Abstract
A single dose of cocaine abolishes endocannabinoid-mediated long-term depression (eCB-LTD) in the nucleus accumbens (NAc) within 24 h of administration. However, it is uncertain whether this altered neuroplasticity entails a behavioral deficit. As previously reported, after a single dose of cocaine (20 mg/kg), mice displayed impaired eCB-LTD in the NAc. Such cocaine-induced neuroplastic impairment was accompanied by an altered preference for saccharin and social interactions and a reduction in mRNA levels of the anandamide-catabolizing enzyme NAPE-PLD. The pharmacological increase of anandamide through the fatty acid amide hydrolase (FAAH) inhibitor URB597 (1 mg/kg) reversed the cocaine-induced loss of eCB-LTD in the NAc and restored normal social interaction in cocaine-exposed mice, but it did not affect saccharin preference. Overall, this research underlines the neuroplastic and behavioral alterations occurring after the initial use of cocaine and suggests a potential role for anandamide.
Similar content being viewed by others
Introduction
The initiation of drug addiction stems from the first exposure to a rewarding substance. As drug consumption becomes frequent, the initial voluntary and goal-directed use can evolve into habitual and compulsive patterns, ultimately resulting in addiction in vulnerable people1. Researchers have long studied the factors contributing to this transition1, but even a single drug encounter can induce enduring changes in the central nervous system, persisting after the drug is no longer present2,3.
The nucleus accumbens (NAc) plays a crucial role in drug-related and natural reward learning, with the endogenous cannabinoid (eCB) system (ECS) and synaptic plasticity in dopamine receptor 1 (D1)- and 2 (D2)-expressing medium spiny neurons (SPNs) implicated in reward-seeking behavior4,5. The ECS comprises cannabinoid receptors 1 (CB1R) and 2 (CB2R), eCB ligands (anandamide, AEA, and 2-arachidonoylglycerol, 2-AG), and enzymes for synthesis (N-acylphosphatidylethanolamine-specific phospholipase D, NAPE-PLD, and diacylglycerol lipase, DAGL) and degradation (fatty acid amide hydrolase, FAAH, and monoacylglycerol lipase, MAGL)6. In the NAc, the ECS modulates neuroplasticity in synapses through eCB-mediated long-term depression (eCB-LTD), a mechanism that downregulates excessive synaptic activity7. eCB-LTD governs the strength of synapses from distal glutamatergic projections (e.g., the medial prefrontal cortex and the amygdala) onto SPNs8. Activation of mGluR5 mediates eCB-LTD by triggering the synthesis of eCBs, which in turn induce LTD via two mechanisms: presynaptic inhibition of neurotransmitter release through CB1R activation7, and postsynaptic internalization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) through transient receptor potential vanilloid 1 (TRPV1) activation8. While the specific eCB responsible for the cocaine-induced loss of plasticity is a subject of ongoing debate, AEA has emerged as a promising candidate because it activates CB1R6 and possesses ideal characteristics for facilitating TRPV1-mediated plasticity, including its function as an intracellular messenger9 and its full agonist activity at TRPV1 channels10.
The molecular and physiological underpinnings of reward-seeking behavior remain incompletely understood. Pharmacological and genetic approaches have demonstrated that eCB-LTD in D1-expressing SPNs of the NAc is crucial for the expression of cocaine, natural reward (i.e., sucrose), and brain-stimulation-seeking behaviors11. Rodents display various behavioral changes following cocaine exposure. However, it is unclear whether a single cocaine administration can induce behavioral changes beyond its acute effects. For example, repeated pairings of saccharin with cocaine decrease saccharin consumption12,13. Similarly, both acute14,15,16 and chronic17 cocaine use in rodents reduces social interaction during drug use and even after one day of withdrawal from repeated exposure18. However, although a single cocaine administration abolishes eCB-LTD in mice3, it remains unclear how a single cocaine exposure affects NAc-related behaviors once the drug's acute effects have subsided.
In this study, we report that a single dose of cocaine can alter behavior 24 h after exposure, with disrupted social interactions and diminished saccharin preference. Additionally, our data suggest that elevating AEA levels through the FAAH inhibitor URB597 elicits beneficial effects, rescuing physiological eCB-LTD and adapted behavior. Therefore, we propose that the FAAH inhibitor URB597 could have a potential therapeutic effect for addressing initial impairments caused by cocaine.
Results
A single cocaine exposure leads to loss of eCB-LTD in the NAc core
As expected, a single cocaine (20 mg/kg i.p.) administration abolished plasticity in the NAc 24 h after its administration3 (Fig. 1B–C; repeated measures (RM) two-way ANOVA, time F(49, 980) = 51.25, p < 0.001, cocaine F(1, 20) = 2.174, p = 0.155, time x cocaine F(49, 980) = 1.491, p = 0.017), and eCB-LTD was present in the saline group (Fig. 1D; Student’s t test, t(11) = 3.426, p = 0.005), but not in the cocaine group (Fig. 1E; Student’s t test, t(9) = 0.947, p = 0.369).
