Abstract
Rosa damascena is one of the most important medicinal and ornamental plants in Iran which is tolerant of salinity to some extent. However, the selection of genotypes that are more tolerant to salinity will influence on Damask cultivation in salt stress-affected regions. For this purpose, a factorial experiment in a completely randomized design with three replicates was performed under in vitro conditions on four Damask rose genotypes (Atashi, Bi-Khar, Chahar-Fasl and Kashan) at 5 concentrations of NaCl (0, 25, 50, 75, and 100 mM), and the physico-chemical traits were measured 14 and 28 days after treatment.The results showed that Atashi genotype with high levels of Chl a, Chl b, total Chl content, carotenoids, relative leaf water content, proline, total soluble protein, TPC, TFC, TAA, and the highest increase in the activity of antioxidant enzymes such as GPX, APX, CAT, SOD, and POD as well as the lowest amount of hydrogen peroxide showed a better protection mechanism against oxidative damage than the other three genotypes (Bi-Khar, Chahar-Fasl and Kashan) in the 14th and 28th days by maintaining the constructive and induced activities of antioxidant enzymes, it was shown that Bi-Khar genotype had moderate tolerance and Kashan and Chahar-Fasl genotypes had low tolerance to salinity stress. In vitro selection methods can be used effectively for salt tolerant screening of Damask rose genotypes, although the same experiment should be conducted in open filed cultures to verify the in vitro experimental results.
Similar content being viewed by others
Introduction
Damask rose (Rosa damascena) is known as an aromatic plant, and most researchers consider its origin to be Bulgaria, Iran, Turkey and India1. Considering the various uses of Damask rose in medicinal products, perfumery and cosmetics, it has caused a lot of attention to develop its cultivation2. According to the ability of Damask rose to be somewhat tolerant to environmental stresses3 it seems that the selection of a more resistant genotypes has a greater advantage for the development of its cultivation in the mentioned regions. Different degrees of resistance to salinity have been seen among rose4 and pistachio5 genotypes which depends on various factors, including cultivar, rootstock, irrigation and planting system. Therefore, it is of vital need to screen and choose the salt tolerance genotypes for sustainable agriculture.
In recent years, drought stress is going to be widespread through the world and subsequently salinity is un-avoidable phenomenon as a secondary stress, especially in arid and semi-arid regions. Iran is one of the countries where major abiotic stresses such as drought and salinity cause yield reduction, loss of soil fertility and impossibility of agriculture in most of its parts6. Salinity is the major limiting factors in the soil which effect on plant performance and its chemical constituents. The harmful effects of salinity on plant growth are categorized in four items; (1) osmotic effect, (2) specific ion effects, (3) nutritional imbalance, and (4) production of reactive oxygen species (ROS)7. Salinity caused to increase in the cellular antioxidant pool components such as carotenoids, ascorbate, proline, total phenol content (TPC), total flavonoid content (TFC), and total antioxidant activity (TAA) especially in the tolerant variety of A. tricolor, while the sensitive variety accumulated the proline much more than other variety8. Against negative effect of salinity on morphology and physiology of plant, increase in superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), ascorbate peroxidase (APX), and peroxidase (POD) activity under salinity have been reported also in plants9. It is suggested that the increment of ascorbate and APX is the sign of tolerance in plants under stress9. On the other hand, decrease in carbohydrates was expected because of prevention of the Rubisco and phosphoenol pyruvate carboxylase and malate dehydrogenase activities in plants under salinity10. Attia et al.11 concluded that the salt resistance of R. damascena at 100 mM NaCl was correlated the maintenance of high water and chlorophyll contents, although lipid peroxidation was occurred by increasing malondialdehyde (MDA) and hydrogen peroxide (H2O2) contents in Damask rose at 100 mM NaCl. It is also demonstrated that treatment of R. rubiginosa with 150–200 mM NaCl resulted in higher extent of leaf chlorosis compared to lower concentrations (25–50 mM), and it is more tolerant to salinity- induced by NaCl than CaCl212. Our previous study showed that the tolerance threshold of Damask rose ʻKashanʼ to salinity was 50 mM (about 6.5 dS m−1), although the antioxidant capacity of explants exposed to 100 mM NaCl was higher than 50 mM13. According to the our previous studies14,15,16 the Damask rose genotypes enriched with antioxidants were screened across to drought stress. Due to the high economic value of the Damask rose as a suitable medicinal plant in dry regions16, it is necessary to evaluate some developmental and performance under salinity. In addition, one of the ways to select the genotypes with the best performance under abiotic stresses is to use of the tissue culture, which requires very little space and time17. Considering that Iran as the main origin of Damask rose, it is located in the arid belt of the world and had the ancient history of growing the mentioned medicinal and valuable ornamental plant in saline areas, it shows that Damask rose can be an effective option for the development of cultivation in saline areas.
Therefore, the aim of the present study was to investigate the effect of salinity on some physiological and biochemical traits, under in vitro culture after 14th and 28th days salinity treatment to better elucidate non-enzymatic and enzymatic antioxidative defense pathways, regarding salt-tolerant by comparing four genotypes of R. damascena.
Results
Physiological traits
Salt stress in all Damask rose genotypes caused to a gradual reduction in leaf relative water content (RWC) and membrane stability index (MSI) in a concentration and time-dependent manner up to 100 mM NaCl (Fig. 1a,b) RWC decreased significantly (p < 0.05) in four genotypes under the different salt concentrations (Fig. 1a). At the 14th day, the reduction percentage in RWC in ‘Atashi’ was less (180%) at 100 mM NaCl, while ‘Chahar-Fasl’ showed a greater reduction (348%). Significant differences were observed between the 14th and 28th days, ‘Atashi’ had 244% loss and ‘Chahar-Fasl’ had 434% loss in the shoots water content (Fig. 1a). The membrane stability index (MSI) was significantly reduced in the Damask rose genotypes due to salinity stress (Fig. 1b). ‘Chahr-Fasl’ genotype had the maximum decrease (334 and 264%) while the ‘Atashi’ genotype had the minimum decrease (260 and 170%) compared to the control shoots (Fig. 1b) and other genotypes on the 14th and 28th days, respectively.
