Abstract
This study evaluated the lethal, sublethal, and toxic of a commercial formulation of cypermethrin in the anuran species Physalaemus gracilis. In the acute test, concentrations of 100–800 μg L−1 were tested over 96 h. In the chronic test, cypermethrin concentrations recorded in nature (1, 3, 6, and 20 μg L−1) were tested for mortality and then used for the micronucleus test and erythrocyte nuclear abnormalities over a 7-days period. The LC50 determined for P. gracilis for the commercial cypermethrin formulation was 273.41 μg L−1. In the chronic test, a mortality of more than 50% was observed at the highest concentration (20 μg L−1), as it caused half of the tadpoles studied to die. The micronucleus test showed significant results at concentrations of 6 and 20 μg L−1 and recorded the presence of several nuclear abnormalities, indicating the genotoxic potential of the commercial cypermethrin formulation for P. gracilis. Cypermethrin presented a high risk to the species, indicating that it has the potential to cause several problems in the short and long term and to affect the dynamics of this ecosystem. Therefore, it can be concluded that the commercial formulation of cypermethrin had toxicological effects on P. gracilis.
Similar content being viewed by others
Introduction
Aquatic fauna is frequently exposed to pesticides due to the constant expansion of agricultural activities and intensive use for pest control1,2. Contamination of water resources near agricultural lands can affect the development and survival of non-target organisms, such as amphibians3,4,5,6,7.
Amphibians has been gaining importance toxicity bioassays for de evaluation of envirolmental matrices8,9. Anuran amphibians are considered good bioindicators of environmental pollutants due to their distinctive characteristics such as complex life cycles, rapid larval growth rates, trophic position, permeable skin10,11, water-dependent reproduction12 and unprotected eggs11,13,14. Physalaemus gracilis, popularly known as the weeping frog, has been shown to be a bioindicator species for pesticide contamination4,5,6,7,15. The species occurs in Argentina, Uruguay, Paraguay, and Brazil16 in lentic waters, protected areas, or areas of environmental change17 and is considered stable by the IUCN due to its wide distribution and tolerance to a wide range of habitats18.
Global insecticide use reached 600,000 tons in 2020, with Brazil being the third largest consumer, behind the United States, which together account for 48% of total global consumption19. Cypermethrin is one of the most widely used pyrethroids for agricultural and domestic applications such as cotton crops, peanuts, rice, potatoes, coffee, onions, citrus, peas, beans, snap beans, tobacco, cassava, watermelon, millet, corn, cucumber, cabbage, soybean, sorghum, tomato and tobacco20. With the increased use of this insecticide in agricultural areas, the likehood of contamination in nearby water bodies increases21,22. Cypermethrin ((RS)-α-cyano-3phenoxybenzyl (1RS,3RS;1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylate) is classified as highly toxic (class II)20.
Sublethal effects reported in amphibians due to exposure to cypermethrin include behavioral, morphological, and biochemical changes in tadpoles23,24,25, mortality and alteration of metamorphosis duration, enzymatic changes, decrease in hatching success24,25, hyperactivity26, inhibition of cholinesterase activity27, and changes in swimming activity7,28. However, there is limited research addressing the genotoxic effects of cypermethrin in amphibians. Therefore, it is important to evaluate the susceptibility of anuran species to cypermethrin.
Environmental contaminations affect normal growth and development of amphibians, but induction of genetic DNA damage following exposure to pesticides is ultimately the most important adverse effect13. Analysis of blood cell morphology is an important bioindicator of pollution and toxic potential of substances to wildlife species29. Micronucleus test is one of the most commonly used methods to detect genotoxicity of chemical substances in the environment30. It is a rapid, effective, and inexpensive method and a good indicator of chemical contamination in organisms such as amphibians31,32 and can provide information on exposure to genotoxic contaminants33.
The objective of this study was to evaluate the toxic potential of a commercial formulation of cypermethrin in Physalaemus gracilis tadpoles using the micronucleus test and an ecological risk assessment.
Results
Mortality
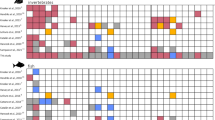
The commercial formulation of cypermethrin studied showed an LC50 of 273.41 µg L−1 (− 95% = 211.22 µg L−1; + 95% = 353.90 µg L−1) for Physalaemus gracilis tadpoles in 96 h exposure. Tadpole mortality was significant compared to exposure time in 72 h (F3;24 = 5.25, p = 0.006; Tukey p = 0.043), indicating that the longer the exposure time, the greater the mortality, but not compared to concentration (F6;35 = 2.15, p = 0.07) (Fig. 1).
In the chronic test, mortality of P. gracilis tadpoles was significant compared to concentrations 6 µg L−1 (F3;20 = 9.35, p = 0.026) and 20 µg L−1 (F3;20 = 9.35, p = 0.0004); and compared to time after 168 h (F6;21 = 2.97, p = 0.02) (Fig. 2).
