Abstract
Overuse of chemical fertilizer and pesticides in agricultural activity is frequently damaging to soil health and can accumulate heavy metals in the soil environment, causing harm to plants, humans, and the ecosystem. This study was done to evaluate the effectiveness of biofertilizers in reducing heavy metal levels in contaminated soil and enhancing the activity of soil enzymes that are crucial to plant growth and development. Two bacteria strains, Pseudomonas aeruginosa. and Bacillus firmus, were chosen to develop biofertilizers based on molasses. The pot experiment was setup using a completely randomized design with four treatments and five levels; Bacillus firmus and Pseudomonas aeruginosa were used separately, and they were combined for the biofertilizer dose (20, 40, 60, 80, and 100 mL). Utilizing contaminated soils taken from a greenhouse farm the effect of biofertilizer on heavy metal bioremediation and soil enzyme activity was examined. Methods of soil agrochemical analysis were used to determine the soil physiochemical properties and the concentrations of heavy metals Cu, Fe, Zn, Cd, Mo, Mn, were determined by inductively coupled plasma–mass spectrometry ICP-MS, following DTPA extraction methods. In results, soil pH decreased from 8.28 to 7.39, Ec increased from 0.91 to 1.12, organic matter increased from 18.88 to 20.63 g/kg, N increased gradually from 16.7 to 24.4 mg/kg, and K increased from 145.25 to 201.4 mg/kg. The effect of biofertilizer treatment on soil physiochemical characteristics was significantly positive. Application of biofertilizer significantly increased the heavy metal bioavailability and the activities of soil enzymes. Soil pH were positively correlated with soil Zn (0.99819*), APK (0.95869*) activity and negatively correlated with Fe (0.96759*) also statistically significant at (p < 0.05). The soil Cu positively correlated with Fe (0.99645*), Cd (0.97866*), β.D.GLU (0.99769*) and negatively correlated with PAK (− 0.9624*). Soil ARY had positive correlation with soil Mn (0.99683*), Cd (0.95695*), and negative correlation with PAK (− 0.99424*) at (p < 0.05). Soil enzyme activities were negatively correlated to heavy metals at a significant level. Collectively, the study highlights the potential of biofertilizers as a sustainable and effective approach to enhance soil health and remediate heavy metal-contaminated soils in greenhouses.
Similar content being viewed by others
Introduction
Biofertilizers sustain agricultural land’s soil structure and biodiversity and are eco-friendly, cost-effective, non-toxic, and simple to apply. This makes them an effective alternative to chemical fertilizers1,2. Biofertilizers, also known as microbial inoculants, are organic preparations containing particular microorganisms from plant roots and root zones. They increase the plant’s growth and yield by 10 to 40%3. When applied to the seed, plant surface, or soil, these bioinoculants colonize the rhizosphere and the inside of the plant, which helps the plant grow4. They supply nutrients to the soil and protect the plant from pests and diseases, improving soil fertility and crop yield2. Another benefit is that biofertilizers are no longer needed after 3 to 4 years since parental inoculants are enough for reproduction5. Biofertilizers increase agricultural output and soil fertility and they increase soil structure, crop production, and nutrient cycling when applied to soil6,7,8. The use of potential biofertilizers will improve soil efficiency and sustainability, reduce agricultural pollution, and improve food quality9. Biofertilizers dissolve the key nutrients and make them available to the plants10,11. Biofertilizers improve plant nutrition and stress tolerance by fixing atmospheric nitrogen and solubilizing soil nutrients12,13. Field studies have revealed that the yield of food crops can be increased by approximately 25% applying biofertilizers while reducing the use of nitrogenous and phosphatic fertilizers by about 25–50% and 25%, respectively14.
Heavy metals (HMs) are a common cause of soil pollution all over the world. HMs pollutants in the soil have become a major concern due to their toxic effects on human health and the environment15. These pollutants are mainly introduced into the soil through human activities such as mining, use of agrochemicals, burning of fossil fuels, industrial waste discharge, and waste disposal16,17. High levels of HMs are directly toxic because they stop intracellular enzymes from working and cause oxidative stress, which damages cellular structures18. Accumulated zinc (Zn), cadmium (Cd), copper (Cu), manganese (Mn), and iron (Fe) are considered to be major pollutants in soil and water, and these metals are not readily degraded into useful chemicals19,20. Farmers worldwide are now focusing on the protection of decreasing agricultural land and the restoration of resources to their pristine condition in response to the increasing issue of soil contamination21. Changes in the physical, chemical, and biological properties of the soil and an increase in secondary contamination are also big problems. Due to these issues, physicochemical approaches have been considered unable to be applied in agriculture.