Photosynthetic pigments
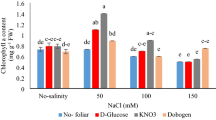
As well as increase in the NaCl concentration, the photosynthetic pigments content including chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (total Chl,), and carotenoids (CARs) in the shoots of the plant decreased, which is an indicator of stress. The least and the highest decrease in Chl a were related to the ‘Atashi’ and ‘Chahar-Fasal’ with a 170% and 340%, respectively (Fig. 2a) compared to the control explants (Fig. 2a). In the 14th and 28th days, the highest Chl a was observed in the ‘Atashi’ and the lowest amount in both times was obtained in the ‘Chahar-Fasal’ and ‘Kashan’ (Fig. 2b). ‘Bi-Khar’ had the lowest decrease up to 7% compared to the other genotypes on the 14th day (Fig. 2b). On the other hand, ‘Chahar-Fasl’ displayed the lowest Chl b on the 28th day, at sever salinity stress (100 mM NaCl) compared to the control. The highest amount of total Chl (1.441 mg g−1 FW) was related to the ‘Atashi’ in the control shoots while the lowest amount (0.179 mg g−1 FW) was related to the 100 mM NaCl and ‘Chahar-Faslʼ (Fig. 2c). In comparison with the control shoots, the ‘Atashi’ and ‘Chahar-Fasl’ had the least and maximum decrease by 170% and 460%, respectively. (Fig. 2c). In the 14th and 28th days, the ‘Chahar-Fasl’ had the least total Chl (0.499 and 0.417 mg g−1 FW) and the ‘Atashi’ had the most total Chl (1.383 and 0.912 mg g−1 FW) (Fig. 2d). According to the Table 1 ‘Atashi’ displayed the least decrease, with a reduction of 150% (Table 1) in Chl b compared to the control on 14th day. ‘Atashi’ had the most CARs content also on 14th day under control condition, while ‘Chahar-Fasl’ and ‘Kashan’ at 100 mM NaCl on the 28th day, had the lowest CARs content. The greatest reduction in CARs were related to the ‘Chahar-Fasl’ (411%) and ‘Kashan’ (586%) on the 14th and 28th days (Table 1).
The effect of salt stress × four Damask genotypes on (a) Chl a, (b) sampling time (14th and 28th day) × four Damask genotypes (Atashi, Bi-Khar, Chahar-Fasl, and Kashan) on Chl a, (c) salt stress × four Damask genotypes on Chl b, and (d) sampling time × four Damask genotypes on Chl b. Different letters indicate significant differences according to LSD test at P < 0.05.
Antioxidant enzyme activities
Antioxidant enzyme activities in Damask rose genotypes were affected by NaCl concentration and time (Table 2) as in the 14th day up to 100 mM NaCl and in the 28th day up to 75 mM NaCl, all of the salt-treated Damask rose genotypes showed a substantial increase in their guaiacol peroxidase (GPX) and SOD concentration (Table 2). Furthermore, ‘Bi-Kharʼ and ‘Kashanʼ at 100 mM NaCl showed the maximum (626%) and the minimum (387%) increment in GPX on the 14th day, respectively compared to the control. At 75 mM NaCl, ʻAtashiʼ and ʻKashanʼ had the greatest (258%) and the lowest (119%) increment on the 28th day (Table 2). The activity of SOD in ʻAtashiʼ and ʻBi-Kharʼ showed the greatest and lowest amount (429% and 200%, respectively) in comparison with the control shoots on the 14th day, which were followed up to 204% and 160%, respectively on the 28th day (Table 2). Polyphenol oxidase (PPO) activity in all genotypes was increased at 75 and 50 mM level of NaCl on the 14th and 28th days, although it was decreased at higher concentrations of NaCl (Table 2). The highest PPO activity was obtained in ‘Atashi’ under 50 mM NaCl on the 28th day, while the lowest activity was obtained in the control shoots on the 14th day. Finally, the highest increment was recorded in ‘Atashi’ (630%), and the lowest was recorded in ‘Kashan’ (390%) on the 28th day (Table 2).
The activity of CAT and APX was increased in all Damask rose genotypes on 14th day and then reduced at higher concentrations of NaCl on 28th day. The most and the least CAT activity was detected in ‘Atashi’ under 75 mM NaCl on the 28th day and control treatment, respectively (Table 2). Our findings revealed that the highest increase was noted in ‘Atashi’ (420%) and the lowest was recorded in ‘Chahar-Fasl’ (250%) on 14th day (Table 2). The most APX activity was observed in ‘Atashi’ under 75 mM NaCl on 28th day, while the least was observed in ‘Kashan’ under control conditions on 14th day. Our findings showed that the highest and the lowest increase in APX were obtained in ʻAtashiʼ (470%) and ‘Kashan’ (330%) on 28th day, respectively (Table 2).
POD enzyme activity also increased along with increase in the level of NaCl, so that it showed the maximum and minimum value respectively in ʻAtashiʼ and ʻKashanʼ at 100 mM NaCl (Fig. 3a). ʻAtashiʼ had the most POD activity on both times after treatment, while the least POD activity was related to ʻChahar-Faslʼ. (Fig. 3b). POD showed the maximum activity 28 days after sever salinity treatment while the lowest POD activity was observed in the control shoots on 14th day (Fig. 3c).
The effect of salt stress × four Damask genotypes on (a) POD activity, (b) sampling time (14th and 28th day) × four Damask genotypes (Atashi, Bi-Khar, Chahar-Fasl, and Kashan) on POD activity, and (c) salt stress × sampling time on POD activity. Different letters indicate significant differences according to LSD test at P < 0.05.
Total phenol content (TPC) and total flavonoid content (TFC)
The triple interaction between time, genotype, and salinity had a statistically significant (P ≤ 0.05) impact on TPC and TFC content (Table 3) of Damask rose genotypes. Along with increase in the concentration of NaCl, the level of TPC also increased in all genotypes as the highest TPC was obtained in Atashi genotype under 100mM NaCl on 28th day (Table 3). The lowest TPC content was observed in the control explants of ‘Chahar-Fasl’ and ‘Kashan’, on 14th day. A marked enhancement of TPC content (324%) belonged to ‘Atashi’ under 100 mM NaCl on 14th day and the lowest was displayed in ‘Bi-Khar’ (176%) on 28th day (Table 3). In the 14th day up to 100 mM NaCl and in the 28th day up to 75 mM salinity, all of the salt treated Damask rose genotypes showed a substantial increase in their TFC (Table 3). Also, ‘Atashi’ and ‘Kashan’ at 100 mM NaCl showed the greatest (270%) and the lowest (387%) increment in TFC on 14th day, respectively compared to the control shoots. On the other hand, under 75 mM NaCl, ‘Atashi’ and ‘Chahar-Fasl’ had the greatest (258%) and the lowest (265%) increment in TFC on 28th day (Table 3).