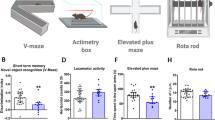
Single cocaine (20 mg/kg) exposure perturbates NAc core plasticity, related behaviors and NAPE-PDL gene expression. (A) Mice were treated with cocaine/saline and 24 h later were tested for eCB-LTD in the NAc, saccharin preference, and social behavior and gene expression levels. (B) Representative traces during baselyne (− 10–0 min) and LTD expression period (30–40 min). Scale: 0.1 mV, 5 ms. (C) Average time courses of eCB-LTD 24 h after receiving either saline (n = 12) or cocaine (n = 10). (D) fECSP during baseline and LTD expression period in saline and (E) cocaine groups. (F) Preference for saccharin measured for 24 h following cocaine/saline administration. During the habituation day and the test day, the measurements were performed at 1, 2, 6 and 24 h. (G) Cocaine administration decreased preference for saccharin during the following day, especially after (H) 1 and (I) 24 h (SAL n = 24, COC n = 22). (J) Time spent in each compartment during the habituation phase in the three-chamber social interaction test. Mice did show preference for the left/right compartment (SAL n = 13, COC n = 12). (K) Cocaine-treated mice did not differ in their locomotor activity during the habituation phase. (L) Time spent in the interactive zones surrounding the novel mouse or the empty holder during the sociability phase. Cocaine-treated mice showed an increase in social interaction. (M) Gene expression levels of CB1R, (N) FAAH, (O) mGluR5 and (P) NAPE-PLD in the vSTR of saline- (n = 9–11) and cocaine-treated (n = 8–10) after the social interaction test. SAL: saline, COC: cocaine, SI: social interaction, LTD: long term depression, fEPSP: field postsynaptic excitatory potential, CB1R: cannabinoid receptor 1, FAAH: fatty acid amide hydrolase, mGluR5: metabotropic glutamate receptor 5, NAPE:PLD: N-acylphosphatidylethanolamine-specific phospholipase D.
A single cocaine exposure perturbates social behavior the day after use
Previous research has suggested that cocaine use alters social interaction and reward processing, but it is uncertain whether initiating cocaine use affects such behaviors in rodents beyond its acute effects. To investigate this, mice were compared in terms of their preference for consuming saccharin, a potent natural reward. Throughout the habituation phase, mice exhibited a notable inclination towards saccharin, and their preference for saccharin increased along the time as shown in Fig. 1F. Following administration of cocaine/saline, mice treated with cocaine displayed a reduction in saccharin preference (Fig. 1G; RM Two-way ANOVA, time F(3, 132) = 92.19, p < 0.001, cocaine F(1, 44) = 5.224, p = 0.027, cocaine x time F(3, 132) = 2.687, p = 0.049, Sidak 1 h p = 0.014), which was particularly noticeable at 1 h (Fig. 1H; Student’s t test, t(44) = 2.849, p = 0.006), but also evident after 24 h (Fig. 1I; Student’s t test, t(44) = 2.301, p = 0.026). Such effects of cocaine were specific to saccharin consumption, as water consumption was not altered (Supplementary Fig. 1).
Next, mice were given either cocaine or saline 24 h before being tested for social interaction. During the habituation phase, there were no group differences in preference for either compartment or locomotor activity (Fig. 1J–K). In the sociability phase, mice spent more time interacting with the novel mouse than with the empty holder (Fig. 1L; Two-way ANOVA, compartment F(1, 22) = 113.6, p < 0.001). In particular, cocaine pre-treatment seems to alter mice behavior (Two-way ANOVA, cocaine F(1, 22) = 4.421, p = 0.047) mainly due to an increase in the time spent interacting with the novel mouse.
A single cocaine exposure decreases the NAPE-PLD gene expression levels 24 h within administration
We investigated possible changes in the gene expression of key components of the ECS that contribute to the eCB-mediated plasticity in the NAc (Fig. 1M–P). qPCR analyses performed on the vSTR 24 h following cocaine/saline administration revealed a reduction of NAPE-PLD gene expression in cocaine-treated mice (Fig. 1P; Student’s t test, t(16) = 2.757, p = 0.013), but no significant effect on CB1R (Fig. 1M; Student’s t test, t(19) = 0.255, p = 0.801), FAAH (Fig. 1N; Student’s t test, t(16) = 0.870, p = 0.397) and GluR5 (Fig. 1P; Student’s t test, t(16) = 1.819, p = 0.088).
URB597 prevents the cocaine-induced loss of eCB-LTD in the NAc core
The observed decline in NAPE-PLD gene expression after the onset of cocaine use suggests a reduced AEA synthesis. Based on these findings, we hypothesized that the correction of AEA levels could reverse the synaptic and behavioral impairments caused by cocaine.
Therefore, the day after administering either cocaine or saline, we euthanized the mice and assessed the expression of eCB-LTD in the NAc in the presence of URB597 (2 µM). In the group that received saline, eCB-LTD was induced both in the presence or absence of URB597, although URB597 enhanced it (Fig. 2B–C; RM Two-way ANOVA, time F(49, 1078) = 66.24, p < 0.001; URB597 F(1, 22) = 1.992, p = 0.172; time x URB597 F(49, 1078) = 1.565, p = 0.008). On the other hand, in cocaine-treated mice the expression of eCB-LTD was absent, and the application of URB597 rescued it (Fig. 2B and D; RM Two-way ANOVA, time F(49, 931) = 53.33, p < 0.001; URB597 F(1, 19) = 7.630, p = 0.012, URB597 x time F(49, 931) = 3.662, p < 0.001). The comparison of the fEPSC recorded during the LTD-expression period (30–40 min) indicates a global effect of URB597 in enhancing LTD expression (Fig. 2E; Two-way ANOVA, cocaine F(1, 40) = 1.149, p = 0.290; URB597 F(1, 40) = 9.759, p = 0.003; cocaine x URB597 F(1, 40) = 1.945, p = 0.171).