Various HMs resistant microorganisms have recently been identified by researchers from polluted areas, mine dumps and abandoned sites, industrial, waste dumping yards, and the rhizosphere of plants growing in HMs-contaminated soil22,23. The isolated bacterial genera, such as Arthrobacter, Enterobacter, Corynebacterium, Stenotrophomonas, Bacillus, and Pseudomonas play an important role in the bioremediation process. Bacillus-based biofertilizers are more active than Pseudomonas-based ones because Bacillus spp. produces more metabolites and forms spores, which improves cell viability in commercially prepared products24,25. Bacillus-based biofertilizers increase plant-available nutrients in rhizospheres, reduce pathogenic microbe development, and activate pest defense mechanisms26. Further, the microbial communities in these contaminated soils are disrupted, which affects their important roles in recycling organic matter, controlling plant diseases, boosting plant growth, and getting clear of harmful chemicals in the soil27,28. The inoculation of Bacillus spp. into heavy metal-contaminated soil can reduce the harmful effects of these metals on plant growth through support increasing water uptake and reducing electrolyte to reduce Cd stress29.
Due to the viability and efficiency of utilizing bacteria in bioremediation, especially the use of bacteria to remove HMs from contaminated soil, attention has been drawn to this strategy. Bacteria have several ways to process HMs through general resistance mechanisms, bioremediation, and efflux mechanisms. Lead (Pb), chromium (Cr), arsenic (As), zinc (Zn), cadmium (Cd), copper (Cu), mercury (Hg), and nickel (Ni) are the most common HMs. Unlike other pollutants, HMs are mostly stored in the soil. At high concentrations, these contaminants are very harmful to both plants and microorganisms30. Bioremediation encompasses all biological approaches for pollution reduction. Bioremediation is a potential approach to clean up polluted soil economically and sustainably31. Bioremediation uses plants, animals, and microbes to remove contaminants such heavy metals. It is one of the most effective, non-invasive, and economically viable strategies for substantially reducing heavy metal contamination and restoring many ecosystems’ natural environments32. As a consequence, bioremediation has been widely accepted and recommended as a modern technology to resolve the issues correlated with other remediation methods to remove contaminants from the soil in an eco-friendly and sustainable approach33,34. The future profitability of bioremediation with biofertilizers such as nitrogen fixing organisms and nutrient mobilizers including phosphate, potassium, and zinc solubilizers, iron sequesters, and sulfur oxidizers, on the other hand, is dependent on biological considerations like competence and ability to effectively remove the stated or native biofertilizers and abiotic factors such as nutrients, pH, and temperature35,36.
Microorganisms and plant or animal wastes are the main sources of soil enzymes. Enzymes accumulate in soil as free enzymes or stabilized on clay surfaces or soil organic matter37. Furthermost enzymes that are regularly used to assess the impact of heavy metals pollution may be separated into two groups: oxidoreductases [such as dehydrogenase (DH)] and hydrolases [such as β-D-glucosidase (β-D-GLU), phosphatase (PHO), urease (URE) and arylsulfatase (ARYL)]38. HMs concentration and soil enzyme activity are usually negatively correlated. Thus, soil enzyme investigations have been used to identify HM contamination in soils39,40. Bioavailability of HMs are the most important factor in microbial processes and enzyme activity. Extensive research have established that the biological impacts of contaminants are not connected to the overall concentration of a contaminant in soils41,42. Alternatively, organisms also respond to the part of the signal that is biologically available to them. ISO 1107442 defines the concept of bioavailability as follows: “Bioavailability is the degree to which chemicals in the soil can be absorbed or decomposed by humans or other organisms, or can interact with biological systems. However, according to Lee et al.43, soil enzyme (DH, PHO, and UR) activity are significantly correlated with soil physio-chemical characteristics.
Molasses is the most commonly applied organic carbon substrate in biofertilizer44. Indeed, molasses is used to help bacteria generate anaerobic reducing conditions as part of the bioremediation process45. Briefly explained, molasses provides more organic carbon to soil bacteria, allowing them to develop and remove contaminants from the soil46. Considering organic carbon is the most essential building material for bacteria, it may be the most visible and valuable component of biofertilizer47.
The chinese celery cabbage (Brasica rapa var chinensis L.) it’s one of the most economically important vegetable families in the world. However, this vegetable has high sensitive to heavy metal concentration in the soil.
In this study, we hypothesized that (I) to investigate the influence of biofertilizers on heavy metal bioremediation and enzyme activities in the soil. (II) to determine if biofertilizers can successfully remediate heavy metal-contaminated soil and restore its fertility in a sustainable manner. Specifically, (III) to assess the potential of biofertilizers in enhancing the degradation and immobilization of heavy metals in the soil, as well as their effects on soil enzyme activities, (IV) to evaluate the effectiveness of biofertilizers on the soil physiochemical properties, and their correlation between soil heavy metal and enzyme activities.
Materials and methods
Site description and soil sample collection
This experiment used greenhouse farm soil from the Sha Tou region of Yangzhou city (32°16′23″ N, 119°31′48″ E). Bulk soil samples collected randomly with hand auger from 0 to 20 cm depth was air-dried at room temperature for 14 days. Dry samples were homogenized and sieved (< 2 mm) for future use.