Micronucleus and erythrocyte nuclear abnormalities
The increase in micronuclei was significant in individuals exposed to concentrations of 6 µg L−1 (F4,70 = 7.72; p < 0.01) and 20 µg L−1 (F4,70 = 7.72; p < 0.01) compared to the control treatment (Table 1). The concentration of 20 µg L−1 promoted 4 times more micronuclei compared to the control treatment.
All tadpoles studies exhibited some nuclear abnormalities (Fig. 3), with 20.16 ‰ of abnormalities at the highest concentration (20 µg L−1) after 168 h of exposure. The most common ENAs at all concentrations was the notched nucleus (6.64‰ of the total cells analyzed), followed by a lobed nucleus (2.65‰) and a nucleus with bubble (1.17‰). Notched (F4,70 = 27.16; p < 0.05) and lobed cells (F4,70 = 13.94; p < 0.01) were significant at a dose of 3 µg L−1, while bubble cells (F4,70 = 38.04; p < 0.01) and microcytosis were significant from 6 µg L−1 (F4,70 = 24.47; p < 0.01) and apoptosis at the highest dose (20 µg L−1) (F4,70 = 11.82; p < 0.01) compared to control.
Micronucleus and erythrocyte nuclear abnormalities in P. gracilis tadpoles exposed to concentrations of the commercial formulation of cypermethrin. (A; control) cells with normal nuclei, (B) cell with micronucleus; (C) cells with apoptosis; (D) binucleated cell; (E) cell with bubble or bud; (F) cells with karyolysis; (G) notched cell; (H) lobed cells; (I) cells with microcytosis.
Total cell changes (‰) were significantly different from control treatment at concentrations of 3 µg L−1 (F4;70 = 53.4, p < 0.05), 6 µg L−1 (F4;70 = 53.4, p < 0.01), and 20 µg L−1 (F4;70 = 53.4, p < 0.01) (Table 1).
Ecological risk analysis
Cypermethrin exhibited high acute (AHQ > 0.5) and chronic (CHQ > 1) ecological risk to P. gracilis for all variables evaluated (Table 2). The MATC value ranged from 1.73 to 10.95 μg L−1.
Discussion
Toxicity evaluation showed that the insecticide cypermethrin has a high acute toxicity to P. gracilis. The LC50 determined was 273.41 µg L−1, a value below 10,000 µg L−1, a reference value for the classification of the substance as very toxic according to GHS34. Similar results were also found for P. cuvieri (LC50 = 240 µg L−1)28 and lower for P. biligonigerus (LC50 = 129 µg L−1)35 and Duttaphrynus. melanostictus (LC50 = 3.34 µg L−1)23, indicating that cypermethrin can be lethal to several amphibian species. In addition, concentrations approaching those observed for several species have already been detected in natural waters (194 µg L−1)21, strongly suggesting that pesticides play an important role in the decline of populations of these aquatic species. Concentrations of cypermethrin considered sublethal or chronic also caused high mortality in P. gracilis. At a concentration of 20 µg L−1 cypermethrin, the mortality rate was more than 50% after a seven-day exposure to the insecticide. It is noteworthy that this result has characteristics of acute toxicity, since it caused the death of more than half of the cypermethrin-exposed individuals. Vanzetto et al.7 also confirmed the occurrence of changes in swimming activity and mouth morphology of P. gracilis tadpoles exposed to pyrethroids, affecting their ability to escape from predators and to feed, influencing the viability of anuran population36,37.
The observed high mortality was a consequence of genotoxic effects observed in amphibians exposed to different subconcentrations of cypermethrin (6 and 20 µg L−1), which was confirmed by the presence of micronuclei (MN) and erythrocyte nuclear abnormalities. The formation of MN indicates the occurrence of errors in mitotic division associated with weak binding of chromosomes to microtubules, a defect in the protein complex responsible for chromosome capture and transport, errors in chromosome segregation, and errors in DNA damage repair38,39 that may be associated with pesticide-induced oxidative stress40,41. Other abnormalities were also observed at all concentrations evaluated. Increasing cypermethrin concentrations resulted in a 5% and 20% increase in erythrocyte nuclear abnormalities at the lowest (1 µg L−1) and highest (20 µg L−1) doses assessed, respectively. Changes in species DNA, for example, can have significant effects on short- and long-term survival, declines in population size, changes in reproductive ability, inbreeding, loss of genetic diversity, and changes in migration rates. All of these factors affect species survival and conservation42,43. The formation of abnormalities in erythrocytes may indicate a blockade of cytokinesis leading to abnormal cell division (binucleated erythrocytes)44,45; lobed nuclei are protrusions of the nuclear membrane with multiple lobesarcau46, while other erythrocyte abnormalities may be related to DNA amplification, such as nuclear buds/bubbles47. The presence of anucleated erythrocytes may indicate effects on oxygen transport, especially in polluted waters48,49. While apoptosis is indicative of cell death50.