URB597 (1 mg/kg) prevents the cocaine (20 mg/kg)-induced impairment in eCB-LTD and sociability. (A) Mice were treated with cocaine/saline and 24 h later eCB-LTD was measured with the presence of absence of URB597. Other cohorts of mice were treated with cocaine/saline and URB597/vehicle 24 h and 30 min, respectively, prior to being tested saccharin preference, social behavior, and gene expression levels. (B) Representative trace during baseline (− 10–0 min) and LTD expression period (30–40 min) of a SAL-treated mouse. Scale: 0.1 mV, 5 ms. (C) Average time courses of eCB-LTD for SAL-ACSF (n = 13) and SAL-URB (n = 10) groups. (C) Representative trace during baseline and LTD expression period of a COC-treated mouse. Scale: 0.1 mV, 5 ms. (D) Average time courses of eCB-LTD for COC-ACSF (n = 12) and COC-URB (n = 11) groups. (E) fECSPs during the LTD expression period showing that URB597 rescues the cocaine-induced loss of eCB-LTD. (F) Gene expression levels of CB1R, (G) FAAH, (H) mGluR5 and (I) NAPE-PLD 24 h and 30 min following cocaine/saline and URB597/vehicle administration, respectively (SAL-VEH, n = 10–11; SAL-URB, n = 8–9; COC-VEH, n = 8–10; COC-URB, n = 10–12). (F) Cocaine-induced decrease in saccharin preference is not affected by URB597 at (G) 1 or (H) 24 h post URB597 administration (SAL-VEH n = 13, SAL-URB n = 11, COC-VEH n = 12 and COC-URB n = 10). (I) The time spent in each compartment during the habituation phase showed that mice did not show initial preference for neither the left nor the right compartment (SAL-VEH, n = 13; SAL-URB, n = 10; COC-VEH, n = 11; COC-URB, n = 12). (J) Locomotor activity during the habituation session was not affected by either treatment. (K) Time spent in the interactive zones for each group. URB597 prevented the cocaine-induced increase in the time spent interacting with the novel mouse. (L) Gene expression levels of CB1R, (M) FAAH, (N) mGluR5 and (O) NAPE-PLD after the social interaction test (SAL-VEH, n = 10–11; SAL-URB, n = 8–9; COC-VEH, n = 8–10; COC-URB, n = 10–12). SAL: saline, COC: cocaine, VEH: vehicle, URB: URB597, SI: social interaction, LTD: long term depression, fEPSP: field postsynaptic excitatory potential, CB1R: cannabinoid receptor 1, FAAH: fatty acid amide hydrolase, mGluR5: metabotropic glutamate receptor 5, NAPE:PLD: N-acylphosphatidylethanolamine-specific phospholipase D.
URB597 treatment normalizes social interaction in cocaine-exposed mice
Regarding the saccharin test, the measurements took place 24 h following cocaine (20 mg/kg i.p.)/saline administration and 30 min after URB597 (1 mg/kg i.p.)/vehicle treatment and occurred at 1, 2, 6, and 24 h. After receiving cocaine/saline, mice treated with cocaine displayed a decrease in saccharin preference, persisting even after 48 h (Fig. 2F–H). However, URB597 did not have any effect on saccharin preference, despite the clear effect of cocaine (Fig. 2F; RM Three-way ANOVA, time F(3. 126)= 58.71, p < 0.001, cocaine F(1. 42)= 1.877, p < 0.178; URB597 F(1. 42)= 0.527, p < 0.472; time x cocaine F(3, 126) = 3.288, p = 0.023, Sidak 24 h p = 0.012; time x URB597 F(3, 126) = 0.334, p = 0.801; cocaine x URB597 F(1, 42) = 0.001, p = 0.981; time x cocaine x URB597 F(3, 126) = 1.069, p = 0.365; Fig. 2H; Two-way ANOVA, cocaine F(1 42) = 6.821, p = 0.012; URB597 F(1 42) = 0.001, p = 0.971; cocaine x URB597 F(1 42) = 0.354, p = 0.555). Similarly, URB597 did not alter the total saccharin or water consumption (Supplementary Fig. 2).
Besides, we tested the effects of URB597 on the cocaine-induced increase in social interaction. During the habituation phase, none of the groups exhibited a preference for either the left or right compartment (Fig. 2I) or an alteration in locomotor activity (Fig. 2J). During the sociability phase, we observed that mice spent more time interacting with the novel mouse than with the empty holder (Fig. 2K; Three-way ANOVA, compartment F(1, 42) = 250.6, p < 0.001; cocaine F(1, 42) = 0.529, p = 0.471; URB597 F(1, 42) = 0.763, p = 0.387) and, in particular, cocaine pre-treatment increased social interaction while URB597 treatment prevented such increase in the cocaine-treated mice (Three-way ANOVA, cocaine x URB597 F(2, 84) = 312.8, p < 0.001; Sidak, SAL-VEH vs COC-VEH p = 0.018, COC-VEH vs COC-URB p = 0.016).
URB597 treatment does not modify NAPE-PLD gene expression
qPCR analyses conducted on the vSTR (Fig. 2L–O) showed that cocaine administration resulted in a decrease in the gene expression of NAPE-PLD, regardless of URB597 treatment (Fig. 2O; Two-way ANOVA, cocaine F(1, 33) = 7.127, p = 0.011, URB597 F(1, 33) = 2.045, p = 0.162; cocaine x URB597 F(1, 33) = 21.587, p = 0.217), indicating that the URB597-induced increase in AEA is likely due to reduced degradation rather than increased synthesis. No differences due to cocaine or URB597 treatment were found in CB1R (Fig. 2L; Two-way ANOVA, cocaine F(1, 35) = 0.224, p = 0.637, URB597 F(1, 35) = 0.101, p = 0.752; cocaine x URB597 F(1, 35) = 0.003, p = 0.955), FAAH (Fig. 2M; Two-way ANOVA, cocaine F(1, 34) = 0.162, p = 0.690, URB597 F(1, 34) = 0.654, p = 0.424; cocaine x URB597 F(1, 34) = 1.949, p = 0.172) and mGluR5 (Fig. 2N; Two-way ANOVA, cocaine F(1, 35) = 1.012, p = 0.321, URB597 F(1, 35) = 1.162, p = 0.288; cocaine x URB597 F(1, 35) = 0.666, p = 0.420).