Total antioxidant activity (TAA)
TAA was significantly affected by time, genotype and salt stress (Table 3). In all four genotypes, an enhancement in TAA was observed by increasing in NaCl concentration compared to the control. The highest TAA was obtained at 100 mM NaCl of the ‘Atashi’ on 28th day and the lowest was recorded for ‘Chahar-Fasl’ and ‘Kashan’ control shoots on 14th day. The most significant increase in TAA was obtained in ‘Atashi’ (277%) under the highest concentration of NaCl on 14th day (Table 3).
Protein, proline, malondialdehyde (MDA) and hydrogen peroxide (H 2 O 2 ) content
The mean comparison for the interaction and main effects of salinity, time and genotype on protein, proline, H2O2, and MDA is presented in Table 4. Up to 25 mM NaCl, the amount of total soluble protein increased in all genotypes except for ‘Atashi’. As well as increase in the NaCl concentration, there was a decreasing trend in the amount of total soluble protein in all Damask rose genotypes (Table 4). ‘Atashiʼ had the minimum decrease in total protein up to 39.7% and 60.6% on 14th and 28th days, respectively, while ‘Chahar-Fasl had the maximum decrease with 230% and 340% on 14th and 28th days, respectively, compared to the other genotypes (Table 4). On 14th and 28th days of the experiment, increasing in the NaCl concentration triggered a substantial increase in the amount of proline in all Damask rose genotypes. ‘Atashi’ and ‘Bi-Khar’ had the maximum and minimum proline at 100 mM NaCl, compared to the control on 28th and 14th days, respectively (Table 4). As well as increase in the concentration of NaCl from 0 to 100 mM, the amount of H2O2 increased dramatically in all Damask rose genotypes. At 100 mM NaCl, ‘Kashan’ showed the maximum H2O2 with 236% and 264% compared to the control conditions on 14th and 28th days, respectively while ‘Atashi’ showed the minimum increase up to 212% compared to the control Damask explants (Table 4). All of the salt-treated Damask rose genotypes showed a significant increment in their MDA content on 14th and 28th days (Table 4) as ‘Bi-Khar’ and ‘Atashi’ showed the most (380%) and the least (230%) increment at 100 mM NaCl compared to the normal condition (no salinity) on 28th and 14th days, respectively (Table 4).
Multivariate analysis of Damask rose genotypes under NaCl treatment and sampling time
Heat map analysis based on the response of Damask rose genotypes to salt stress simulated by NaCl applications under in vitro condition revealed that the traits including MDA, H2O2, proline, TAA, and TPC had positive correlation to salt stress tolerance. On the other hand, some traits such as enzymatic antioxidant including POD, SOD, CAT, APX, and PPO along with TFC, photosynthetic pigment, protein, RWC and MSI were also correlated positively (Fig. 4a). Cluster analysis and dendrograms in the heat map (Fig. 4b) revealed three major classes in the assessed traits of the plants under salt stress. Class I contained PPO, Proline, APX, TFC, SOD, GPX, TAA, POD CAT and TPC; class II contained H2O2 and MDA and class III contained MSI, RWC, CARs, Protein, Chl a, Chl b and Total Chl (Fig. 4b). Moreover, biplot of variables confirmed the heat map cluster analysis in which traits classified in three groups (Fig. 4a). In general, cluster analysis of heat maps for interaction between NaCl and sampling time indicated three classes. Class I contained the Damask rose genotypes was treated by NaCl and 14th day after treatment. Class II contained the Damask rose genotypes was treated by NaCl and 28th day. Finally, class III R. damascena genotypes under severe salt stress which was induced by 100 mM NaCl (Fig. 4a).
Heat map (a), loading biplot of the evaluated traits (b) and principal component analysis heat map (c) of the enzymatic antioxidants, the physiological and the biochemical changes in Damask rose genotypes. Genotypes under in vitro-induced salt stress condition. Heat map representing of relative water content (RWC), membrane stability index (MSI), Chlorophyll a (Chl a), Chlorophyll b (Chl b), Total chlorophyll (Total Chl), Carotenoids (CARs), Proline, Malondialdehyde (MDA), hydrogen peroxide (H2O2), Total soluble protein, Guaiacol peroxidase (GPX), Ascorbate peroxidase (APX), Superoxide dismutase (SOD), Peroxidase (POD), Catalase (CAT), Polyphenol oxidase (PPO), Total flavonoids content (TFC), Total phenol content (TPC) and Total antioxidant activity (TAA).
The principal component analysis (PCA) and the proportion of total variance (Fig. 4c) explained that three PCA were contributing 87.5% of total variation. The first PCA was the most influential with the variance of 57.9% and contained the traits of CARs, TPD, PPO, Proline, SOD, CAT, APX, GPX and POD activity. The second PCA explained 24% of the total variance, and consisted of RWC, MSI, CARs, Protein Chl a, Chl b and total Chl. The third PCA explained 5.6% of the total variance with H2O2 and MDA (Fig. 4c).
Difference between Damask rose genotypes (Atashi, Bi-Khar, Chahar-Fasl, and Kashan) under 100 mM salinity on 14th day and 28th day after salinity treatment were illustrated in Fig. 5a,b which is confirmed that Atashi genotype have had better performance than others 28 days after salinity treatment.
Discussion
Significant difference in Damask rose explants physiological traits such as leaf RWC and MSI was found among the four genotypes in this research (Fig. 1). RWC is undoubtedly one of the first plant traits influenced by salinity stress, because water stress occurs frequently when plants are exposed to high concentrations of salt in soil solution. Our findings revealed that salt stress affected on the water state of Damask rose genotypes, resulting in a decrease in RWC in saline conditions (Fig. 1a). Under control conditions, ʻAtashiʼ had the maximum leaf RWC (66.52%) (Fig. 1a). RWC decrease has been documented in several melon species, which is consistent with our findings18. Stevens et al.19 previously found that maintaining cell membrane integrity is important in salinity tolerance. MSI was reduced in this research when plants were exposed to salinity stress. Under saline conditions, ‘Atashi’ and ‘Bi-Khar’ had the most stable cell membranes (Fig. 1b). Several other researchers have confirmed these findings20, which stated that salinity increased cellular electrolyte leakage, so that selecting genotypes with superior leaf MSI may result in improved salinity tolerance in plants.