Other studies have also demonstrated the genotoxic effects of cypermethrin. Cabagna et al.51 demonstrated the presence of micronuclei and nuclear changes in cells such as binucleated cells and apoptotic cells in Odontophrynus americanus after exposure to high concentrations of cypermethrin (5000 and 10,000 µg L−1) for up to 96 h. Cypermethrin-induced apoptosis of cells was also detected in P. biligonigerus52 and Rhinella arenarum53. These results indicate that cypermethrin has a genotoxic effect on several species of aquatic organisms, and analysis of MN and ENAs may be an indicator of sublethal effects in amphibians and can be applied to native species and wild populations exposed to toxic substances12.
The commercial formulation of cypermethrin had a high ecological risk, both acute and chronic, with HQ above USEPA54 level of concern, and is likely to have adverse effects on species if present in the environment. In the chronic risk assessment, the NOEC for mortality was 3 µg L−1, confirming that concentrations already found in water may pose a risk to the species55. determined a NOEC of 500 µg L−1 for lethality of R. arenarum larvae after 168 h of exposure to a mixture of endosulfan and cypermethrin; this value was reduced to 0.0005 µg L−1 after 336 h. The authors show that the longer the exposure time, the lower the concentration that can have harmful effects on the species. It is also important to highlight that the NOEC value was higher than that observed for P. gracilis for the same exposure time, indicating species-specific responses to cypermethrin. Moreover, in terms of mortality, exposure of P. gracilis to cypermethrin caused a CHQ value of 64.67, a value higher than the reference value established by the USEPA54 and also reported for the larvae of R. arenarum (CHQ > 388.00 in 336 h), indicating that the insecticide studied poses a high risk to several amphibian species. Considering that P. gracilis completes metamorphosis in approximately 30 days56, it can be inferred that the cypermethrin concentrations tested can potentially promote population decline by preventing infected individuals from reaching the adult or reproductive stage at an early stage.
In the calculated risk assessment for micronuclei and other erythrocyte nuclear abnormalities, CHQ values ranged from 14.92 to 97.00, suggesting that cypermethrin poses a potential genotoxic risk to P. gracilis, even in its natural habitat. The maximum acceptable concentration of the xenobiotic for P. gracilis, taking into account mortality, is 4.24 µg L−1. However, concentrations as low as 1 µg L−1 have shown genotoxic effects. This fact may lead to an increase in abnormal individuals57 and affect the development and reproduction of the species in its habitat, contributing to the decline of the amphibian population.
The commercial formulation of the insecticide cypermethrin showed high acute and chronic toxicity to P. gracilis. A high mortality rate was observed, possibly due to the toxic effects evidenced by the presence of micronuclei and erythrocyte nuclear abnormalities, especially notched nuclei, lobed nuclei, and nuclei with bubble. In addition, high ecological risk, both acute and chronic, was demonstrated for the species studied. These data combined with previous studies by our research group showing that even various commercial formulations of cypermethrin still cause a reduction in acetylcholinesterase (AChE) and butyrylcholinesterase (BchE) activities and oxidative stress58 and cause changes in swimming activity and mouth malformations59 in P. gracilis indicate the high lethal and sublethal toxicity of the commercial formulation of cypermethrin for this species. Hartmann et al.60 considered that commercial formulations of cypermethrin were most toxic to P. gracilis and another species of the same genus (P. cuvieri), compared to nine other pesticides. This suggests that environmentally relevant legally approved concentrations of cypermethrin may cause high mortality rates and contribute to long-term population declines.
Further studies are needed to evaluate the toxicity of the insecticide to amphibians, as the concentrations found in the environment caused high mortality rates and pose a potential risk to P. gracilis. Research with amphibian species should be encouraged because there are few data on these organisms, especially Brazilian species.
Materials and methods
Chemicals
Toxicity tests were conducted with the commercial formulation of the insecticide cypermethrin: Cyptrin 250 EC (Nufarm Indústria Química e Farmacêutica S.A., Maracanaú/CE, Brazil) consisting of 250 gL−1 of the active ingredient (a.i.) cypermethrin and 723 g L−1 of inert ingredients. The insecticide has a density of 1.12 g mL−1 at 22 °C, a melting point of 60–80 °C (pure isomers), a boiling point of 170–195 °C, and a vapor pressure of 4 × 10–8 mm Hg at 70 °C61. Under normal temperature and pH conditions, the product is stable to hydrolysis with a half-life greater than 50 days and stable to photolysis with a half-life greater than 100 days62,63.