Discussion
The current findings reveal that a single exposure to cocaine can alter saccharin preference and social behavior, as well as disrupt the neuroplastic mechanisms regulating synaptic strength in the NAc, even after the drug has been eliminated from the system. These outcomes are accompanied by a decrease in the AEA-catabolizing enzyme NAPE-PLD, suggesting a deficit in AEA signaling that could account for the suppression of plasticity induced by cocaine. Enhancing AEA levels pharmacologically through FAAH inhibition restores the cocaine-induced loss of eCB-LTD in the NAc and normalizes social behavior in cocaine-exposed mice but has no effect on the cocaine-induced decrease in saccharin preference.
Consistent with our results, cocaine-induced loss of plasticity in the NAc has been largely documented for the last 20 years, occurring after single cocaine exposure2,3, repeated non-contingent cocaine administration19 and cocaine self-administration20. However, how this loss of plasticity is related behavioral alteration after the first cocaine exposure remains unclear.
We found that a single dose of cocaine leads to a reduction in saccharin preference for the subsequent 48 h. The decrease in saccharin preference observed during the first hour after cocaine administration could be attributed to the stimulant's effects on locomotor activity, which may interfere with consumption behavior. However, the fact that cocaine administration did not interfere with water consumption suggests a different explanation. Furthermore, the prolonged suppressive effects beyond this period cannot be explained by an acute effect of the drug. Instead, we hypothesize that cocaine exposure induces long-lasting neuroplastic adaptations in the mesocorticolimbic system, responsible for natural reward processing, leading to the observed changes in saccharin preference. In line with this, previous studies have extensively documented that pairing cocaine with saccharin dramatically decreases saccharin intake12,13,21,22,23. This effect appears to be due to a decrease in the perceived value of saccharin as a reward, rather than an impairment in sweet taste sensitivity13. The ability of cocaine to decrease natural reward is supported by studies using intracranial self-stimulation. While cocaine appears to facilitate brain reward function when present in the organism24,25, the opposite effect is observed during withdrawal25,26. Interestingly, pharmacological27 or genetic deletion of mGluR5 in D1-expressing SPNs11 also increases intracranial self-stimulation thresholds, suggesting impaired reward function. This evidence supports the idea that cocaine decreases saccharin preference by disrupting natural reward function, likely due in part to an impairment in mGluR5 signaling. Alternatively, cocaine has been observed to have a negative impact on appetite28,29, often resulting in significant weight loss among cocaine users30. This could potentially contribute to the decrease in saccharine preference caused by cocaine. However, it is still uncertain whether this effect could account for the continued decrease in saccharine preference observed up to 48 h after cocaine administration.
Furthermore, a single dose of cocaine led to increased social interaction 24 h after administration. Given that the half-life of cocaine in the mouse brain is 16 min31, this behavioral finding cannot be attributed to the drug's acute effects. Instead, it implies that the central nervous system undergoes neuroplastic adaptations due to cocaine administration, leading to behavioral changes even when the drug is no longer present. In contrast, previous studies have indicated that social interaction is reduced during acute14,15,16 or repeated non-contingent cocaine administration17. These discrepancies suggest that the immediate behavioral effects of the drug are distinct from those produced by cocaine-induced neuroplastic changes. Wang et al. (2014) reported decreased social interaction after a 4-day cocaine treatment (20 mg/kg) when measured 24 h after the last administration18, contrasting with our findings. Possible explanations for these disparities include the use of different measurement models for social behaviors (three-chamber test versus open arena) or varying effects of cocaine on social behavior depending on whether it is administered acutely or repeatedly.
Cocaine-treated mice showed a decrease in NAPE-PDL gene expression levels after undergoing the social interaction test. Similarly, acute cocaine administration was associated with a decrease in gene and protein expression of NAPE-PDL and DAGLα in the hippocampus, resulting in an overall reduction in eCB synthesis32. Based on these findings, we hypothesize that a single exposure to cocaine may result in a reduction in the production of AEA, thus playing a role in the impairment of eCB-LTD in the NAc. Therefore, we pharmacologically inhibited FAAH activity with URB597 to test whether an increase in AEA levels could restore the cocaine-induced plasticity impairments as well as behavioral alterations.
We found that the enhancement of AEA caused by FAAH inhibition is sufficient to restore the loss of eCB-LTD after a single cocaine exposure, indicating a prominent role of AEA in mediating this kind of plasticity. Congruently, Grueter et al. (2010) found an enhancement of both TRPV1- and CB1R-mediated LTD in the NAc following URB597 administration. Considering that the function of TRPV1 and CB1R appears unaffected following cocaine exposure2,33 and that mGluR5 signaling is hindered due to receptor internalization by the homer scaffolding proteins3,34, it is proposed that the decline in eCB-LTD observed after cocaine use is primarily due to a reduction in mGluR5's capability to convert anterograde glutamate transmission into eCB production, including AEA. However, a complementary role of 2-AG in mediating this type of plasticity and the cocaine-induced alterations cannot be ruled out. Based on these observations, we propose that a single cocaine exposure disrupts eCB-LTD by impairing mGluR5 signaling and decreasing AEA synthesis. Reduced AEA levels lead to decreased activation of both presynaptic CB1R, resulting in a blunted inhibition of glutamate release, and postsynaptic TRPV1, causing an impaired AMPAR internalization (Fig. 3). It is yet to be determined whether the effects of AEA are primarily targeted towards CB1R or TRPV1, or whether other NAEs also play a role in this process.