Pot experiment
The pot experiment was done under greenhouse conditions, during summer season (May 2022 to July 2022) to investigate the impact of biofertilizer on plant growth and heavy metal bioremediation on Chinese celery cabbage growth. The study was designed as factorial arranged in complete randomized design (CRD) with four treatments and three replications. Treatments were four different biofertilizer rates including control treatment, Bacillus firmus (20, 40, 60, 80, and 100 mL/ pot), Pseudomonas aeruginosa (20, 40, 60, 80, and 100 mL/ pot), and a combination of Bacillus firmus and Pseudomonas aeruginosa (20, 40, 60, 80, and 100 mL/ pot) respectively. The pot dimensions were measured at 30 cm in height, 20 cm in diameter at the top, and 16 cm in diameter at the bottom. 10 kg of contaminated clay loam soil from the top layer (0–25 cm) was added to the pot. At a depth of 1 cm, five seeds were spread. The soil was sampled in four different treatments following harvest. Each treatment's soil was collected and well mixed before being taken for analysis.
Determination of soil physiochemical properties
The soil samples were analyzed for pH (1:1 in water) using a pH meter (Shanghai Leici). The soil electrical conductivity (EC) of 1:25 (w/v) in water was measured with an EC meter (TZS-EC-I; Zhejiang Top Instrument Co., Ltd., Hangzhou, China). The soil total nitrogen (TN) and available nitrogen (N) were measured following the semi-micro Kjeldahl method48. Sodium bicarbonate extraction and the molybdenum-antimony resistance colorimetry method were used to determine soil available phosphorus (P) content49. Soil available potassium (K) and sodium (Na+) were determined by potassium dichromate-external heating with a film photometry (WGH-Shanghai) method50. The soil organic matter (O.M) was determined using dichromate oxidation methods. The total phosphorus (TP) was determined using a UV-spectrophotometer (MTHSH, UV-5800PC, Shanghai, China) after being extracted by H2SO4-HClO4. The Olsen-P was extracted with a NaHCO3 solution containing 0.5 mol L−1 and measured at 710 nm with the molybdenum blue method. The exchangeable Ca2+, Mg2+, K+, Na+, and NH4+ were measured at pH 7 with 1 M ammonium acetate. The concentration of soil-available sulfur (S) was determined by the spectrophotometric method51. For all soils, physiochemical properties determinations were performed according to soil agrochemical analysis methods52.
Determination of the soil heavy metals concentration
After spending 24 h in a 5% (v/v) nitric acid solution, all glass and plastic containers were rinsed with ultrapure water and stored for use. 20.00 g of air-dried soil passing through 2 mm screen was placed in a 250 mL Erlenmeyer flask, combined with with 40 mL of DTPA extraction at 25 ± 2 °C, and was thoroughly shaken. Oscillate at a frequency of 180 rpm/min ± 20 rpm/min for 2 h and filter to determine. ICP-MS inductively coupled plasma–mass spectrometry was used to conduct a heavy metal analysis on the dried samples according to standard operating protocols53. In basic terms, samples were digested in a solution of HNO3-HClO4 (80/20, v/v) and Cu, Fe, Zn, Cd, Mo, and Mn concentrations were determined following DTPA extraction methods54. Each batch sample's analysis included method blanks, duplicate samples, and soil standard samples (GBW07456, Geophysical and Geochemical Exploration Institute of the Chinese Academy of Geological Sciences) for quality assurance and control. Using DTPA solution, the instrument's absorbance was calibrated to the spectrometer to zero point. The properties of the initial soil analysis were described in Table 1.
Determination of soil enzyme activities
Urease activity was measured using urea as a substrate, as described by Kandeler and Gerber55. 5 g of moist soil contained 20% of water content were incubated for 24 h at 37 °C with 1 mL of methylbenzene, 10 mL of 10% urea, and 20 mL of citrate buffer (pH 6.7). Then, 1 mL of filtered soil solution, 1 mL of sodium phenolate, and 3 mL of sodium hypochlorite were added and diluted to 50 mL, and absorbance was determined at 578 nm using a spectrophotometer (MTHSH, UV-5800PC, Shanghai, China). For dehydrogenase activity, iodonitrotetrazolium formazan (INTF) was used as a substrate56,57, whereby 1 g of moist soil was mixed with 100 mL of INTF (0.2% w/v) solution and incubated for 24 h at 30 °C. After incubation, 40 mL of acetone was added, and absorbance was determined at 464 nm. The analysis of alkaline phosphatase activity was performed as stated by Tabatabai and Bremner58. The P-nitrophenyl phosphate (p-NPP) was used as the substrate. 1 g of moist soil contained 20% of water content were mixed with 20 mL of 100 mM p-NPP in acetate buffer (pH 5.2) and incubated at 30 °C for 30 min. After incubation, 1 mL of CaCl2 and 4 mL of 0.5 M NaOH were added to terminate the reaction and the absorbance was measured at 405 nm. For the β-D-glucosidase activity, nitrophenyl-β-D-glucoside (PNG) was used as a substrate. Briefly, 1 g of soil was mixed with 0.2 mL toluene, 4 mL modified universal buffer (pH 6), and 1 mL PNG solution (25 mM) and incubated for 1 h at 37 °C as previously descried by Eivazi and Tabatabai59. After incubation, 1 mL of CaCl2 solution and 4 mL of Tris buffer (pH 12) were added, and the absorbance was measured at 405 nm. The activity of arylsulfatase was tested using p-nitrophenyl sulfate solution (p-NSS) as described by Tabatabai and Bremner60. 1 g of moist soil was mixed with 1 mL of p-NSS sulfate solution (0.05 M) and incubated for 24 h at 30 °C. After incubation, 1 mL of CaCl2 0.5 M and 4 mL of NaOH 0.5 M were added and the absorbance was measured at 420 nm.