The commercial formulation was used to prepare the stock solution (500 mg a.i.a. L−1), which was diluted in distilled water to obtain the concentrations to be used in the acute toxicity study: 100 µg L−1, 200 µg L−121, 350 µg L−1, 400 µg L−1, 600 µg L−1, 700 µg L−1, and 800 µg a.i. L−1; and chronic toxicity: 1 µg L−1, 3 µg L−164, 6 µg L−165, and 20 µg a.i. L−166.
Test organism
Spawns of Physalaemus gracilis (Boulenger, 1833) were collected in natural environment in lakes in the city of Erechim, RS, Brazil (latitude: 27° 43′ 46.11″ South; longitude: 52° 16′ 54.40″ West), with egg laying lasting less than 24 h.
Immediately after collection, spawnings were placed in aquaria with a capacity of 15 L containing water with drinking water standards: temperature 22 °C ± 2 °C, pH 7.0 ± 0.5, dissolved oxygen 4.0 ± 1.0 mg L−1, turbidity < 5, conductivity 160 ± 10 µS cm−1, alkalinity (CaCO3) 9.74 mg L−1, Ca 6.76 mg L−1, Na 44.1 mg L−1, Mg 1.35 mg L−1, Fe 0.08 mg L−1, Ni < 0.001 mg L−1; under constant aeration and controlled laboratory conditions with temperature of 24 ± 2 °C, relative humidity between 60–80% and 12/12 h light–dark. Tadpoles were reared under these conditions until they reached developmental stage 2567. The tadpoles used in the tests had an average weight of 0.037 g ± 0.012 and an average length of 15.82 mm ± 1.59.
Ethical approval and informed consent
The present study was approved by the Ethics Committee for the Animal Use (CEUA) of the Federal University of Fronteira Sul, Erechim, RS, Brazil, under protocol number 23205.003634/2017-70.
Experimental design
Toxicity tests
The acute toxicity test was performed on subjects exposed to six concentrations of the commercial formulation of cypermethrin: 100, 200, 350, 400, 600, 700, and 800 µg a.i. L−1 for 96 h. Water temperature (22 ± 2 °C) and dissolved oxygen (4 ± 2 mg L−1) were kept constant throughout the test period, and tadpole mortality was assessed every 24 h. Acute toxicity was classified according to the Globally Harmonized System of Classification and Labeling of Chemicals34 as: high toxicity (LC50 < 1 mg L−1), moderate toxicity (LC50 between 1 and 10 mg L−1), or low toxicity (LC50 > 10 mg L−1).
The chronic toxicity test lasted 168 h (7 days) under static conditions and using the sublethal concentrations: 1, 3, 6, and 20 µg a.i. L−1. In both tests, 10 tadpoles were evaluated per treatment with six replicates, resulting in a total of 60 individuals for each concentration. In parallel, a treatment was performed as a negative control using only water. Each experimental unit consisted of a sterile glass with a capacity of 500 mL and a density of 1 tadpole per 50 mL of solution. The flasks were covered with plastic film to prevent evaporation and were constantly aerated.
The present study was approved by the Ethics Committee for the Animal Use (CEUA) of the Federal University of Fronteira Sul, Erechim, RS, Brazil, under protocol number 23205.003634/2017-70. All methods were carried out in accordance with relevant guidelines and regulations. The study is reported in accordance with ARRIVE guidelines.
The water was chemically analyzed to determine pesticide concentrations at 0, 96, and 168 h. Analysis was performed at the Laboratory for Analysis of Pesticides (LARP), Federal University of Santa Maria, using gas chromatography coupled with triple quadrupole mass spectrometry (Varian model 1200, Palo Alto, CA, USA), according to Sabin et al.68 and Martins et al.69. The pesticide quantification in the water was shown as Supplementary material (Table SM1).
Micronucleus and erythrocytes nuclear abnormalities
For micronucleus assay (MN) and erythrocyte nuclear abnormalities (ENAs), 15 tadpoles from each treatment were analyzed. Tadpoles were anesthetized with 5% lidocaine (50 mg g−170, and blood samples were collected by cardiac puncture with disposable heparinized syringes. Blood smears were prepared on sterilized slides for microscopy, air dried, fixed with 100% methanol (4 °C) for 2 min, and then stained with 10% Giemsa solution in the dark for 15 min. At the end of the process, the slides were washed with distilled water to remove excess dye and dried at room temperature.
The presence of MN and ENAs was determined by analyzing at least 1000 erythrocytes from each tadpole using a 100 × microscope objective71. A total of 75,796 tadpole erythrocytes were evaluated considering cypermethrin concentrations and control. Genotoxicity was analyzed according to Carrasco et al. and Fenech et al.38,72 by determining the frequency of the following nuclear lesions: (1) anucleated cells: cells without nuclei; (2) apoptosis cells: fragmented nuclei, with cells programmed to die; (3) binucleated cells: cells with two nuclei; (4) nuclear bud or bubble cells: nucleus with a small protrusion of the nuclear membrane, bubble size similar to the size of micronuclei; (5) karyolysis cells: nucleus that has only the outline, without internal material; (6) notched cells: nuclei that have a well-defined slit or cut in their shape, also called kidney-shaped nuclei; (7) lobed cells: nuclei with protrusions larger than the bubbles described above; (8) cells with microcytosis: condensed nucleus and reduced cytoplasm; and. The changes were compared with the negative control results.