Salinity as well as drought stress, influenced on plant pigmentation as a decrease in the amount of photosynthetic pigments under salt stress is thought to be caused by delay in pigments biosynthesis or broken down quickly21. Reduced Chl a and Chl b levels, as well as total Chl content at higher NaCl concentrations, were measured in all Damask rose genotypes shoot (Fig. 2a–d). These findings are consistent with earlier research, which found that salt stress induced by NaCl, caused to decrease in total Chl concentration in Phaseolus vulgaris22. Salinity substantially reduced Chl content and photosynthetic efficacy of peppermint23. The enhanced activity of photosynthetic enzymes and the breakage of the pigment-protein complex could explain the decrease in Chl under salt stress24. According to Khan25, salt stress delayed the synthesis of photosynthetic pigments. The total carotenoid content of all Damask rose genotypes grown in vitro culture supplemented with 100 mM NaCl was reduced (Table 1). The findings were consistent with those found in Cornus sericea26 and melons27. Salt stress caused to β-carotene breakdown, which led to a lower carotenoid concentration28. Carotenoids are thylakoid membrane components that absorb and transmit light to Chl, protecting it from photooxidation29. As a result, the breakdown of carotenoids may also induce Chl degradation. The breakdown of photosynthetic pigments caused by salt is most likely due to higher toxic ions, increased chlorophyllase enzyme activity, and damage to the photosynthetic apparatus30. According to the recent study, a high amount of toxic ions because of the various salts in effluent caused to the loss of photosynthetic pigments in wheat, including Chl a, Chl b, and carotenoids31. Given that salinity changed photosynthetic pigment metabolism, it is evident that under abiotic stress, Chl content is tightly controlled32.
Salt stress can boost the actions of APX, CAT, and GR in P. popularis leaves33. The antioxidant enzymes (GPX, SOD, CAT, APX, PPO, and POD) in this study responded differently to salt treatment, exposure duration, and NaCl concentration (Table 2). Our findings indicated that antioxidant enzymes such as GPX, SOD, CAT, APX, and PPO in the leaf stayed active after prolonged activation (14th and 28th days), (Table 2). The activities of SOD, CAT, GPX, and APX in Damask rose explants decreased as well as increased in salinity duration (28th day) at sever salt stress (100 mM NaCl). These findings were in line with those reported for Brassica juncea L. in which antioxidant system activities increased after brief intervals of 30 min to 5 days of NaCl treatment34, and for maize seedlings treated for 21 days except for POD activity35. Numerous studies have shown that when plants were treated with NaCl, the activities of APX, SOD, CAT, and GR increased compared to control plants36. SOD and APX in mustard and maize37, and GPX, SOD, and CAT in UCB-138. Under salinity, the transcript levels of the SOD, CAT, and APX genes stress significantly increased39. Furthermore, it has been demonstrated that GPX activity increased in corn under salinity stress40. When plants are subjected to salinity stress, ROS accumulation increased, resulting in an imbalance between ROS generation and scavenging, and cellular oxidative damage. Plants have been equipped with an effective scavenging ROS defense system that includes enzymatic (APX, SOD, CAT, GPX, POD) and non-enzymatic (carotenoids, flavonoids, and anthocyanin)40. Most likely, the levels of APX, GPX, CAT, APX, POD, and SOD in this research increased in order to reduce the amount of ROS generated under salinity stress. An increase in PPO activity is most likely an induced defensive response that delays senescence. The activity of PPO increased in response to salt stress with the highest activity in older leaves (Table 2). As an adaptive approach under stress, this could selectively promote cell death in these tissues, reducing further water loss and allowing limited nutrients to be partitioned to younger tissues. Previous research has shown that polyphenol production is influenced by abiotic variables such as ambient influences41. According to Radi et al.42, salinity-induced changes in plant metabolism could result in an accumulation in various phenolic compounds.
Under salt-induced stress, the total phenolic content increased in this research. Our findings revealed that as well as increase in NaCl level on the 14th and 28th days the total phenolic contents were also increased (Table 3). According to earlier study, increase in salt concentration significantly caused to increase in total phenolic contents level43. Furthermore, it has been demonstrated that salt stress can alter the phenolic molecules of plants, which are sensitive to salt44. The synthesis pathway of total phenolic content may be linked to its buildup under salinity stress. The phenylpropanoid pathway is responsible for the production of phenolic compounds in plants, and this pathway can be triggered in saline conditions45. Plants produced several hormones under salinity stress conditions, including methyl-jasmonic acid and jasmonic acid, which trigger phenylalanine ammonia lyase activity which is the primary enzyme in phenolic metabolism in plants results in the production of phenolic compounds46. In this study, flavonoid content was tested at various salt levels, and along with increase in NaCl concentration, flavonoid content increased on the 14th and 28th days in all Damask rose genotypes (Table 3). Gengmao et al.47 demonstrated that as well as increase in NaCl concentrations up to 150 mM, flavonoid levels increased in safflower plant. It was also stated that under abiotic stresses, plants boost flavonoid synthesis to protect themselves from unfavorable stress circumstances48. Plants use secondary metabolites in reaction to salinity stress to eliminate and cope to the harmful effects of salt stress. Plants use oxidative defense metabolism, compatible solutes, inorganic nutrients, hormonal control, and secondary compounds in reaction to salt stress49. Flavonoids are essential secondary metabolites that decrease the negative effects of osmotic stress, such as salinity50. Flavonoids accumulate in vacuoles, chloroplasts, and nuclei, and vacuole flavonoids interfere with hydrogen peroxide detoxification51. Salt stress effects on plant water status by lowering the osmotic potential of the soil solution52. As previously reported53, the total antioxidant capacity (TAC) system increased with increasing in salinity level. At moderate salinity, a small increase in antioxidant molecules and their activity implies a regulatory response. Significantly greater quantity and activity of these compounds, on the other hand, represent an apparent state of urgency to deal with oxidative stress under high salinity, which is also reflected in a significant growth decrease (Table 3).
Lower protein loss in of ‘Atashi’ may be attributable to its increased tolerance to NaCl stress conditions. Furthermore, these findings showed that extending the time exposure of salinity, reduced the amount of proteins, particularly in ‘Chahar-Fasl’ (Table 4). Plants have a dual reactivity to protein patterns when exposed to salt. Salt stress decreases overall protein concentration while simultaneously initiating the synthesis of other specialized proteins required for salt tolerance via ABA activation54. The increased activity of protease may be responsible for the breakdown of protein content in a saline soil. Degraded proteins may provide a storage form of nitrogen, which is important in osmotic regulation under stress55.