Production and preparation of the biofertilizer
Bacillus firms and pseudomonas aeruginosa were cultured in different broths: nutrient broth and beef extract peptone medium broth. 250 mL of each medium was placed into a 500 mL Erlenmeyer flask and autoclaved for 20 min61. Before use, one agar plate of each pure bacterial culture was suspended in 10 mL sterilized 0.85% sodium chloride. Liquid medium was inoculated with 0.1% pure bacterial culture, shaken at 115 rpm, and incubated at 30 °C for 3 days. The media that increases the spores of Bacillus firmus and Pseudomonas spp. cells will be used in the pot experiment.
Growth of Bacillus firmus and Pseudomonas aeruginosa
Bacteria were grown individually in molasses, contain between 0.1% and 2.5% of the mother liquid inoculant was mixed with 1000 mL of molasses-based liquid media in a 2 L Erlenmeyer flask at room temperature for 72 h on a 115 rpm gyratory shaker. The Bacillus firmus and pseudomonas aeruginosa liquid inoculants were then mixed at a volume ratio of 1:10 for the final volume of 100 mL. Bacillus firmus spore and Pseudomonas aeruginosa cell counts were performed on nutrient agar for Bacillus firmus and beef extract peptone medium broth for Pseudomonas aeruginosa at 10, 8 by serial dilution plate method62. The optimal composition was stored at room temperature for 28 days for the determination of acidity, electrical conductivity, E. coli and Salmonella population, and phytohormone.
Application of biofertilizer
Molasses, Bacillus firmus, and Pseudomonas aeruginosa were among the organic substances used to produce liquid biofertilizers. To estimate the approximate concentration of heterotrophic bacteria (4 × 105/mL), a serial dilution was carried out using the supernatant. The dilutions were then plated. This estimated concentration was utilized to adjust the concentration of the biofertilizer in order to standardize the final concentration of biofertilizer treatments for implantation (4 × 105/mL). Each treatment got a different amount of biofertilizer at five levels: 20, 40, 60, 80, and 100 mL of prepared biofertilizer on the first day after planting chinese celery cabbage. Molasses was obtained from a sugar factory. The raw materials were analyzed for pH, Ec, total nitrogen (TN), total phosphorus (TP), and total potassium (TK), organic matter, organic carbon (O.C), and the C/N ratio. The chemical properties of the initial substrate, molasses, were descript in Table 2.
Statistical analysis
The statistical analysis was performed using origin lab pro software 202163. All experiments were performed in triplicate. Error bars on graphs show the standard error. Pearson correlation significance was calculated among various soil HMs concentrations parameters and soil enzyme activity at 95% confidence. The data were analyzed by analysis of variance (ANOVA) and the means were compared using the Fisher LSD test (p < 0.05).
Results
Effects of biofertilizer on the soil physiochemical properties
Biofertilizer treatments resulted in significant changes in the physiochemical properties of the soil. The effects of biofertilizer treatments on soil pH levels were significant at p < 0.05. Biofertilizer treatments reduced pH levels (Table 3). While the greatest soil pH value was observed in the B treatment, the pH was decreased from (8.28) in the Ck treatment to (7.39) in the B treatment. Biofertilizer treatments increased soil Ec levels (Table 3). The greatest Ec value (1.12 ds m−1) was obtained from combined BP treatment, and the lowest Ec value (0.91 ds m−1) was obtained from Ck treatment without biofertilizer. The effects of biofertilizer treatments on total N levels were found to be non-significant at p < 0.05 (Table 3). Increased available N contents were observed with biofertilizer treatments (Table 3). While the highest available N content (24.4 mg/kg) was observed in combined (BP) treatments, the lowest available N content (16.7 mg/kg) was observed in CK treatments. The effects of biofertilizer treatments on available P contents were significant at p < 0.05 (Table 3). While the highest P content (51.72 mg/kg) was observed in B treatment, the minimum P content (35.06 mg/kg) was observed in BP treatment (Table 3). Biofertilizer treatments had a significant at p < 0.05 effect on available K levels (Table 3). Biofertilizer treatments increased the available K contents of the soils. The greatest K content (453.3 mg/kg) was obtained from BP treatment, and the lowest value (125.5 mg/kg) was obtained from CK treatment. The effects of biofertilizer treatments on soil organic matter content were significant at p < 0.05 (Table 3). Increasing O.M levels were observed with biofertilizer treatments (Table 3). The highest O.M level (20.63 g/kg) was obtained from BP treatments, and the lowest O.M level (15.19 g/kg) was observed in B treatments. The effects of biofertilizer treatments on total N levels were found to be non-significant at p < 0.05 (Table 3). A significant effect was found on total P between Ck and treatment at p < 0.05 (Table 3).
