fluid using synthetic absorptive matrices (SAM) are less invasive and have been used successfully for paediatric sampling for both bacterial17 and viral18 detection.
Our group established self-sampling at home using saliva and SAM for studying pneumococcal carriage after experimental exposure in adults19. We have also investigated the use of SAM for the detection of pneumococcal carriage in hospitalised children aged 1–5 years under anaesthesia20, showing that SAM has equal sensitivity to the current gold standard NPS21. Moreover, we have demonstrated that hands can be vehicles for transmission of pneumococcus and lead to acquisition of nasopharyngeal carriage22. This study employs our previously established methodologies of saliva, nasal fluid and hand sampling and adapts them for longitudinal monitoring of the oral, nasal and hand microbiota in families. Our monitoring platform also included questionnaires for capturing risk factors for RTI occurrence and transmission. Here, we present the study design and its acceptability to study participants. We seek to establish saliva, nasal lining fluid using SAM and hand sampling for monitoring microbial presence and transmission in the community with a scope to identify an “at risk” microbiome for RTIs.
Material and methods
Study design and subjects
The FAMILY Micro study (ISRCTN 52814289) was a collaboration between the Liverpool School of Tropical Medicine and the local paediatric hospital, Alder Hey Children’s Hospital, that was ethically approved by the North West–Greater Manchester West Research Ethics Committee (20/NW/0304). The study was conducted in accordance with Good Clinical Practice regulations and the principles of the Declaration of Helsinki. Healthy families comprising of two adults aged 18–60 years and between one to three children aged 28 days–18 years living in or close to Liverpool, UK were enrolled between October 2020 and August 2021 (Supplementary Table S1). Briefly, we excluded any families where at least one member had a history of serious respiratory infections, a current respiratory infection or any condition that directly or indirectly (through medication) affected their immune responses or microbiome. However, if a family member developed an illness during the study, they were not excluded. In our previous home sampling study in adults, 61/63 (97%) participants accepted and complied successfully with self-testing at home19. A sample size of 125 participants was therefore required to detect a 97% compliance rate with a 95% confidence interval and an error margin of 3%. Anticipating a dropout rate of approximately 20%, we adjusted the sample size target to a maximum of 160 participants.
The study design was adapted to adhere to COVID-19 restriction measures. Recruitment was conducted via email to staff in local hospitals and universities and parents in local schools and nurseries, flyers at clinics in Alder Hey Hospital, social media, and word of mouth. Consent and training appointments took place virtually over Microsoft Teams. First, a member of the clinical team assessed participants’ eligibility. Eligible participants were then trained in study procedures by a member of the research team who described sample collection by sample type and explained how to complete the questionnaires. A bag with sample kits and a laminated sheet with sample collection kit contents, an information sheet on sample collection, a laminated step-by step instructions sheet, a dated collection schedule and a complete set of questionnaires were provided to participants after the consent appointment. Informed consent was obtained from all participants and/or their legal guardians. Participants aged 16 years and above provided written informed consent prior to study commencement, as a photo or scanned email attachment before sending in the post if necessary. Children aged 11 to 15 years were asked to sign an assent form and for all children under 16 years, parental consent was covered by the parent’s consent form and verbal assent during the initial consent appointment as confirmed by the study doctors. Communication between the study team and participants was maintained throughout the study via the WhatsApp platform, including reminders of sampling dates, arranging times for sample pick-ups, and answering any queries.
Sampling procedures
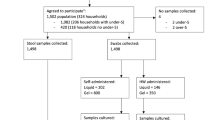
Parents and older children (5 years or older) self-collected saliva, nasal lining fluid and hand swabs at home once every two weeks for six months (Fig. 1). Parents helped younger children (< 5 years) to collect samples. Each sample kit was clearly labelled with study week number and distinguished between family members by participant ID (Parent A, Parent B, Child A, Child B or Child C) and a colour-coded label. Tubes with bacteria preservative media were kept in domestic fridges (4 °C) until use. Family members were asked to collect samples on the same day as per their collection schedule or if that wasn’t possible, one day before or after the scheduled date. Participants were asked to send a WhatsApp picture of the samples to the study’s dedicated, password-protected phone to confirm collection. Samples were stored in domestic freezers (-20°C) until pick-up and transport to the research laboratory at the Liverpool School of Tropical Medicine. Pick-ups were arranged half-way through and at the end of the study with samples transported on cold packs. Participants were also given the option to request more frequent pick-ups as required.