Proline content of in vitro cultivated Damask rose genotypes increased dramatically with increasing in salinity and time (Table 4). Our findings are consistent with previous studies of figure56. Proline performs critical functions such as osmoregulation and lowering the negative effects of ROS during salinity stress. As a result, more proline accumulation in plants can result in greater saline tolerance43. Under extreme salt stress, ‘Atashi’ had the greatest proline concentration in the current research (Table 4).
Based on what we found, increase in the level of NaCl causes H2O2 levels to increase in all genotypes on days 14 and 28 after salinity treatment. Several studies have shown that when plants are under stress from salt, the amount of H2O2 in their tissues increases more36. Under stress conditions, plants produce ROS, and an excessive buildup of ROS in response to salt stress can cause oxidative stress and severely damage natural metabolism via membrane lipid peroxidation57. In this study, salinity increased the MDA level of treated genotypes significantly on 14th and 28th days (Table 4). Increased lipid peroxidation is a frequent sign of salt stress, which is caused by an increase in ROS production. It has been said that increased ROS formation may harm cellular organelles and components such as membrane lipids, whereas genotypes with lower MDA concentration are more resistant to salty conditions58. In this aspect, ‘Bi-Khar’ has the lowest MDA content.
Conclusion
The change in weather conditions due to global warming and increase in drought has led to an increase in soil salinity. Therefore, it is so important to measure the threshold of tolerance of different Damask roses to salt stress regarding to the way of defense mechanism. According to the results, it is clear that despite the severe stress applied to the Damask rose genotypes, the mechanisms involved in stress tolerance have worked well in these samples and the osmotic and antioxidant protection mechanisms against the death and deterioration of the explants. Although PPO was not activated under more concentrations of NaCl, POD and APX were the most common enzymes which kept their activation in 100 mM NaCl until 28th day after salinity treatment. Also TPC and TFC was in higher content until 28th day in tolerant Damask rose genotype, while other antioxidative enzymes as well as other osmolytes were up-regulated more at 14th day after salinity treatment. Based on the results we have selected the Atashi > Bi-Khar > Kashan > Chahar-Fasl genotypes as the most tolerant Damask rose under salt stress, respectively. So, it was concluded that Atashi genotype under 100 mM NaCl, was the most favorable and tolerant genotype with the highest optimal level of antioxidant activity and the accumulation of compatible osmolytes, as well as the high concentration of total soluble protein compared to the other genotypes. In conclusion, in vitro selection methods can be used effectively for salt tolerance screening of Damask rose genotypes, although the same experiment should be done in the field to verify the in vitro experimental results.
Materials and methods
Plant materials and culture medium
One-year branches with active growth of four genotypes of R. damascena including Chahar-fasal, Kashan, Atashi, and Bi-khar were taken from a research farm in Pakdasht, Iran. The plant material and shoots for wild collections were obtained under the supervision and permission of the Maragheh University guidelines and according to the national guidelines and all authors complied with all the local and national guidelines. Damask rose cuttings were washed 20 min under running tap-water, then they were surface-sterilized in 30% (v/v) NaClO for 20 min. The branches recut into the one node shoot explants (1.5–2 cm), and placed in the MS free hormone media59, for the initial establishment which solidified with 7.5 g of agar. The pH value was adjusted to 5.7 before autoclaving at 121 °C for 20 min. Five Shoot nodes were cultured, in 150 mL culture vessels containing 25 mL of establishment medium as one replication, then kept at 25 ± 2 °C and 16 h-photoperiod (light intensity, 8.85 W/m2) and 60–70% RH. Following explants were screened every day for fungal or bacterial contamination then any contaminated vessels were removed from the experiment collection. Shoots were repeatedly sub-cultured three times at a constant three-week subculture interval.
Proliferation medium and growth of culture
The basic medium for proliferative media was the same as the MS establishment medium59, plus 130 mg of iron sequester and 332 mg of CaCl2, as well as 0.36 mg L−1 benzyl adenine (BA) and 0.03 mg L−1 Indole 3-butyric acid (IBA). The pH was then adjusted to 5.7 and sterilized in an autoclave. After sterilization and transfer to the flow-box, 25 mL of medium was added to each culture vessels to be used for subculture. Finally, five explants were placed in 150 mL culture vessels containing the proliferation medium as one replication and transferred to the growth chamber to be propagated and then the mentioned propagated shoots have been used.
Preparing the treatment medium including NaCl to induced salinity
NaCl was added to the culture medium at five levels (0, 25, 50, 75 and 100 mM) to simulate salt stress. Shoot explants with only four young leaves were placed in culture medium, and then five explants were placed in each culture vessel and transferred to the growth chamber and after 14 and 28 days, the explants were collected and evaluated for physiological, and biochemical traits.
Physiological traits
Relative water content (RWC)
Fresh leaf samples were weighted to determine fresh weight (FW), and then they were immersed in dH2O at 4 °C for 24 h. After absorption of surface water, they were weighed again for turgid weight (TW). Leaf samples were then dried in the oven at 75 °C for 48 h and their DW was measured and RWC was calculated according to the Barrs and Weatherley60 protocol.
Membrane stability index (MSI)
Membrane stability of Damask rose explants were evaluated by 0.1 g of leaves. Then similar disks were detached of them. The samples were put in test tubes having 10 mL of dH2O in two sets. Then the electrical conductivity of the samples were recorded after incubation at 40 °C for 30 min (C1) and 100 °C for 15 min (C2) by means of EC meters (Jenway model, UK) and the protocol of Almeselmani et al.61.
Chlorophyll a, b, total chlorophyll and carotenoids (CARs)
About 0.5 g of finely mixed of leaves were extracted using 10 mL of 80% acetone. After 24 h incubation in dark place chlorophyll was extracted from completely blanched leaves. Then the amount of absorbance of extracted samples was recorded at 649, 665, and 470 nm by a spectrophotometer (Shimadzu UV-2550, Kyoto, Japan). For evaluating the concentration of chlorophyll, a standard method similar to Arnon62, was applied.