URB597 actions in the NAc synapses. (A) In naïve mice, eCB-LTD exerts negative control over glutamatergic synapses in the NAc through mGluR5 signaling. When glutamate binds to mGluR5, it triggers the production of AEA, which in turn suppresses glutamate release through CB1R binding and causes AMPAR internalization through TRPV1 signaling. (B) One day following cocaine exposure, mGluR5 internalization impairs the downstream pathway and suppresses AEA release, resulting in a loss inhibitory control over SPNs activation. (C) The inhibition of AEA degradation by URB597 rescues the AEA-dependent activation of CB1R and TRPV1R and restores eCB-LTD in the NAc. eCB-LTD: endocannabinoid-mediated long term depression, CB1R: cannabinoid receptor 1, NMDAR: N-methyl-D-aspartate receptor, AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, TRPV1: transient receptor potential vanilloid 1, mGluR5: metabotropic glutamate receptor 5, FAAH: fatty acid amide hydrolase, NAPE:PLD: N-acylphosphatidylethanolamine-specific phospholipase D, AEA: anandamide. Created with biorender.com.
However, URB597 treatment did not affect cocaine-induced suppression of saccharin intake. A previous study reported that URB597 (0.5 mg/kg) reduced saccharin preference in mice35, which is consistent with the finding that URB597 raises intracranial self-stimulation thresholds36. Such effect was not observed here as URB597 did not affect saccharin preference in either cocaine- or saline-treated mice. The reasons why an increase in AEA appears to prevent cocaine-induced changes in social interaction but not saccharin preference remain unclear. One possible hypothesis is that the brain processes these two types of natural rewards differently, and therefore, they rely on distinct neural circuits that may involve varying levels of AEA in the synapses. Thus, AEA may play a different role in the neural pathways involved in processing social rewards versus those involved in processing sweet rewards.
In the case of social behavior, we found that URB597 treatment 30 min before the test prevented the cocaine-induced increase in sociability. To our knowledge, this is the first evidence of the impact of FAAH inhibition on social interaction after cocaine administration. Prior examinations of the role of URB597 on sociability have yielded inconclusive results. While some studies reported a URB597-induced decline in social behaviour37,38,39, others found the opposite outcome40,41. In line with the present results, two independent studies reported a reduction in social interaction following systemic administration of URB597 that is mediated through TRPV1 rather than CB1R38,39. Moreover, an effective reduction in operant responding for social play was observed with a dosage of 0.2 mg/kg of URB59742. Further research is required to elucidate the involvement of AEA in cocaine-induced alterations in social behavior, as well as the precise mechanisms underlying such modulation.
The present study has two main limitations. Firstly, while the impairments induced by cocaine in both accumbal plasticity and related behaviors follow the same administration regimen, the current experimental design does not allow us to determine a causal relationship between these outcomes. Therefore, it is crucial to experimentally manipulate the expression of eCB-LTD11 after cocaine exposure in order to uncover whether the observed behavioral alterations are dependent on this plasticity. Secondly, conducting a broader pharmacological examination by using antagonists targeting different NAEs receptors, such as CB1R or TRPV1, would provide valuable information regarding the mechanism through which URB597 restores eCB-LTD.
In conclusion, the results of the present study indicate that acute cocaine administration enhances social behavior and reduces saccharin preference after the drug has left the system. Parallelly, a single dose of cocaine disrupts the neuroplastic mechanisms that control synaptic strength in the NAc and decreases the gene expression of the AEA-catabolizing enzyme NAPE-PLD. We evidenced that the cocaine-induced loss of eCB-LTD is mediated by AEA, as FAAH inhibition is sufficient to restore the impairment caused by cocaine. Furthermore, FAAH inhibition normalizes the cocaine-induced alterations in social behavior. Future investigations should establish whether the effects of cocaine and URB597 on eCB-LTD and social behavior are causally connected. Overall, this study demonstrates that a single exposure to cocaine can induce neuroplastic changes that result in behavioral alterations even in the absence of the drug and highlights the involvement of the ECS, especially AEA, in the early impairments induced by cocaine.
Materials and methods
Animals
Behavior and biochemistry: Male C57BL/6J mice (postnatal day, PD, 56) were purchased from Janvier (Barcelona, Spain) and maintained in the animal facility (UBIOMEX, PRBB) in a 12 h light–dark cycle. Mice were housed at a stable temperature (22 °C ± 2) and humidity (55% ± 10%), with food and water ad libitum. They were allowed to acclimatize to the new environmental conditions for at least five days prior to experimentation. Animal care and experimental protocols were approved by the Barcelona Biomedical Research Park – Universitat Pompeu Fabra Ethical Committee for Animal Research (CEEA-PRBB-UPF).
Electrophysiology: Male C57Bl/6J mice (PD60) were purchased from Janvier (Le Genest-Saint-Isle, France) and maintained in the animal facility in a 12 h light–dark cycle. Mice were housed at stable temperature (20 ± 1 °C) and humidity (60%), with food and water ad libitum. They were allowed to acclimatize to the new environmental conditions for at least one week prior to experimentation. All experiments were performed on male C57BL/6J mice between PD70 and PD110. The French Ethical committee authorized this project (APAFIS#3279-2015121715284829 v5).
In all the studies, animals were treated in compliance with the European Communities Council Directive (86/609/EEC) and the ARRIVE guidelines for the care and use of laboratory animals.
Materials
Cocaine HCl (20 mg/kg i.p.; Cocaine was kindly provided by the National Institute on Drug Abuse, Bethesda, MD; USA or purchased from Alcaliber S.A., Madrid, Spain) was dissolved in 0.9% NaCl. The FAAH inhibitor URB597 (1 mg/kg i.p. or 2 µM) was purchased in Merck Life Science (Madrid, Spain) or Tocris Bioscience (Illkirch, France) and was dissolved in a vehicle solution composed by ethanol, cremophor E.L. (Sigma-Aldrich) and 0.9% NaCl (1:1:18).