Effects of biofertilizer application on the soil exchangeable nutrients
The impact of biofertilizer application on the soil exchangeable nutrient Ca2+, Mg2+, K+, Na+, NH4+ and sulfur (S) was significantly different were compared between treatments and control, and interaction between dose and treatments except for Ca2+ was non-significantly different between treatments and dose. The soil Na+ and K+ contents were increased by the application of biofertilizers. The effects of biofertilizer and treatments on exchangeable Ca2+ levels were non-significant at p < 0.05 (Table 4). Treatments with biofertilizer had a significant effect on exchangeable Mg+ contents at p < 0.05 (Table 4). The highest Mg2+ content (73.66 g/kg) was obtained from combined BP treatment, and the lowest Mg+ content (61.77 g/kg) was obtained from Ck treatment (Table 4). Biofertilizer treatments had a significant at p < 0.05 effect on exchangeable K+ contents (Table 4). Exchangeable K+ contents increased with biofertilizer treatments (Table 4). The highest K+ content (201.50 g/kg) was obtained from combined BP treatment, and the lowest K+ content (145.25 g/kg) was obtained from Ck treatment. The effects of biofertilizer treatments on exchangeable Na+ contents were found to be significant at p < 0.05 (Table 4). The greatest Na+ content (230.25 g/kg) was obtained from combined BP treatment, and the lowest Na+ content (65.75 g/kg) was obtained from Ck treatment (Table 4). Table 4 shows that biofertilizer treatments had a significant effect on ammonium nitrogen NH4+ at p < 0.05 and soil S at p < 0.05.
Effects of biofertilizer on the soil heavy metal contents
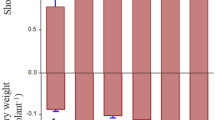
The application of biofertilizer containing Pseudomonas aeruginosa and Bacillus firmus was significantly affected by the treatments with different doses of biofertilizer. When compared to the control, the use of biofertilizer treatments and dose levels had a significant effect on Cu bioremediation at p < 0.05 (Fig. 1A). Application of biofertilizers on the Zn content were significantly affected by biofertilizer and dose treatments at p < 0.05 (Fig. 1B). While the Cd bioremediation content was observed in all treatments with a dose-significant effect compared to the control at p < 0.05 (Fig. 1C). Biofertilizer treatments and dose levels did not have clear significant effects on the Mo content at p < 0.05 but there is siginificant differences among treatments at p < 0.05 (Fig. 1D). Biofertilizer treatments increased soil Zn contents were compared the dose and treatment to control without biofertilizer. The greatest Fe bioremediation level was observed at doses of 80 mL with B treatments and dose 60 mL with B treatments compared to the control (Fig. 1E). While the greatest Mn content was obtained from BP treatment at 40 mL dose compared to the control (Fig. 1F).
Graphs (A) Cu, (B) Zn, (C) Cd, (D) Mo, (E) Fe, (F) Mn. The interaction between dose and treatments of biofertilizer application, effect of dose concentration (20, 40, 60, 80 and 100/mL) on heavy metal concentration in the soil. After application: (CK) control, (B) Bacillus firmus, (P) Pseudomonas aeruginosa (BP) combination of Bacillus firmus and Pseudomonas aeruginosa Values are means ± SE (n = 3). Bars with different letters represent significantly (p < 0.05) differences after ANOVA and an LSD (Least significant difference) test.
Effects of biofertilizer on the soil enzyme activities
Soil enzyme activities were determined in the soils treated with CK, B, P, and BP, the influences of different doses of biofertilizer on rhizosphere soil enzyme activities are described in Fig. 2. The soil urease activity was significantly affected at (p < 0.05) were compared between treatment and control (Fig. 2A). The application of biofertilizer treatments and dose levels significantly affected on the dehydrogenase activity at (p < 0.05) (Fig. 2B). Biofertilizer application was significantly affected on the soil alkaline phosphatase activity at (p < 0.05) were compare between treatment and control; but there was no significant difference between treatments or dose levels at (p < 0.05) (Fig. 2C). The effect of biofertilizer application on the soil β-D-glucosidase activity was found to be significant at (p < 0.05), when compared the treatments and dose levels (Fig. 2D). Furthermore, the effect of biofertilizer application on soil arylsulfatase activity was significant effect at (p < 0.05) were compared to control resulted in (Fig. 2E).