Ecological risk analysis
Ecological risk was determined using the hazard quotient (HQ) according to the United States Environmental Protection Agency54. The HQ is calculated by the ratio between the estimated environmental concentration (EEC) and LC50 for acute risk (AHQ = EEC/LC50), and between ECC and no observed effect concentration (NOEC) for chronic risk (CHQ = EEC/NOEC). When it was not possible to statistically calculate NOEC, lowest observed effect concentration (LOEC) values were used to calculate HQ73. The HQ was compared to the level of concern (LOC—level of concern)54, where AHQ > 0.5 and CHQ > 1 indicate the existence of an ecological risk. The EEC is an estimated (or maximum) environmental contaminant concentration next to the geographic range of the species, using the maximum level of cypermethrin reported in Argentina (0.194 mg L−1)25. The maximum acceptable toxicant concentration (MATC) was calculated from the geometric mean of the LOEC and NOEC values.
Statistical analysis
Acute toxicity test results (LC50) were analyzed by the TSK—Trimmed Spearman-Karber method74 using GBasic software. Chronic test data were previously tested for normality of errors (Shapiro Wilks) and homogeneity of variances (Bartlett). Results were analyzed with a one-way analysis of variance (ANOVA). Tukey’s test was used to compare data with each other, and Dunnett’s test was used to compare treatment data with the negative control.
LOEC and NOEC data were analyzed with the Dunnett test. Statistical tests were performed using Statistica 8.0 software (StatSoft) with a significance level of 95% (p < 0.05).
Data availability
Data are available in Supplementary Information and on request from the corresponding author.
References
Diamond, M. L. et al. Exploring the planetary boundary for chemical pollution. Environ. Int. 78, 8–15 (2015).
Pérez-Iglesias, J. M., Soloneski, S., Nikoloff, N., Natale, G. S. & Larramendy, M. L. Toxic and genotoxic effects of the imazethapyr-based herbicide formulation Pivot H® on montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol. Environ. Saf. 119, 15–24 (2015).
Beebee, T. J. C. & Griffiths, R. A. The amphibian decline crisis: A watershed for conservation biology?. Biol. Conserv. 125, 271–285 (2005).
Herek, J. S. et al. Can environmental concentrations of glyphosate affect survival and cause malformation in amphibians? Effects from a glyphosate-based herbicide on Physalaemus cuvieri and P. gracilis (Anura: Leptodactylidae). Environ. Sci. Pollut. Res. 27, 22619–22630 (2020).
Rutkoski, C. F. et al. Lethal and sublethal effects of the herbicide atrazine in the early stages of development of Physalaemus gracilis (Anura: Leptodactylidae). Arch. Environ. Contam. Toxicol. 74, 587–593 (2018).
Macagnan, N. et al. Toxicity of cypermethrin and deltamethrin insecticides on embryos and larvae of Physalaemus gracilis (Anura: Leptodactylidae). Environ. Sci. Pollut. Res. 24, 20699–20704 (2017).
Vanzetto, G. V., Slaviero, G., Sturza, P. F., Rutkoski, F. & Macagnan, N. Toxic effects of pyrethroids in tadpoles of Physalaemus gracilis (Anura: Leptodactylidae). Ecotoxicology 28, 1105–1114 (2019).
Peluso, J. et al. Metals, pesticides, and emerging contaminants on water bodies from agricultural areas and the effects on a native amphibian. Environ. Res. 226, 115692 (2023).
Pérez Coll, C. S., Aronzon, C. M. & Svartz, G. V. Chapter 17. Developmental Stages of Rhinella arenarum (Anura, Bufonidae.) in Toxicity Studies: AMPHITOX, a Customized Laboratory Assay 407–424 (2017). https://doi.org/10.1039/9781782629887-00407.
Jing, X. et al. Exposure of frogs and tadpoles to chiral herbicide fenoxaprop-ethyl. Chemosphere 186, 832–838 (2017).
Slaby, S., Marin, M., Marchand, G. & Lemiere, S. Exposures to chemical contaminants: What can we learn from reproduction and development endpoints in the amphibian toxicology literature?. Environ. Pollut. 248, 478–495 (2019).
Fanali, L. Z., Franco-Belussi, L., Bonini-Domingos, C. R. & de Oliveira, C. Effects of benzo[a]pyrene on the blood and liver of Physalaemus cuvieri and Leptodactylus fuscus (Anura: Leptodactylidae). Environ. Pollut. 237, 93–102 (2018).