Saliva collection
Participants were asked to spit into a 50mL centrifuge tube (Appleton Woods, UK) to collect approximately 1ml saliva at least 30 min after last drinking, eating, or brushing their teeth. Saliva was preserved in 1mL STGG (Skimmed milk–Tryptone–Glucose–Glycerine) 10% glycerol media23, poured into the tubes after collection. For children < 2 years that were likely unable to spit, chewable paediatric absorbent swabs (Salimetrics, USA) were used for saliva collection by holding them in their mouth for 2 min. Swabs were stored in accompanying centrifuge tubes (Sarstedt, Germany) pre-filled with STGG. Children aged 2–5 years were given the option between the two methods.
Nasal lining fluid collection
Participants were asked to hold the SAM strip (Hunt Development LTD, UK) inside one nostril for up to 2 min. The diameter of the strip used differed according to participants’ age (adults 7mm and children 4.5mm) as per manufacturer instructions. Infants (< 1yr) were given an extra option for a smaller strip of 3mm diameter.
Hand swabs collection
Participants were asked to swab their unwashed dominant hand, using a sterile cotton swab (MWE, UK) after soaking it with sterile saline (Laboratoires Gilbert, France). Swabs were stored in accompanying tubes pre-filled with STGG.
Quantitative and qualitative data collection
Questionnaires (quantitative)
Participants completed questionnaires throughout the study (Fig. 1). Demographic and epidemiological (environmental log, contact log, dietary habits) data were collected at the beginning of the study. For assessing the feasibility of the sample collection methods, participants completed a questionnaire after every sampling time point to monitor each participant’s level of discomfort and pain and time taken to collect each sample type. For monitoring each family member’s health, participants were asked to complete a symptom log for each episode of illness. The family’s routines, such as means of transport and time spent at work, school, or home, were also recorded in monthly dairies. At the end of the study, participants completed an exit questionnaire on the acceptability of sample collection methods, indicating the overall opinion of the family, that of individual participants, and the key reasons for it.
Interview (qualitative)
At the end of the study, adult participants were invited for an optional interview to share their experiences (Fig. 1). Interviews were performed via Microsoft Teams and recorded using the record meeting function. Recordings were stored in a secure study file on the research team’s shared drive and were deleted post-transcription. Results from interviews were used to assess the feasibility of our approach and are reported elsewhere24.
Data analysis
Baseline demographics were analysed using summary statistics. Continuous variables were summarised using the number of observations, mean, median, standard deviation, and minimum and maximum values, and for categorical (nominal) variables, the number and percentage of subjects was used. Data from children were discussed overall and in five age groups: infants (28 days–1 year), toddlers (1–2 years), pre-schoolers (3–5 years), school-aged children (6–12 years) and adolescents (13–17 years).
The primary endpoints of the study were participants’ compliance and acceptability of the sample collection methods. To assess participant compliance, returned samples were counted and expressed as a percentage (%) of the total number of expected samples per sample type for each participant, based on the number of weeks their family took part in the study. Average compliance was calculated for each age group. Acceptability of home sampling was assessed based on the exit questionnaire. Sampling acceptability was assessed overall and per sample type. Overall acceptability was expressed as the % of families or participants who accepted home sampling out of the total number of families or participants, with 95% binomial confidence intervals. The reasons for acceptability were reported as % of families who indicated each reason. Fourteen families scored their top three reasons in order from most (with a score of 1) to least (with a score of 3) important. Here, we also present the % of these families who gave number 1 score per reason. We also calculated the participant acceptability per individual sample type using comments written on the exit questionnaire. Sample type acceptability was expressed as % of the total number of participants accepted each sample type.
Results
Recruitment approach summary
Eighty-four families were approached or expressed interest in participating in the FAMILY Micro study and were sent the Patient Information Leaflet (Fig. 2). Of these, twenty-eight (33%) either refused or never replied, while a further fifteen (18%) were deemed ineligible. One family was recruited but never returned any samples or documents to the study team. Therefore, forty families (48%), representing a total of 157 participants, were monitored in the FAMILY Micro study (Fig. 2). The study was conducted while COVID-19 restrictions were changing in UK. Twenty-five participating families (62%) were recruited through word of mouth by the study team and by existing participants. Communication emails to staff in local hospitals and universities (12 families, 30%) and to parents in local schools and nurseries (3 families, 8%) were also a successful recruitment strategy and these remained open despite COVID-19 restrictions. Recruitment via social media or flyers at Alder Hey Hospital clinics was unsuccessful.