Effects of biofertilizer application on the soil enzyme activity five dose levels (20, 40, 60, 80 and 100/mL) on heavy metal concentration in the soil after application and four treatments represent as (CK) control, (B) Bacillus firmus, (P) Pseudomonas aeruginosa (BP) combination of Bacillus firmus and Pseudomonas aeruginosa Values are means ± SE (n = 3). Graphs (A) URE, (B) DEH, (C) APK, (D) β-D-GUL, (E) ARY. Bars with different letters represent significantly (p < 0.05) differences after ANOVA and an LSD (Least significant difference) test.
Correlation between soil heavy metal content and enzyme activities
The Pearson correlation coefficients between soil heavy metals and enzyme activity are provided in Table 5. Soil pH were positively correlated with soil Zn (0.99819*), APK (0.95869*) activity and negatively correlated with Fe (0.96759*) also statistically significant at (p < 0.05). The soil Cu positively correlated with Fe (0.99645*), Cd (0.97866*), β.D.GLU (0.99769*) and negatively correlated with PAK (− 0.9624*). The URE activity were negatively correlated with Mo (− 0.98885*), pH, PAK and positively correlated with other metals and enzyme activity nevertheless had no significant at (p < 0.05). Therefore, soil pH was an important factor among all soil heavy metals and enzyme activity. The negative correlation between soil pH and soil enzyme activity except PAK shows positive correlation with soil pH. However, soil ARY had positive correlation with soil Mn (0.99683*), Cd (0.95695*), and negative correlation with PAK (− 0.99424*) activity. DEH had slight change negatively and positively but did not have any statistically significant relationship with all parameters at (p < 0.05). This result indicated that the relationship between soil heavy metals and soil enzyme activities was more closely.
Discussion
In this study, particularly Bacillus firmus and Pseudomonas aeruginosa, which are used as biofertilizers to bioremediate soil, have been polluted with heavy metals due to human activities. This research investigated the effects of biofertilizer on heavy metal bioremediation. These include soil enzyme activity, soil physiochemical parameters, and the correlation between heavy metals in the soil and enzyme activity. Further biofertilizer was applied in five doses (20 mL, 40 mL, 60 mL, 80 mL, and 100 mL) to greenhouse soil grown with Chinese celery cabbage. The results of the current study showed that the application of biofertilizer was directly related to the improvement of soil physiochemical parameters, including soil organic matter and total P. Biofertilizers improve plant nutrition and stress tolerance by fixing atmospheric nitrogen and resolving soil nutrients12,13. Biofertilizer promotes soil organic carbon content by releasing organic molecules via the roots19,64 found that the organic carbon content of pot soils significantly increased. The current results are consistent with the findings of Faye et al.65, who found that using biofertilizer significantly increased the amount of soil organic carbon. Biofertilizer application at five different doses in greenhouse conditions significantly increased the soil available P and K levels in the single treatments, especially P. The soil nutrients were enhanced by low-dose 40 mL and medium-dose 60 mL levels Table 3. These findings demonstrated a direct correlation between the application of Bacillus firmus and Pseudomonas aeruginosa and their ability to enhance soil nutrient availability. The use of biofertilizer has the potential to increase the amount of P that is easily available in soil since it supports a higher population of a variety of bacteria that are capable of solubilizing soil P66. The amount of P in the soil solution may change as root exudates, such as organic ligands, are released67. According to literature cited microorganisms primarily solubilize insoluble P by the formation of organic acids and chelating compounds. The use of Bacillus firmus and Pseudomonas aeruginosa may have resulted in more solubilization of insoluble phosphates in the soil and as a result, increased phosphate uptake68. According to Tak et al.69 biofertilizer significantly changed ion selectivity, increasing K+ and Ca2+ uptake while decreasing Na+ uptake. It was observed that biofertilizer caused distinct buildup of N, P, and K, therefore maintaining nutritional balance70,71. Bacillus firmus and Pseudomonas aeruginosa have been shown to enhance soil nutrients and consequently fertility in soils72. In this study, the soil Na+ and K+ contents Table 4 were increased by application of biofertilizers.
According to this research, Table 3 soil pH values under greenhouse conditions for Chinese celery cabbage reduced with biofertilizer application, under the five dosage as compared to the treatments without biofertilizer. According to Berger et al.73, biofertilizer decreased soil pH and increased soil available P and K levels, due to enhanced K release from organic components and minerals. Numerous parameters, including soil pH and fertility, are associated with the presence of Bacillus firmus and Pseudomonas aeruginosa in soil74. Reference75 discovered that biofertilizer inoculation caused a pH shift, slightly lowering alkalinity, and slightly increasing organic matter content in all biofertilizer inoculated treatments compared to uninoculated soil. In this investigation, biofertilizer treatments caused a decrease in soil pH; the overall mean decrease in pH when compared to soils not treated with biofertilizer was 7.71%. The BF + 40 treatment had the lowest pH (7.16). Due to the application of biofertilizer, there was a slight change in the pH of the soil. The pH of the soils decreases as a result of microbial inoculants’ increased amounts of organic acid. The current results are consistent with those of 76,77, who found that applying bio-fertilizers reduced the pH of the soil. These bacteria can produce organic acids as byproducts of their metabolic processes, which can lower the pH of the soil. This acidification can influence the pH levels and make them more acidic.