Experimental design
We used three different cohorts of mice (Fig. 1A and 2A). One cohort was used for the measurement of eCB-LTD. Mice were injected with saline or cocaine (20 mg/kg) 24 h before being euthanized and extracellular recordings of the NAc core were measured with the presence or absence of URB597. A second cohort of mice was used to test the effects of cocaine and URB597 on saccharin preference. After the habituation phase, mice were injected with either cocaine or saline, and 24 h later with URB597 or vehicle. A third cohort of mice was used to test the effects of cocaine and URB597 on social behavior in the three-chamber social interaction test and to assess the mRNA levels of different components that participate in the eCB-LTD. Mice were pre-treated with cocaine or saline 24 h before the test, and with URB597 or vehicle 30 min before the test. Immediately after the test, mice were euthanized by cervical dislocation, the brain were quicky removed, and the ventral striatum (vSTR) was dissected for qPCR analysis.
Slice preparation and electrophysiology
Adult male mice were deeply anesthetized with isoflurane 4% and sacrificed by decapitation as described7,43,44,45. The brain was sliced (300 μm) on the coronal plane with a vibratome (Integraslice, Campden Instruments) in a sucrose-based solution at 4 °C (in mm as follows: 87 NaCl, 75 sucrose, 25 glucose, 2.5 KCl, 4 MgCl2, 0.5 CaCl2, 23 NaHCO3 and 1.25 NaH2PO4). Immediately after cutting, slices containing the NAc were stored for 1 h at 32 °C in a low calcium ACSF that contained (in mM) as follows: 130 NaCl, 11 glucose, 2.5 KCl, 2.4 MgCl2, 1.2 CaCl2, 23 NaHCO3, 1.2 NaH2PO4, and were equilibrated with 95% O2/5% CO2 and then at room temperature until the time of recording. Interleaved slices were incubated in a ACSF alone or ACSF containing 2 µM URB597 for 30 min prior to and during the recording.
Field potential recordings were made in coronal slices containing the NAc core as previously described7,43. Recordings were made in the medial ventral accumbens core close to the anterior commissure7,43.For recording, slices were placed in the recording chamber and superfused (1.5–2 ml/min) with ACSF (same as low Ca2+ ACSF with the following exception: 2.4 mM CaCl2 and 1.2 mM MgCl2). All experiments were done at 25 °C. Picrotoxin (100 µM) was added to the superfusion medium to block gamma-aminobutyric acid type A (GABA-A) receptors. All drugs were added at the final concentration to the superfusion medium. For field excitatory postsynaptic potential (fEPSP), the recording pipette was filled with ACSF, and afferents were stimulated with a glass electrode filled with ACSF and placed ~ 200 µm in the dorsal-medial direction of the recording pipette. The stimulus intensity was adjusted around 60% of maximal intensity after performing an input–output curve (baseline fEPSP amplitudes ranged between 0.15 mV and 0.4 mV). Stimulation frequency was set at 0.1 Hz. Recordings were performed with an Axopatch-200B amplifier (Axon Instrument, Molecular Device, Sunnyvale, USA). Data were lowpass filtered at 2 kHz, digitized (10 kHz, DigiData 1440A, Axon Instrument, Molecular Device, Sunnyvale, USA), collected using Clampex 10.2 and analyzed using Clampfit 10.2 (Axon Instrument, Molecular Device, Sunnyvale, USA). Both fEPSPs’ area and amplitude were analyzed.
Saccharin preference test
The test was adapted from previous studies46 with minor modifications. Mice were individually housed and exposed to two bottles: one containing a 0.2% saccharin solution (Sigma-Aldrich, Madrid, Spain) and the other containing tap water. No previous food or water deprivation was applied before or during the test. During day 1 and 2, mice were habituated to the experimental conditions (the presence of two bottles and the saccharin taste). During the experiment, mice were injected with either cocaine or saline on day 3, and with URB597 or vehicle on day 4. Afterwards, they were placed back in their home cage. Throughout the 4-day experiment, the two bottles containing either water or saccharin solution were continuously available for the mice, except for a 30 min period following the URB597/vehicle injection, which allowed time for URB597 to increase AEA levels47. The consumption of water and saccharin solution was measured by weighting the bottles at 1, 2, 6 and 24 h on days 2, 3 and 4. A control cage without animals was placed to control the amount of liquid spontaneously lost from the bottles. Saccharin preference was calculated according to the following ratio: saccharin intake(g)/[saccharin intake (g) + water intake (g)] × 100.
Three-chamber social interaction test
The test was adapted from previous studies48,49. Briefly, it consisted of two 10 min phases: habituation and sociability. The box consisted of a rectangle divided into three connected chambers. The right and left chambers had a holder located in one of the corners. For habituation, mice were introduced to the central chamber and were free to move around the box. For sociability phase, a novel mouse was trapped inside one of the holders, and the experimental mouse was reintroduced into the central chamber and allowed to freely explore the box. The novel mice were male, juvenile (5-week-old), C57BL/6 mice. Time spent in each compartment and in the interactive zones (around the holders) was recorded using the SMART software (Panlab s.l.u., Barcelona, Spain) for subsequent analysis.
qPCR
Total RNA extraction from NAc samples was conducted using the trizol method as previously described48,50. We evaluated the expression of CB1R, FAAH, mGluR5, NAPE-PLD and GAPDH as a housekeeping gene. RT-PCR was performed by High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) using random primers and following standardized protocols. As per the qPCR, 20 ng of sample were loaded in addition to the following reagents: LightCycler SBYR green 480 Master Mix (Roche LifeScience, Product No. 04707516001) and the specific primers for the target genes (Integrated DNA Technologies, Inc.) (Table 1). The qPCR was performed in QuantStudio 12K Flex (Thermofisher).