Soloneski, S., Ruiz de Arcaute, C. & Larramendy, M. L. Genotoxic effect of a binary mixture of dicamba- and glyphosate-based commercial herbicide formulations on Rhinella arenarum (Hensel, 1867) (Anura, Bufonidae) late-stage larvae. Environ. Sci. Pollut. Res. 23, 17811–17821 (2016).
Agostini, M. G., Roesler, I., Bonetto, C., Ronco, A. E. & Bilenca, D. Pesticides in the real world: The consequences of GMO-based intensive agriculture on native amphibians. Biol. Conserv. 241, 108355 (2020).
Rutkoski, C. F. et al. Morphological and biochemical traits and mortality in Physalaemus gracilis (Anura: Leptodactylidae) tadpoles exposed to the insecticide chlorpyrifos. Chemosphere 250, 126162 (2020).
Frost, D. Amphibian Species of the World 6.2, an Online Reference. https://amphibiansoftheworld.amnh.org/ (2023).
Borges-Martins, M., Colombo, P., Zank, C., Becker, F. G. & Melo, M. T. Q. Biodiversidade: Regiões da Lagoa do Casamento e dos Butiazais de Tapes. in Biodiversidade: Regiões da Lagoa do Casamento e dos Butiazais de Tapes, Planície Costeira do Rio Grande do Sul (eds. Becker, F. G., Ramos, Ri. A. & Moura, L. de A.) 276–291 (2007).
Lavilla, E., Kwet, A., Segalla, M. V. & Baldo, D. Physalaemus gracilis. The IUCN Red List of Threatened Species 2010 (2010).
FAOSTAT. Food and Agriculture Organization of the United Nations. Pesticides Use. https://www.fao.org/faostat/en/#data/RP (2022).
ANVISA. Índice Monográfico Nome c10 Cipermetrina. (2023).
Marino, D. & Ronco, A. Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada Argentina. Bull. Environ. Contam. Toxicol. 75, 820–826 (2005).
Paravani, E. V., Simoniello, M. F., Poletta, G. L., Zolessi, F. R. & Casco, V. H. Cypermethrin: Oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat. Res. Genet. Toxicol. Environ. Mutagen 826, 25–32 (2018).
David, M., Marigoudar, S. R., Patil, V. K. & Halappa, R. Behavioral, morphological deformities and biomarkers of oxidative damage as indicators of sublethal cypermethrin intoxication on the tadpoles of D. melanostictus (Schneider, 1799). Pest. Biochem. Physiol. 103, 127–134 (2012).
Greulich, K. & Pflugmacher, S. Differences in susceptibility of various life stages of amphibians to pesticide exposure. Aquat. Toxicol. 65, 329–336 (2003).
Greulich, K. & Pflugmacher, S. Uptake and effects on detoxication enzymes of cypermethrin in embryos and tadpoles of amphibians. Arch. Environ. Contam. Toxicol. 47, 489–495 (2004).
Saha, S. & Kaviraj, A. Acute toxicity of synthetic pyrethroid cypermethrin to some freshwater organisms. Bull. Environ. Contam. Toxicol. 80, 49–52 (2008).
Khan, M. Z. et al. Effect of Cypermethrin and permethrin on cholinesterase activity and protein contents in Rana tigrina (Amphibia). Turk. J. Zool. 27, 243–246 (2003).
Wrubleswski, J., Reichert, F. W., Galon, L., Hartmann, P. A. & Hartmann, M. T. Acute and chronic toxicity of pesticides on tadpoles of Physalaemus cuvieri (Anura, Leptodactylidae). Ecotoxicology 27, 360–368 (2018).
López González, E. C., Larriera, A., Siroski, P. A. & Poletta, G. L. Micronuclei and other nuclear abnormalities on Caiman latirostris (Broad-snouted caiman) hatchlings after embryonic exposure to different pesticide formulations. Ecotoxicol. Environ. Saf. 136, 84–91 (2017).
Feng, S., Kong, Z., Wang, X., Zhao, L. & Peng, P. Acute toxicity and genotoxicity of two novel pesticides on amphibian Rana N. Hallowell. Chemosphere 56, 457–463 (2004).
Ghisi, N. C., de Oliveira, E. C. & Prioli, A. J. Does exposure to glyphosate lead to an increase in the micronuclei frequency? A systematic and meta-analytic review. Chemosphere 145, 42–54 (2016).
Josende, M. E. et al. Genotoxic evaluation in two amphibian species from Brazilian subtropical wetlands. Ecol. Indic. 49, 83–87 (2015).
Pérez-Iglesias, J. M. et al. The genotoxic effects of the imidacloprid-based insecticide formulation Glacoxan Imida on Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae). Ecotoxicol. Environ. Saf. 104, 120–126 (2014).
GHS. Globally Harmonized System of Classification and Labelling of Chemicals (GHS). https://doi.org/10.1265/jjh.65.5. (2017).