Biochemical traits
Antioxidative enzymes
Fresh leaves (1 g) were homogenized in 5 mL of 50 mM K–phosphate buffer (pH 7.0), brought to 5 mM Na–ascorbate and 0.2 mM EDTA by the addition of concentrated stocks. The homogenate extract was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was used for assessment of enzyme activities. Guaiacol Peroxidase (GPX) was evaluated by monitoring the increase in absorbance at 470 nm (ε = 26.6 mM −1 cm −1) during polymerization of guaiacol. One unit of activity was defined as the amount of enzyme producing 1 µmol of tetraguaiacol per min at 25 °C. Superoxide dismutase (SOD) was assayed according to the protocol of Beauchamp and Fridovich63, which is based on prevention of the photochemical reduction of nitro blue tetrazolium. The reactants were then placed under a 20-W fluorescent lamp for 15 min and the samples in the tubes were covered with a black cloth. At the end of the reaction, the absorbance at 560 nm was determined. The activity of catalase (CAT) was measured, as previously established by Li et al.64. The Damask rose explant leaves (0.5 g) were grounded in liquid nitrogen and extracted with the following described method: 100 mM potassium phosphate buffer (pH 7.8) including 0.1 mM EDTA, 1% (w/v) PVP and 0.1% (v/v) Triton × 100. The extraction was centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatants were collected and applied for evaluating the enzyme activity. Ascorbate peroxidase (APX) activity was done according to the Yoshimura et al.65 protocol. The reaction solution involved phosphate buffer (250 μL), 1 mM ascorbate (250 μL), 0.4 μM EDTA (250 μL), 190 μL ddH2O, 10 mM trans oxide (10 μL) and 50 μL supernatant. The absorption at 290 nm for 1 min determined the enzyme activity. The activity of polyphenol oxidase (PPO) was measured by Nicoli et al.66 protocol. The enzymatic solution was extracted the same as peroxidase. In this case, a reaction complex was prepared of 100 μL of enzymatic extraction, 2.5 mL of potassium phosphate buffer (pH 6.8), 200 μl of 0.02-M pyrogallol as the enzyme precursor and was recorded at 420 nm with a spectrophotometer (Shimadzu, Japan).
Total phenol content (TPC)
Using the Folin-Ciocalteu reagent, the total phenolic concentration of Damask explants was determined as Singleton et al.67 method. The plant extract was combined with 0.5 cc of the Folin-Ciocalteu reagent, and the mixture was then held at 25 °C for 8 min. After 8 min, 2 mL of sodium carbonate solution (7.5% concentration) was added to this solution, and its volume was then increased with water to 8 mL. After two hours, the total phenolic concentration was determined using a 725 nm wavelength. A calibration curve was created using gallic acid as the reference. The amount of mg gallic acid equivalents per gram of sample was used to indicate the overall phenolic content.
Total flavonoid content (TFC)
Using quercetin as a reference standard, the total flavonoid was measured using the aluminum chloride technique68. To accomplish this, 1 mL of quercetin extract or standard solution (20, 40, 60, 80, and 100 μg mL−1) was added to a 10 mL volumetric flask containing 4 mL of purified water. After 5 min, 0.30 mL of 5% NaNO2 was added to the flask, followed by 0.3 ml of 10% AlCl3. After 5 min, 2 mL of 1 M NaOH was added, and the amount was increased to 10 mL with dH2O. The overall flavonoid concentration was determined at 510 nm. Total flavonoid amounts were recorded as mg quercetin equivalent (mg QE 100 g−1 FW).
Total antioxidant activity (TAA)
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) technique was used to calculate TAA. 1 g of the fresh leaf samples was centrifuged at 21,913 g for 15 min after being separated with 2 mL of methanol at a concentration of 80%. After that, 100 µL of the extract was combined with 180 µL of DPPH (0.1 M), the mixture was left to sit in the dark for 30 min, and then a UV spectrophotometer was used to read the absorbance at 517 nm. The TAA was determined by applying the following equation69:
Protein
Protein content was assayed following the Bradford method70, which was calibrated with the standard bovine serum albumin curve. So, 100 mg of the treated explants were placed in a test tube with 2 mL of 50 mM potassium phosphate buffer at pH 7.0. The solution was centrifuged at 7000–12,000 rpm. Then, the supernatant was taken and centrifuged at 3000 rpm for 15 min at 4 °C. The samples were prepared with 1:100 dilution ratios and read at 595 nm.
Proline
Proline content was evaluated by homogenizing 0.2 g of leaf fresh weight in 2 mL of 3% aqueous sulfosalicylic acid and then centrifuged at 10,000 rpm for 30 min. Then, the pellet was washed with 3% aqueous sulfosalicylic acid twice. The supernatant was pooled, and the amount of proline was evaluated using ninhydrin reagent and toluene extraction71.
Hydrogen peroxide (H2O2)
Explants H2O2 content was determined according to the Liu et al.72 protocol. So, 0.5 g of leaf tissue was grounded with potassium phosphate buffer (KPB) (pH 6.8), and then centrifuged at 7000 rpm for 25 min at 4 °C. A 100-µL aliquot of the supernatant was added to 1 mL of Xylenol solution. The solution was then completely mixed and left to rest for 30 min and the extract absorption was read by a spectrophotometer (Shimadzu, Japan) at 560 nm.
Malondialdehyde (MDA)
MDA content was determined as 2-thiobarbituric acid (TBA) reactive metabolites73. So, 1.5 mL of the leaf extracts were homogenized in 2.5 mL of 5% TBA made in 5% Trichloroacetic acid. The mixture was warmed at 95 °C for 15 min and then cooled on ice quickly and centrifuged at 5000 rpm for 10 min. Then the absorbance of supernatant was read at 532 nm. MDA content was calculated as in the below equation:
Statistical analysis
The factorial experiment was carried out based on a completely randomized design with 3 replications. Data were statistically analyzed by MSTAT-C ver 2.1 software and means were separated using the Fischer’s protected least significant difference (LSD) test at p = 0.05. Pearson correlation and cluster dendrogram heat maps were performed in R foundation for sta-tistical computing (version 4.1.2), Iran (2021). URL https://cran.um.ac.ir/, accessed on3 June 2022.
Plant permission statement
We obtained permission for collection of plant material used in the study.
Data availability
Correspondence and requests for the datasets generated and/or analyzed during the current study and materials should be addressed to H.S.H.
References
Khosh-Khui, M., & Jabbarzadeh, Z. Effects of several variables on in vitro culture of damask rose (Rosa damascena Mill.). In IV International Symposium on Rose Research and Cultivation 751 pp. 389–393 (2005).
Omidi, M. et al. Biochemical and molecular responses of Rosa damascena mill. cv. Kashan to salicylic acid under salinity stress. BMC Plant Biol. 22, 1–20 (2022).