Households and participants demographics
Most of the households were comprised of four family members (83%), were based in the Liverpool City Region (78%) and had an income range of £50,000–100,000 (78%) (Table 1). The mean age of parents was 39.8 years (range of 31–54 years) with a male:female ratio of 1:1. Most parents had completed university-level education (80%) and a high proportion (46%) were employed in medical, research and life science professions, as expected from the recruitment strategy (Table 2). The mean age of children (N = 77) was 6.5 years (range of 2 months–17 years) including 9 infants (mean age = 5.0 months), 11 toddlers (mean age = 1.5 years), 21 pre-schoolers (mean age = 4.2 years), 21 school-aged children (mean age = 8.5 years) and 15 adolescents (mean age = 14.1 years). Male children were 55% with the distribution varying by age group from 37% of infants being male compared to 62% of adolescents (Table 3).
Methodology compliance
All 40 families and 157 participants were included in the analysis of compliance, with a total of 1612 hand, 1585 saliva and 1557 nasal lining fluid samples returned to the research laboratory. Compliance was over 80% for all sample types and age groups (Fig. 3A–C). Compliance was highest for hand swabs. In the youngest age groups (infants, toddlers, and pre-schoolers), compliance was lower for saliva and lowest for nasal lining fluid sampling (Fig. 3C).
Participant compliance with collection of (A) hand swab, (B) saliva and (C) nasal lining fluid samples at home. Data is expressed as percentage of the mean of the compliance of each participant per age group, with compliance the number of samples collected from a participant as a proportion of the number of samples expected based on the number of weeks their family participated in the study. Bars represent 95% Confidence Intervals. Infants n = 9, toddlers n = 11, pre-schoolers n = 21, school-aged n = 21, adolescents n = 15, adults n = 80.
Family and participant acceptability to methodology
Only families who completed all six months of sampling and returned their acceptability questionnaire were included in this analysis (n = 33). Twenty-five families (76% CI [58%,89%]) indicated their overall acceptance of our methodology; families who did not find it acceptable were more likely to have at least one child < 5 years (Fig. 4A).
Overall, 73% CI [64%,80%] of individual participants accepted our methodology. Participant acceptability varied with age, with acceptability highest amongst adults (86% CI [76%,94%]) and lowest for toddlers (30% CI [7%,65%]) (Fig. 4B). 85.19% of parents who had attended university found our method acceptable compared to 87.50% of adults who didn’t have a tertiary education (p = 0.86). 81.25% of parents who worked in a healthcare or research profession found the method acceptable compared to 91.18% of parents who worked in other areas (p = 0.24).
Our methodology was found to be fast to complete (76% CI [55%,91%]), easy for sample collection (76% CI [55%,91%]) and painless (60% CI [39%,79%]). Furthermore, 20% CI [7%,41%] of families commented that sampling at home was preferable to traveling to a testing center. Based on the most important reason (with a score of 1), acceptability varied with family composition with fast completion (29% CI [8%,58%]), ease of sample completion (43% CI [18%,71%]), painless collection (21% CI [5%,51%]) and sampling at home (7% CI [0.2%,34%]) being the primary reasons for accepting our methodology (Fig. 4C).
Participant acceptability to sample type
Hand swabs were universally accepted, and most participants also accepted saliva sampling . Nasal sampling was the most challenging, with a steady decline in acceptability with decreasing age and only 14% CI [0.4%,58%] acceptability in the youngest age group of infants (Fig. 5).
Participant acceptability to nasal lining fluid, saliva and hand swabsampling per age group. Data is expressed as percentage of the number of participants accepted in one age group divided by the total number of participants in that age group. Infants n = 7, toddlers n = 10, pre-schoolers n = 17, school-aged n = 17, adolescents n = 12, adults n = 66.
Discussion
We have developed a methodology comprising of minimally invasive biological self-sampling with questionnaires for monitoring of the human oral, nasal and hand microbiota, and assessed its acceptability in a family setting after six months. We demonstrated that our methodology was acceptable for many parents and children across a wide age range, with highest acceptability amongst adults and school-aged children. To our knowledge this is the first study that employs longitudinal, minimally invasive sampling at home in a family setting, with collection of samples from three different human niches.