This research found that biofertilizer treatments significantly affected soil Ec values Table 3, which ranged from 1.79 to 3.29 ds m1. Ec is a major indicator of the soil’s quality and is influenced by the ion concentrations in the soil solution 78. As ion concentrations rise, soil Ec values rise as well. The Ec values for soils treated with biofertilizer were higher than among control soils without bio-fertilizers, according to Singh et al.79. Reference80 reported decreased pH and increased Ec values with bio-compost treatments along with Azotobacter. The current research showed that biofertilizer treatments enhanced the amount of cations, particularly Ca2+, Mg2+, and K+. Azotobacter-treated plots maintained soil available nutrients and organic carbon concentrations. Our study results showed that the application of biofertilizer increased amount of Mg2+, and K+ and decrease in the Ca2+ at single treatments Table 4, this may be lead to ability of this bacteria to transform the Ca2+.
Biofertilizer application improved heavy metals bioremediation in the Cu, Cd, Mo, Fe, and Cd; nonetheless decreased in the Zn at five different doses as compared to treatments without biofertilizer Ck (Fig. 1). Biofertilizers increase heavy metal availability by solubilization, chelation, and oxidation/reduction processes81. Biofertilizer develops iron-chelating compounds, reduces soil pH, releases organic acids into the soils, and potentially increases the availability of Fe75. Consequently, using biofertilizer and plants for remediation is a potential solution to heavy metal contamination. The application was reduce the Cd and Mo content in the soil82, report that the biofertilizer was decrease heavy metals components of Pb, Cd, Mo, in the soil which was extremely intensive for using chemical fertilizer and pesticides. In contrast, the use of biofertilizer increased the bioavailability of Cu in both bacteria strains utilized, Bacillus firmus and Pseudomonas aeruginosa. This study demonstrated the possible application of biofertilizer in the bioremediation of heavy metals in soil, and a similar study was noted by Mesa-Marín et al.83 S. ramosissima plant growth was increased by inoculation with heavy metal resistant PGPB. In our investigation, the effect of different doses of biofertilizer was to decrease the levels of Zn in the soil and increase the levels of Mn, which leads to the use of bacteria spices in this study, Bacillus firmus and Pseudomonas aeruginosa, which may work depending on heavy metal concentration and bacteria mechanisms. According to Abdel-Azis et al.84, biofertilizers have the potential to reduce the content of heavy metals in both polluted soil and plant parts. Beyond their impact on enhancing plant development via plant growth promotion, chemicals and bacteria nitrogen fixing (BNF) mechanisms, a similar function of biofertilizer was found. The bacteria existing in biofertilizers have the ability to immobilize heavy metals. This means that they can bind heavy metals to their cellular components, reducing their availability for uptake by plants. As a result, Fig. 1, heavy metal concentrations in the soil was decrease when biofertilizers are used.
According to this study, the application of biofertilizer had a significant effect on the soil enzyme activity and bioremediation of the HMs, Fig. 2 on both the structure and activity. According to Yang et al.85, the enzyme activity as stated by Oleszczuk et al.86, the toxicological effects of Zn, Cu, Mn, Fe, Mo, and Cd on the structure and activity of soil microbial communities are shown to be highly dependent on concentration of heavy metals content and application duration. Furthermore, soil enzyme activity is a measure of ecosystem health and sustainability87. The C, N, and P looping in soil are closely related to, urease, and phosphatase activity41. According to the current investigation, applying biofertilizer significantly increased the activity of the soil enzymes urease, dehydrogenase, β-D-glucosidase, arylsulfatase, and when compared to Ck (Fig. 2A,B,D,E) and decrease in the phosphatase were compare to Ck (Fig. 2C). this suggests that applying biofertilizer might raise soil enzyme activities. Similarly report that changes in rhizosphere bacteria activity might explain the increase in enzymatic activities88. Many studies mentions that the diversity of native microorganisms may improve during biofertilizer application89,90. According to this study, the use of biofertilizer may have contributed to the increase in soil enzyme activity. The use of biofertilizers is considered to promote cabbage growth by increasing the amount of available phosphorus in the soil, modifying the soil community, and activating soil enzymes. Inclusive, these chosen soil enzymes provide valuable evidence about the effects of heavy metal contamination on important soil biochemical processes involved in nutrient cycling, organic matter decomposition, and soil health. They help in assessing the potential for bioremediation strategies to restore the functionality and fertility of soils heavy metal-contaminated.
Heavy metals and soil enzyme activity were both impacted by the correlation between the various soil characteristics91. Soil enzymes have a significant role in the fundamental biochemical characteristics of soil. Additionally, soil enzymes influence the formation, composition, and biochemical characteristics of enzymes92. The activities of enzymes (acid and alkaline phosphatases, β-D-glucosidase, and arylsulfatase) have been reported to be positively correlated with soil heavy metal concentrations67. The outcomes of this investigation were complex. Table 5 shows that Zn was negatively correlated with AKP and URE activity, but the Mn in the soil was positively correlated with URE, AKP, β-D-GUL, ARY, and DEH activity. The activity of the AKP is negatively correlated with the Cu, Fe, and Cd concentrations in the soil. These results indicated that Mo correlated negatively with all soil enzyme activities except for AKP, which positively correlated. Overall, the duration of the study might not capture long-term effects or variations in the bioremediation process and enzyme activities. Different timeframes might lead to different results, as the efficiency of biofertilizer treatment and enzymatic activities could change over time.