Chowdhury, S. R., Tandon, P. K. & Chowdhury, A. R. Rose oil from Rosa damascena Mill., raised on alkaline soils. J. Essent Oil Bear Plants 12, 213–217 (2009).
Lorenzo, H., Cid, M. C., Siverio, J. M. & Ruano, M. C. Effects of sodium on mineral nutrition in rose plants. Ann. Appl. Biol. 137, 65–72 (2000).
Jamshidi Goharrizi, K., Amirmahani, F. & Salehi, F. Assessment of changes in physiological and biochemical traits in four pistachio rootstocks under drought, salinity and drought+ salinity stresses. Physiol. Plant. 168, 973–989 (2020).
Zaman, M., Shahid, S. A., Heng, L., Shahid, S. A., Zaman, M., & Heng, L. Introduction to soil salinity, sodicity and diagnostics techniques. In Guidel Salin assessment, Mitig Adapt using Nucl Relat Tech Springer; pp 1–42 (2018).
Hasanuzzaman, M. et al. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 28(17), 9326 (2021).
Wang, Q. et al. Model analysing the antioxidant responses of leaves and roots of switchgrass to NaCl-salinity stress. Plant Physiol. Biochem. 58, 288–296 (2012).
Sarker, U., & Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 11, 559876 (2020).
Taiz, L., & Zeiger, E. Fisiologia vegetal. Universitat Jaume I (2006).
Attia, H. et al. Induced anti-oxidation efficiency and others by salt stress in Rosa damascena Miller. Sci. Hortic. 274, 109681 (2020).
Hura, T., Szewczyk-Taranek, B., Hura, K., Nowak, K. & Pawłowska, B. Physiological responses of Rosa rubiginosa to saline environment. Water Air Soil Pollut. 228, 1–11 (2017).
Seyed Hajizadeh, H., & Azizi, S. Evaluation of antioxidative responses and salt tolerance of Damask rose under in vitro culture. In XXXI International Horticultural Congress (IHC2022): International Symposium on In Vitro Technology and Micropropagated Plants 1359 p. 197–202 (2022).
Hajizadeh, H. S., Azizi, S., Rasouli, F. & Okatan, V. Modulation of physiological and biochemical traits of two genotypes of Rosa damascena Mill. by SiO2-NPs under In vitro drought stress. BMC Plant Biol. 22, 1–16 (2022).
Seyed Hajizadeh, H., Azizi, S., Rasouli, F. & Kaya, O. Evaluation of nano-silicon efficiency on compatible solutes and nutrient status of Damask rose affected by in vitro simulated drought stress. Chem. Biol. Technol. Agric. 10, 22 (2023).
Hajizadeh, H. S., Rezaei, S., Yari, F. & Okatan, V. In vitro simulation of drought stress in some Iranian Damask Rose landraces. Hortic. Sci. 1, 1 (2022).
Raoufi, A., Salehi, H., Rahemi, M., Shekafandeh, A. & Khalili, S. In vitro screening: The best method for salt tolerance selection among pistachio rootstocks. J. Saudi Soc. Agric. Sci. 1(20), 146–154 (2021).
Zhang, Z. et al. On evolution and perspectives of bio-watersaving. Colloids Surf. B Biointerfaces 55, 1–9 (2007).
Kavas, M., Balouglu, M. C., Akca, O., Köse, F. S. & Gökçay, D. Effect of drought stress on oxidative damage and antioxidant enzyme activity in melon seedlings. Turk. J. Biol. 37, 491–498 (2013).
Tiwari, J. K. et al. Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol. Plant 32, 103–114 (2010).
Rezaei, M., Arzani, A., Saeidi, G. & Karami, M. Physiology of salinity tolerance in Bromus danthoniae genotypes originated from saline and non-saline areas of West Iran. Crop Pasture Sci. 68, 92–99 (2017).
Turan, M. A., Katkat, V. & Taban, S. Salinity-Induced stomatal resistance, proline, chlorophyll and. Int. J. Agric. Res. 2, 483–488 (2007).
Zhou, M., Wei, Y., Wang, J., Liang, M. & Zhao, G. Salinity-induced alterations in physiological and biochemical processes of blessed thistle and peppermint. J. Soil Sci. Plant Nutr. 21, 2857–2870 (2021).
Trifunović-Momčilov, M. et al. Changes in photosynthetic pigments content in non-transformed and AtCKX transgenic centaury (Centaurium erythraea Rafn) shoots grown under salt stress in vitro. Agronomy 11(10), 2056 (2021).
Khan, N. A. NaCl-inhibited chlorophyll synthesis and associated changes in ethylene evolution and antioxidative enzyme activities in wheat. Biol. Plant 47, 437–440 (2003).
Mustard, J. & Renault, S. Response of red-osier dogwood (Cornus sericea) seedlings to NaCl during the onset of bud break. Botany 84, 844–851 (2006).
Yarsi, G. et al. Effects of salinity stress on chlorophyll and carotenoid contents and stomata size of grafted and ungrafted galia C8 melon cultivar. Pak. J. Bot. 49, 421–426 (2017).
Babaei, M., Shabani, L. & Hashemi-Shahraki, S. Improving the effects of salt stress by β-carotene and gallic acid using increasing antioxidant activity and regulating ion uptake in Lepidium sativum L.. Bot. Stud. 63(1), 22 (2022).
Park, S. et al. Chlorophyll-carotenoid excitation energy transfer in high-light-exposed thylakoid membranes investigated by snapshot transient absorption spectroscopy. J. Am. Chem. Soc. 140, 11965–11973 (2018).
Fatehi, S. F., Oraei, M., Gohari, G., Akbari, A. & Faramarzi, A. Proline-functionalized graphene oxide nanoparticles (GO–Pro NPs) mitigate salt-induced adverse effects on morpho-physiological traits and essential oils constituents in Moldavian Balm (Dracocephalum moldavica L.). J. Plant Growth Regul. 41, 2818–2832 (2022).
Hajihashemi, S. et al. Effect of wastewater irrigation on photosynthesis, growth, and anatomical features of two wheat cultivars (Triticum aestivum L.). Water 12, 607 (2020).
Turan, S. & Tripathy, B. C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant 153, 477–491 (2015).
Wang, R. et al. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol. 28, 947–957 (2008).
Ranjit, S. L., Manish, P. & Penna, S. Early osmotic, antioxidant, ionic, and redox responses to salinity in leaves and roots of Indian mustard (Brassica juncea L.). Protoplasma 253, 101–110 (2016).
AbdElgawad, H. et al. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 7, 276 (2016).