Izaguirre, M. F., Lajmanovich, R. C., Peltzer, P. M., Soler, A. P. & Casco, V. H. Cypermethrin-induced apoptosis in the telencephalon of Physalaemus biligonigerus tadpoles (Anura: Leptodactylidae). Bull. Environ. Contam. Toxicol. 65, 501–507 (2000).
Hamilton, P. B. et al. Population-level consequences for wild fish exposed to sublethal concentrations of chemicals - a critical review. Fish Fish. 17, 545–566 (2016).
Cheron, M. et al. Exposure, but not timing of exposure, to a sulfonylurea herbicide alters larval development and behaviour in an amphibian species. Aquat. Toxicol. 254, 106355 (2023).
Fenech, M. et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 26, 125–132 (2011).
Luzhna, L., Kathiria, P. & Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 4, 1–17 (2013).
Costa, M. J., Monteiro, D. A., Oliveira-Neto, A. L., Rantin, F. T. & Kalinin, A. L. Oxidative stress biomarkers and heart function in bullfrog tadpoles exposed to Roundup Original®. Ecotoxicology 17, 153–163 (2008).
Nwani, C. D., Nagpure, N. S., Kumar, R., Kushwaha, B. & Lakra, W. S. DNA damage and oxidative stress modulatory effects of glyphosate-based herbicide in freshwater fish Channa punctatus. Environ. Toxicol. Pharmacol. 36, 539–547 (2013).
Falcão, C. B. R., Pinheiro, M. A. A., Torres, R. A. & Adam, M. L. Spatial-temporal genome damaging in the blue crab Cardisoma guanhumi as ecological indicators for monitoring tropical estuaries. Mar Pollut Bull 156, 111232 (2020).
Thomas, E. G. et al. Effects of freshwater pollution on the genetics of zebra mussels (Dreissena polymorpha) at the molecular and population level. Biomed. Res. Int. 2014, (2014).
de Amaral, D. F. et al. Sub-lethal effects induced by a mixture of different pharmaceutical drugs in predicted environmentally relevant concentrations on Lithobates catesbeianus (Shaw, 1802) (Anura, ranidae) tadpoles. Environ. Sci. Pollut. Res. 26, 600–616 (2019).
Mahboob, S., Al-Balwai, H. F. A. & Fahad Al-Misned, Z. A. Investigation on the genotoxicity of mercuric chloride to freshwater Clarias gariepinus. Pak. Vet. J. 34, 100–103 (2014).
Ruiz de Arcaute, C. et al. Genotoxicity evaluation of the insecticide imidacloprid on circulating blood cells of Montevideo tree frog Hypsiboas pulchellus tadpoles (Anura, Hylidae) by comet and micronucleus bioassays. Ecol. Indic. 45, 632–639 (2014).
Pollo, F. E. et al. Evaluation in situ of genotoxic and cytotoxic response in the diploid/polyploid complex Odontophrynus (Anura: Odontophrynidae) inhabiting agroecosystems. Chemosphere 216, 306–312 (2019).
Glomski, C. A., Tamburlin, J., Hard, R. & Chainani, M. The phylogenetic odyssey of the erythrocyte IV. The amphibians. Histol. Histopathol. 12, 147–170 (1997).
Barni, S. et al. Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat. Toxicol. 81, 45–54 (2007).
Benvindo-Souza, M. et al. Micronucleus test in tadpole erythrocytes: Trends in studies and new paths. Chemosphere 240, 124910 (2020).
Cabagna, M. C., Lajmanovich, R. C., Peltzer, P. M., Attademo, A. M. & Ale, E. Induction of micronuclei in tadpoles of Odontophrynus americanus (Amphibia: Leptodactylidae) by the pyrethroid insecticide cypermethrin. Toxicol. Environ. Chem. 88, 729–737 (2006).
Izaguirre, M. F. et al. Modelos experimentales de anuros para estudiar los efectos de piretroides. Ciencia, Docencia y Tecnol. 2006, 181–206 (2006).
Casco, V. H. et al. Apoptotic cell death in the central nervous system of Bufo arenarum tadpoles induced by cypermethrin. Cell Biol. Toxicol 22, 199–211 (2006).
USEPA. U.S. Environmental Protection Agency. EPA/630/R-95/002F. Guidelines for Ecological Risk Assessment 26846–26924 (Risk Assessment Forum, 1998).
Svartz, G. V., Aronzon, C. M. & Coll, C. S. P. Combined endosulfan and cypermethrin-induced toxicity to embryo-larval development of Rhinella arenarum. J. Toxicol. Environ. Health A 79, 197–209 (2016).
Herek, J. S. Glifosato e seus efeitos sobre duas espécies de anfíbios nativos da américa do sul Physalaemus cuvieri e Physalaemus gracilis (Universidade Federal da Fronteira Sul - Campus Erechim, 2017).