Statistical analysis
Sample size was determined based on previous data and using the G*Power Software51, assuming an α of 0.05, a power of0.8 and a moderate size effect. Data are presented as mean ± SEM. We used GraphPad Prism 9.5.0 and IBM SPSS Statistics 29.0 software for statistical analysis and graphing. For analysis involving two or three factors, we used two- or three-way analysis of variance (ANOVA), respectively. When an experimental condition followed a within-subject design, RM ANOVA was used. Mauchly's test was used to assess the sphericity of the RM ANOVA data and the Geisser-Greenhouse correction was applied whenever appropriate. The factors considered were cocaine treatment (cocaine/saline), URB597 treatment (URB597/vehicle), time (minutes or hours), LTD (baseline/LTD) and compartment (left/center/right, novel/center/empty, or novel/empty). The statistical threshold for significance was set at p < 0.05. Kolmogorov’s test and Levene’s test were used to assess normality of the data and homogeneity of the variances, respectively. When F achieved significance and there was no significant variance in homogeneity, a Sidak’s post hoc test was run. We analyzed the results of single factor experiments with two levels using paired or unpaired two-tailed Student’s t test.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lüscher, C., Robbins, T. W. & Everitt, B. J. The transition to compulsion in addiction. Nat. Rev. Neurosci. 21, 247–263 (2020).
Grueter, B. A., Brasnjo, G. & Malenka, R. C. Postsynaptic TRPV1 triggers cell type–specific long-term depression in the nucleus accumbens. Nat. Neurosci. 13, 1519–1525 (2010).
Fourgeaud, L. et al. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J. Neurosci. 24, 6939–6945 (2004).
Parsons, L. H. & Hurd, Y. L. Endocannabinoid signalling in reward and addiction. Nature 16, 579–594 (2015).
Grueter, B. A., Rothwell, P. E., Malenka, R. C., Sheng, M. & Triller, A. Integrating synaptic plasticity and striatal circuit function in addiction. Curr. Opin. Neurobiol. 22, 545–551 (2012).
Cristino, L., Bisogno, T. & Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16, 9–29 (2019).
Robbe, D., Kopf, M., Remaury, A., Bockaert, J. & Manzoni, O. J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 99, 8384–8388 (2002).
Castillo, P. E., Younts, T. J., Chávez, A. E. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76, 70 (2012).
Van Der Stelt, M. & Di Marzo, V. Anandamide as an intracellular messenger regulating ion channel activity. Prostaglandins Other Lipid Mediat. 77, 111–122 (2005).
Smart, D. et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 129, 227–230 (2000).
Bilbao, A. et al. Endocannabinoid LTD in accumbal D1 neurons mediates reward-seeking behavior. iScience 23, 100951 (2020).
Freet, C. S., Steffen, C., Nestler, E. J. & Grigson, P. S. Overexpression of ΔFosB is associated with attenuated cocaine-induced suppression of saccharin intake in mice. Behav. Neurosci. 123, 397–407 (2009).
Roebber, J. K., Izenwasser, S. & Chaudhari, N. Cocaine decreases saccharin preference without altering sweet taste sensitivity. Pharmacol. Biochem. Behav. 133, 18–24 (2015).
Estelles, J., Rodríguez-Arias, M., Aguilar, M. A. & Miñarro, J. Social behavioural profile of cocaine in isolated and grouped male mice. Drug Alcohol Depend. 76, 115–123 (2004).
Rademacher, D. J., Schuyler, A. L., Kruschel, C. K. & Steinpreis, R. E. Effects of cocaine and putative atypical antipsychotics on rat social behavior an ethopharmacological study. Pharmacol. Biochem. Behav. 73, 769–778 (2022).
Lluch, J., Rodríguez-Arias, M., Aguilar, M. A. & Miñarro, J. Role of dopamine and glutamate receptors in cocaine-induced social effects in isolated and grouped male OF1 mice. Pharmacol. Biochem. Behav. 82, 478–487 (2005).
Morisot, N., Monier, R., Le Moine, C., Millan, M. J. & Contarino, A. Corticotropin-releasing factor receptor 2-deficiency eliminates social behaviour deficits and vulnerability induced by cocaine. Br. J. Pharmacol. 175, 1504–1518 (2018).
Wang, J.-L., Wang, B. & Chen, W. Differences in cocaine-induced place preference persistence, locomotion and social behaviors between C57BL/6J and BALB/cJ mice. Zool. Res. 35, 426–435 (2014).
Huang, C.-C. et al. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J. Neurosci. 31, 4194–4203 (2011).
Martin, M., Chen, B. T., Hopf, F. W., Bowers, M. S. & Bonci, A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 9, 868–869 (2006).
Freet, C. S. & Lawrence, A. L. Ceftriaxone attenuates acquisition and facilitates extinction of cocaine-induced suppression of saccharin intake in C57BL/6J mice. Physiol. Behav. 149, 174–180 (2015).
Twining, R. C. et al. The role of dose and restriction state on morphine-, cocaine-, and LiCl-induced suppression of saccharin intake: A comprehensive analysis. Physiol. Behav. 161, 104–115 (2016).
Grigson, P. S. & Twining, R. C. Cocaine-induced suppression of saccharin intake: A model of drug-induced devaluation of natural rewards. Behav. Neurosci. 116, 321–333 (2002).
Fish, E. W. et al. Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcohol. Clin. Exp. Res. 34, 81–89 (2010).
Stoker, A. K. & Markou, A. Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav. Brain Res. 223, 176–181 (2011).
Stoker, A. K., Olivier, B. & Markou, A. Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology (Berl) 221, 317–327 (2012).
Cleva, R. M., Watterson, L. R., Johnson, M. A. & Olive, M. F. Differential modulation of thresholds for intracranial self-stimulation by mGlu5 positive and negative allosteric modulators: Implications for effects on drug self-administration. Front. Pharmacol. 2, 93 (2012).
Murphy, K. G. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief. Funct. Genomic Proteomic 4, 95–111 (2005).
Billing, L. & Ersche, K. D. Cocaine’s appetite for fat and the consequences on body weight. Am. J. Drug Alcohol Abuse 41, 115–118 (2015).