The approach of sample self-collection is not new. Self-taken URT swabs have previously been used for diagnostic purposes, especially during the COVID-19 pandemic where their use increased the testing rate, preserved scarce personal protective equipment (PPE), and reduced illness in health care workers25. Recent research has demonstrated that nasal swab collection by trained staff is not superior to self-collected or parent-assisted swabs26. Importantly, the laboratory yield of samples was not determined by who took the sample but by the anatomical site from which the sample was taken. Self-collection of saliva has been also used for diagnosing SARS‐CoV‐2 and is considered by some as the best method27. This method does not cause discomfort or pain, can reduce healthcare personnel exposure by avoiding coughing, sneezing, and/or aerosolization during sampling and requires fewer consumables, offering a significant benefit during supply shortages.
In our study, as expected, both saliva and hand sampling were acceptable to participants of all ages, whereas nasal sampling was accepted mostly by adults and older children (> 5 years). Nasal and nasopharyngeal swab collection are associated with a certain amount of pain and discomfort, making them especially unpopular with young children. Even though SAM is less invasive than a swab, infants and toddlers were distressed during nasal sampling procedure as reported by their parents24. Therefore, their acceptability was low for these age groups, with a range of time taken for SAM sampling between 1 s and 2 min24. However, compliance was high (above 80%) as parents kept trying to collect samples. A study using SAM in hospitalized children found that 30 s of sampling yielded good results18. Limiting the time taken for sampling to 30 s may improve acceptability in young children, though it is likely that some will not tolerate sampling even for this shorter period or will refuse sampling over time.
A major limitation of our work is that participating adults had much higher annual household incomes and educational status than the national average despite Liverpool being one of the most deprived local authorities in England. Socioeconomic status has previously been seen to affect participation in a study of one-off self-swabbing28. Additionally, a large proportion of the adults in our study worked in medical, research or life science professions and may be more likely to find our methods acceptable. However, there was no difference in acceptability between parents according to highest educational attainment or occupation. Because personal contact was the most successful recruitment method, it is also possible that many participants were more likely to comply with the methodology because of the personal connection. Therefore, our findings may not be generalisable to the UK population but the wealth of information we have gathered enables us to make an educated predication that our methodology will be feasible for larger studies in the future.
Our study has several strengths. Sampling at home was one of the reasons that participants accepted our methodology indicating the usefulness of our approach compared to clinical visits. It was conducted during and after strict COVID-19 restrictions thus we capture the feasibility of our methodology in the challenging context of a pandemic. We recruited 77 children between 2 months and 17 years of age, with a good distribution across the ages. While the number of children in each age group was limited, the numbers were sufficient to observe substantial differences in acceptability and compliance between the groups. Very high compliance rates were observed in all age groups, resulting in a large dataset of regular longitudinal samples which will yield invaluable information on microbial presence and transmission.
At-home specimen collection for respiratory pathogens enables community sampling and households have been highlighted as an important source of microbial spread within the community29,30,31. Thus, considerations of family members of different ages on comfort and ease with which samples can be obtained are critical for informing future large surveillance studies of microbial prevalence and transmission. Overall, the sampling methods used had a high level of acceptance amongst families and our results support their use in larger studies in the future.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding authors on reasonable request.
References
National Institute for Health and Care Excellence. Guidelines, in Respiratory Tract Infections—Antibiotic Prescribing: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care (National Institute for Health and Clinical Excellence (NICE), 2008).
Liu, L. et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379(9832), 2151–2161 (2012).
Ritchie, H., & Spooner, F. & Roser, M. Causes of death. 2018; Available from: https://ourworldindata.org/causes-of-death.
Societies, F. O. I. R. The global impact of respiratory disease. 2017, European Respiratory Society.
Salciccioli, J. D. et al. Respiratory disease mortality in the United Kingdom compared with EU15+ countries in 1985–2015: Observational study. BMJ 363, k4680 (2018).
Ashworth, M. et al. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br. J. Gen. Pract. 55(517), 603–608 (2005).
Lindbaek, M. Prescribing antibiotics to patients with acute cough and otitis media. Br. J. Gen. Pract. 56(524), 164–166 (2006).
Bell, B. G. et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 14, 13 (2014).
Smith, D. K., Seales, S. & Budzik, C. Respiratory syncytial virus bronchiolitis in children. Am. Fam. Phys. 95(2), 94–99 (2017).
Teo, S. M. et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17(5), 704–715 (2015).
de Steenhuijsen Piters, W. A. et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am. J. Respir. Crit. Care Med. 194(9), 1104–1115 (2016).
Alexandrino, A. S. et al. Risk factors for respiratory infections among children attending day care centres. Fam. Pract. 33(2), 161–166 (2016).