Gibbons, J. W. et al. Remarkable amphibian biomass and abundance in an isolated wetland: Implications for wetland conservation. Conserv. Biol. 20, 1457–1465 (2006).
Rutkoski, C. F. et al. Cypermethrin- and fipronil-based insecticides cause biochemical changes in Physalaemus gracilis tadpoles. Environ. Sci. Pollut. Res. 28, 4377–4387 (2021).
Vanzetto, G. V. et al. Toxic effects of pyrethroids in tadpoles of Physalaemus gracilis (Anura: Leptodactylidae). Ecotoxicology 28, 1105–1114 (2019).
Hartmann, M., Hartmann, P. A. & Müller, C. Pesticide effects on tadpole’s survival. In Toxicology of Amphibian Tadpoles (eds Almeida, E. A. & Freitas, J. S.) 204–214 (CRC Press, 2024).
EXTOXNEXT. Cypermethrin. Extension Toxicology Network - Pesticide Information Profile. http://pmep.cce.cornell.edu/profiles/extoxnet/carbaryl-dicrotophos/cypermet-ext.html (1993).
Kamrin, M. A. Section II - Pyrethroids and other botanicals. In Pesticide Profiles: Toxicity, Environmental Impact, and Fate (ed. Kamrin, M. A.) 22–26 (Lewis Publishers, 1997).
USEPA. U.S. Environmental Protection Agency - Pesticide Fact Sheet Number 199: Cypermethrin. (1989).
Belluta, I., Almeida, A. A., Coelho, J. C., do Nascimento, A. B. & da Silva, A. M. M. Avaliação temporal e espacial no córrego do cintra (botucatu-sp) frente aos defensivos agrícolas e parâmetros físico-químicos de qualidade da água – um estudo de caso. Energia Agric. 25, 54–73 (2010).
Etchegoyen, M., Ronco, A., Almada, P., Abelando, M. & Marino, D. Occurrence and fate of pesticides in the Argentine stretch of the Paraguay-Paraná basin. Environ. Monit. Assess. 189, 1–12 (2017).
Carlos, E. A., Neves, A. A., Reis, C. & De Queiroz, M. E. L. R. Determinação simultânea de trialometanos e agrotóxicos em água por cromatografia gasosa. Quim Nova 34, 272–278 (2011).
Gosner, K. L. A simplified table for staging anuran embryos larvae with notes on identification. Herpetologica 16, 183–190 (1960).
Sabin, G. P., Prestes, O. D., Adaime, M. B. & Zanella, R. Multiresidue determination of pesticides in drinking water by gas chromatography-mass spectrometry after solid-phase extraction. J. Braz. Chem. Soc. 20, 918–925 (2009).
Martins, M. L., Donato, F. F., Prestes, O. D., Adaime, M. B. & Zanella, R. Determination of pesticide residues and related compounds in water and industrial effluent by solid-phase extraction and gas chromatography coupled to triple quadrupole mass spectrometry Published in the topical collection (Bio)Analytical Research in Latin America with guest editors Marco A. Zezzi Arruda and Lauro Kubota. Anal. Bioanal. Chem. 405, 7697–7709 (2013).
CONCEA - Conselho Nacional de Controle de Experimentação Animal. Resolução Normativa CONCEA no 37 - Diretriz da Prática de Eutanásia. (2018).
Lajmanovich, R. C. et al. Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water Air Soil Pollut. 224, 1–13 (2013).
Carrasco, K. R., Tilbury, K. L. & Myers, M. S. Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can. J. Fish. Aquat. Sci. 47, 2123–2136 (1990).
Wu, J., Lu, J., Lu, H., Lin, Y. & Chris Wilson, P. Occurrence and ecological risks from fipronil in aquatic environments located within residential landscapes. Sci. Total Environ. 518–519, 139–147 (2015).
Hamilton, M. A., Russo, R. C. & Thurston, R. V. Trimmed spearman-karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 11, 714–719 (1977).
Acknowledgements
The authors thank the Federal University of Fronteira Sul for financial support. C.F.R. and C. are grateful to CAPES, V.F.S and A.F. to the Rio Grande do Sul State Research Foundation (FAPERG) for fellowships. This study is financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior—Brazil (CAPES), finance code 001.
Author information
Authors and Affiliations
Contributions
M.H., P.A.H. and N.M. contributed to the study conception and design. Material preparation, data collection and analysis were performed by N.M., C.F.R., A.F., V.J.S. and C.M. The first draft of the manuscript was written by N.M., and revised and edited by C.M., P.A.H. and M.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macagnan, N., Rutkoski, C.F., Folador, A. et al. Mortality and toxicity of a commercial formulation of cypermethrin in Physalaemus gracilis tadpoles. Sci Rep 13, 17826 (2023). https://doi.org/10.1038/s41598-023-45090-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45090-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.