Ersche, K. D., Stochl, J., Woodward, J. M. & Fletcher, P. C. The skinny on cocaine: Insights into eating behavior and body weight in cocaine-dependent men. Appetite 71, 75–80 (2013).
Benuck, M., Lajtha, A. & Reith, M. E. A. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J. Pharmacol. Exp. Ther. 243, 144–149 (1987).
Blanco, E. et al. Cocaine-induced behavioral sensitization decreases the expression of endocannabinoid signaling-related proteins in the mouse hippocampus. Eur. Neuropsychopharmacol. 26, 477–492 (2016).
Mccutcheon, J. E. et al. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J. Neurosci. 31, 14536–14541 (2011).
Hoffmann, H. M. et al. Long-lasting impairment of mGluR5-activated intracellular pathways in the striatum after withdrawal of cocaine self-administration. Int. J. Neuropharmacol. 20, 72–82 (2017).
Zhou, Y. et al. Blockade of alcohol escalation and “relapse” drinking by pharmacological FAAH inhibition in male and female C57BL/6J mice. Psychopharmacology (Berl) 234, 2955–2970 (2017).
Vlachou, S., Nomikos, G. G. & Panagis, G. Effects of endocannabinoid neurotransmission modulators on brain stimulation reward. Psychopharmacology (Berl) 188, 293–305 (2006).
Matricon, J., Seillier, A. & Giuffrida, A. Distinct neuronal activation patterns are associated with PCP-induced social withdrawal and its reversal by the endocannabinoid-enhancing drug URB597. Neurosci. Res. 110, 49–58 (2016).
Seillier, A. & Giuffrida, A. The cannabinoid transporter inhibitor OMDM-2 reduces social interaction: Further evidence for transporter-mediated endocannabinoid release. Neuropharmacology 130, 1–9 (2018).
Seillier, A., Martinez, A. A. & Giuffrida, A. Phencyclidine-induced social withdrawal results from deficient stimulation of cannabinoid CB 1 receptors: Implications for schizophrenia. Neuropsychopharmacology 38, 1816–1824 (2013).
Manduca, A. et al. Strain-and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur. Neuropsychopharmacol. 24, 1337–1348 (2014).
Trezza, V. et al. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J. Neurosci. 32, 14899–14908 (2012).
Achterberg, E. M., van Swieten, M. M., Driel, N. V., Trezza, V. & Vanderschuren, L. J. Dissociating the role of endocannabinoids in the pleasurable and motivational properties of social play behaviour in rats. Pharmacol. Res. 110, 151–158 (2016).
Neuhofer, D., Lassalle, O. & Manzoni, O. J. Muscarinic M1 receptor modulation of synaptic plasticity in nucleus accumbens of wild-type and fragile X mice. ACS Chem. Neurosci. 9, 2233–2240 (2018).
Jung, K. M. et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Commun. 3, 1080 (2012).
Lafourcade, M. et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 14, 345–350 (2011).
Gracia-Rubio, I. et al. Maternal separation induces neuroinflammation and long-lasting emotional alterations in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 65, 104–117 (2016).
Fegley, D. et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 313, 352–358 (2005).
Castro-Zavala, A. et al. Bmal1-knockout mice exhibit reduced cocaine-seeking behaviour and cognitive impairments. Biomed. Pharmacother. 153, 113333 (2022).
Alegre-Zurano, L., Martín-Sánchez, A. & Valverde, O. Behavioural and molecular effects of cannabidiolic acid in mice. Life Sci. 259, 118271 (2020).
Castro-Zavala, A., Martín-Sánchez, A., Montalvo-Martínez, L., Camacho-Morales, A. & Valverde, O. Cocaine-seeking behaviour is differentially expressed in male and female mice exposed to maternal separation and is associated with alterations in AMPA receptors subunits in the medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 109, 110262 (2021).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Acknowledgements
We thank Inés Gallego-Landín, Mireia Medrano and Xavier Puig-Reyné for his assistance carrying out the experiment and Dr. Adriana Castro-Zavala for her assistance in the use of BioRender.
Funding
This work was supported by the Ministerio de Ciencia e Innovación (#PID2019-104077RB-100 and #PID2022-136962OB-I00 MCIN/AEI/10.13039/501100011033), Ministerio de Sanidad (Government Delegation for the Plan National on Drugs corresponding to Recovery Mechanism Funds, Transformation and Resilience (PRTR) of the European Union #EXP2022/00869, and ISCIII-Feder-RIAPAd-RICORS #RD21/0009/001), the Generalitat de Catalunya, AGAUR (#2021SGR00485) and by the Institut National de la Santé et de la Recherche Médicale (INSERM). L.A-Z received a FPI grant (BES-2017-080066). P.B-S received a FI-AGAUR grant from the Generalitat de Catalunya (2021FI_B00205). The Department of Medicine and Health Sciences (UPF) is a “Unidad de Excelencia María de Maeztu” funded by the AEI (#CEX2018-000792-M). O.V. is recipient of an ICREA Academia Award (Institució Catalana de Recerca i Estudis Avançats, Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
L.A.-Z., O.M. and O.V. were responsible for the study, concept, and design. L.A.-Z. carried out the behavioral procedures. L.A.-Z., A.C.-R, and O.L. performed the electrophysiology experiments and L.A.-Z. and P.B.-S carried out the qPCR. L.A.-Z., O.M. and O.V. drafted the manuscript. All authors critically reviewed the content and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alegre-Zurano, L., Caceres-Rodriguez, A., Berbegal-Sáez, P. et al. Cocaine-induced loss of LTD and social impairments are restored by fatty acid amide hydrolase inhibition. Sci Rep 13, 18229 (2023). https://doi.org/10.1038/s41598-023-45476-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45476-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.