Man, W. H. et al. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case-control study. Lancet Respir. Med. 7(5), 417–426 (2019).
Man, W. H., de Steenhuijsen Piters, W. A. A. & Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 15(5), 259–270 (2017).
Shaw, L. et al. The human salivary microbiome is shaped by shared environment rather than genetics: Evidence from a large family of closely related individuals. mBio 8(5), 10–1128 (2017).
Satzke, C. et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 32(1), 165–179 (2013).
Wyllie, A. L. et al. Streptococcus pneumoniae in saliva of Dutch primary school children. PLoS ONE 9(7), e102045 (2014).
Thwaites, R. S. et al. Nasosorption as a minimally invasive sampling procedure: mucosal viral load and inflammation in primary RSV bronchiolitis. J. Infect. Dis. 215(8), 1240–1244 (2017).
Nikolaou, E. et al. Experimental human challenge defines distinct pneumococcal kinetic profiles and mucosal responses between colonized and non-colonized adults. mBio 12(1), 10–1128 (2021).
Reiné, J. et al. Dynamic changes in innate immune and T cell function and composition at the nasal mucosa across the human lifespan. bioRxiv, 2019: p. 576744.
Nikolaou, E. et al. Minimally invasive nasal sampling in children offers accurate pneumococcal colonization detection. Pediatr. Infect. Dis. J. 38(11), 1147–1149 (2019).
Connor, V. et al. Hands are vehicles for transmission of Streptococcus pneumoniae in novel controlled human infection study. Eur. Respir. J. 52(4), 1800599 (2018).
O’Brien, K. L. et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J. Clin. Microbiol. 39(3), 1021–1024 (2001).
German, E. L. et al., Participant perceptions and experiences of a community-based pilot respiratory longitudinal sampling study in Liverpool, UK. medRxiv (2023).
Adeniji, A. A. “Self-collected upper respiratory tract swabs for COVID-19 test”: A feasible way to increase overall testing rate and conserve resources in South Africa. Afr. J. Prim. Health Care Fam. Med. 12(1), e1–e4 (2020).
Zoch-Lesniak, B. et al. The respiratory specimen collection trial (ReSpeCT): A randomized controlled trial to compare quality and timeliness of respiratory sample collection in the home by parents and healthcare workers from children aged <2 years. J. Pediatr. Infect. Dis. Soc. 9(2), 134–141 (2020).
Byrne, R. L. et al. Saliva alternative to upper respiratory swabs for SARS-CoV-2 diagnosis. Emerg. Infect. Dis. 26(11), 2770–2771 (2020).
Coughtrie, A. L. et al. Evaluation of swabbing methods for estimating the prevalence of bacterial carriage in the upper respiratory tract: a cross sectional study. BMJ Open 4(10), e005341 (2014).
Althouse, B. M. et al. Identifying transmission routes of Streptococcus pneumoniae and sources of acquisitions in high transmission communities. Epidemiol. Infect. 145(13), 2750–2758 (2017).
Dahlgren, F. S. et al. Household transmission of influenza A and B within a prospective cohort during the 2013–2014 and 2014–2015 seasons. Stat Med 40(28), 6260–6276 (2021).
Consortium & M,. Household transmission of Neisseria meningitidis in the African meningitis belt: A longitudinal cohort study. Lancet Glob. Health 4(12), e989–e995 (2016).
Acknowledgements
The authors would like to thank all participants enrolled in the study for their time and effort especially during the pandemic. We would also like to thank Alder Hey Children’s Hospital for their collaboration as a study recruitment site, as well as Kelly Davies and Kelly Convey for their invaluable assistance in trial set-up and management.
Author information
Authors and Affiliations
Contributions
E.N. conceived and designed the study with input from E.L.G., H.M.N., A.M., B.C.U., H.H., A.M.C. and D.M.F. Study delivery and data acquisition were carried out by E.N., E.L.G., A.H., R.R., F.S., K.L., C.M., S.K., K.C., C.M.P., D.B.H. and A.M.C. Data was analysed and interpreted by E.N. and E.L.G. with assistance from J.M.R., S.J.A. and D.M.F. E.N. and E.L.G. drafted the manuscript, which all authors reviewed.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nikolaou, E., German, E.L., Howard, A. et al. Assessing the use of minimally invasive self-sampling at home for long-term monitoring of the microbiota within UK families. Sci Rep 13, 18201 (2023). https://doi.org/10.1038/s41598-023-45574-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45574-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.