Ahmad, P. et al. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J. Plant Growth Regul. 38, 70–82 (2019).
Ahmad, P. et al. Zinc application mitigates the adverse effects of NaCl stress on mustard Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J. Plant Interact 12, 429–437 (2017).
Jamshidi Goharrizi, K., Baghizadeh, A., Kalantar, M. & Fatehi, F. Assessment of changes in some biochemical traits and proteomic profile of UCB-1 pistachio rootstock leaf under salinity stress. J. Plant Growth Regul. 39, 608–630 (2020).
Azevedo Neto, A. D. & de, Prisco JT, Enéas-Filho J, Abreu CEB de, and Gomes-Filho E.,. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 56, 87–94 (2006).
Wei, P., Yang, Y., Wang, F. & Chen, H. Effects of drought stress on the antioxidant systems in three species of Diospyros L.. Hortic Environ. Biotechnol. 56, 597–605 (2015).
Neffati, M., Sriti, J., Hamdaoui, G., Kchouk, M. E. & Marzouk, B. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 124, 221–225 (2011).
Radi, A. A., Farghaly, F. A. & Hamada, A. M. Physiological and biochemical responses of salt-tolerant and salt-sensitive wheat and bean cultivars to salinity. J. Biol. Earth Sci. 3, 72–88 (2013).
Akbari, M., Mahna, N., Ramesh, K., Bandehagh, A. & Mazzuca, S. Ion homeostasis, osmoregulation, and physiological changes in the roots and leaves of pistachio rootstocks in response to salinity. Protoplasma 255, 1349–1362 (2018).
Yuan, G., Wang, X., Guo, R. & Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 121, 1014–1019 (2010).
Lim, J.-H., Park, K.-J., Kim, B.-K., Jeong, J.-W. & Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 135, 1065–1070 (2012).
Rebey, I. B. et al. Relation between salt tolerance and biochemical changes in cumin (Cuminum cyminum L.) seeds. J. Food Drug Anal. 25, 391–402 (2017).
Gengmao, Z. et al. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crops Prod. 64, 175–181 (2015).
Sharma, A. et al. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 24, 2452 (2019).
Noreen, Z., Ashraf, M. & Akram, N. A. Salt-induced regulation of some key antioxidant enzymes and physio-biochemical phenomena in five diverse cultivars of turnip (Brassica rapa L.). J. Agron. Crop Sci. 196, 273–285 (2010).
Buer, C. S., Imin, N. & Djordjevic, M. A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 52, 98–111 (2010).
Shoeva, O. Y., Mock, H.-P., Kukoeva, T. V., Börner, A. & Khlestkina, E. K. Regulation of the flavonoid biosynthesis pathway genes in purple and black grains of Hordeum vulgare. PLoS One 11, e0163782 (2016).
Sheldon, A. R., Dalal, R. C., Kirchhof, G., Kopittke, P. M. & Menzies, N. W. The effect of salinity on plant-available water. Plant Soil 418, 477–491 (2017).
Bekka, S., Tayeb-Hammani, K., Boucekkine, I., Aissiou, M. Y. E. A. & Djazouli, Z. E. Adaptation strategies of Moringa oleifera under drought and salinity stresses. Ukr. J. Ecol. 12, 8–16 (2022).
Arefian, M., Vessal, S. & Bagheri, A. Biochemical changes in response to salinity in chickpea (Cicer arietinum L.) during early stages of seedling growth. J Anim. Plant Sci. 24, 1849–1857 (2014).
Jamil, M. & Rha, E. S. NaCl stress-induced reduction in growth, photosynthesis and protein in mustard. J. Agric. Sci. 5, 114 (2013).
AbdoliNejad, R. & Shekafandeh, A. Responses of two figs (Ficus carica L.) cultivars under salt stress via in vitro condition. Agric. Sci. Dev. 3, 194–199 (2014).
Hasanuzzaman, M. et al. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 22(17), 9326 (2021).
Raoufi, A., Rahemi, M. & Akbari, M. Glycerol foliar application improves salt tolerance in three pistachio rootstocks. J. Saudi Soc. Agric. Sci. 19, 426–437 (2020).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 15, 473–497 (1962).
Barrs, H. D. & Weatherley, P. E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 15, 413–428 (1962).
Almeselmani, M. et al. Effect of drought on different physiological characters and yield component in different varieties of Syrian durum wheat. J. Agric. Sci. 3, 127 (2011).
Arnon, D. I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1 (1949).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Li, J.-T., Qiu, Z.-B., Zhang, X.-W. & Wang, L.-S. Exogenous hydrogen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol. Plant 33, 835–842 (2011).
Yoshimura, K., Yabuta, Y., Ishikawa, T. & Shigeoka, S. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. Am. Soc. Plant Biol. 123, 223–234 (2000).
Nicoli, M. C., Elizalde, B. E., Pitotti, A. & Lerici, C. R. Effect of sugars and maillard reaction products on polyphenol oxidase and peroxidase activity in food. J. Food Biochem. 15, 169–184 (1991).
Singleton, V. L., Orthofer, R. & Lamuela-Raventós, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299, 152–178 (1999).
Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999).
Vattem, D. A., Lin, Y.-T., Labbe, R. G. & Shetty, K. Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innov. Food Sci. Emerg. Technol. 5, 81–91 (2004).
Bradford, N. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 72, e254 (1976).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Liu, Y.-H., Offler, C. E. & Ruan, Y.-L. A simple, rapid, and reliable protocol to localize hydrogen peroxide in large plant organs by DAB-mediated tissue printing. Front Plant Sci. 5, 745 (2014).
Zhang, Z. et al. On evolution and perspectives of bio-watersaving. Colloids Surf. B Biointerf. Elsevier 55, 1–9 (2007).
Acknowledgements
The present study was carried out by the use of facilities and materials at the University of Maragheh and the paper is published as the part of a MSc. Student research at University of Maragheh, research affairs office.
Author information
Authors and Affiliations
Contributions
H.S.H. conceived and designed the experiments; S.A. performed the experiments; S.A. analyzed the data; A. A. run the tissue culture and growth chamber facilities; H.S.H., and O. K. wrote and proof the final paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azizi, S., Seyed Hajizadeh, H., Aghaee, A. et al. In vitro assessment of physiological traits and ROS detoxification pathways involved in tolerance of Damask rose genotypes under salt stress. Sci Rep 13, 17795 (2023). https://doi.org/10.1038/s41598-023-45041-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45041-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.