Conclusions
This study was investigated Bacillus firmus and Pseudomonas aeruginosa as biofertilizers, and their ability to perform heavy metal bioremediation in the soil was confirmed. Positive effects on soil physicochemical porprates, availability of soil macronutrients, exchangeable nutrients, and six heavy metals were observed under greenhouse conditions. When microbial biofertilizer treatments were applied at five different dosage levels (20 mL to 100 mL). Biofertilizer treatments at all dosage levels enhanced soil organic carbon content and aggregate stability considerably as compared to non-fertilizer applications. Biofertilizer treatments had also significantly decreased contributions to the soil pH and Ec. Based on the outcomes of this study, it was determined that biofertilizer might be utilized as an alternative element of integrated nutrient management systems. As a result, it is possible to conclude that soil enzyme activity was increased in the URE, ARY, DEH, β-D-GUL and decreased in the APK during Pseudomonas aeruginosa biofertilizer application. The best dose found in the enzyme activities was 80 mL, and heavy metal bioremediation was increased at a dose of 60 mL with Bacillus firmus biofertilizer application, which was better than Pseudomonas aeruginosa and combination biofertilizer. Furthermore, the use of biofertilizer for bioremediation processes was recommended as a sustainable, particular, and low-cost substitute for heavy metal bioremediation in contaminated soils. In the prospect of using biofertilizer prepared through molasses for contaminated greenhouse soil, we recommended that, based on the findings of this research, 60 mL be used as dose levels with Bacillus firmus for heavy metal bioremediation and 80 mL as dose levels with Pseudomonas aeruginosa, a combination biofertilizer for soil enzyme activities. There are several potential directions for future research: 1. Optimization of biofertilizer formulations, 2. Assessment of long-term effects, 3. Examination of enzyme mechanisms, 4. Field-scale investigations, 5. Environmental and economic assessments. By addressing these research directions, scientists can further enhance our understanding of the influence of biofertilizer on heavy metal bioremediation and enzyme activities in the soil to revealing the potential for sustainable soil restoration, paving the way for more effective and sustainable soil remediation strategies.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKP:
-
Alkaline phosphatase
- β.D. GLU:
-
β-D glucosidase
- RAY:
-
Arylsulfatase
- DEH:
-
Dehydrogenase
- URE:
-
Urease
- HMs:
-
Heavy metals
- Zn:
-
Zinc
- Cd:
-
Cadmium
- Cu:
-
Copper
- Mn:
-
Manganese
- Fe:
-
Iron
- Pb:
-
Lead
- Cr:
-
Chromium
- As:
-
Arsenic
- Hg:
-
Mercury
- Ni:
-
Nickel
- ICP-MS:
-
Inductively coupled plasma–mass spectrometry
References
Khan, M. N., Mobin, M., Abbas, Z. K. & Alamri, S. A. Fertilizers and their contaminants in soils, surface and groundwater. Encycl. Anthr. 5, 225–240 (2018).
Youssef, M. M. A. & Eissa, M. F. M. Biofertilizers and their role in management of plant parasitic nematodes. A review. J. Biotechnol. Pharm. Res. 5, 1–6 (2014).
Kawalekar, J. S. Role of biofertilizers and biopesticides for sustainable agriculture. J. Bio Innov. 2, 73–78 (2013).
Raghuwanshi, R. Opportunities and challenges to sustainable agriculture in India. Nebio 3, 78–86 (2012).
Bumandalai, O. & Tserennadmid, R. Effect of Chlorella vulgaris as a biofertilizer on germination of tomato and cucumber seeds. Int. J. Aquat. Biol. 7, 95–99 (2019).
Yadav, K. K. & Sarkar, S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 37, 89–93 (2019).
Mishra, D., Rajvir, S., Mishra, U. & Kumar, S. S. Role of bio-fertilizer in organic agriculture: A review. Res. J. Recent Sci. 2277, 2502 (2013).
Malusà, E., Pinzari, F. & Canfora, L. Efficacy of biofertilizers: Challenges to improve crop production. In Microbial Inoculants in Sustainable Agricultural Productivity (eds Singh, D. et al.) (Springer, 2016).
Singh, M. et al. Role of biofertilizers in conservation agriculture. In conservation Agriculture (eds Bisht, J. et al.) (Springer, 2016).
Singh, S. P., Singh, S., Dubey, A. N. & Rajput, R. K. Biofertilizers and plant growth regulators as key player in sustainable agriculture by enhancing soil fertility and crop productivity. Environ. Agric. Heal. 12–18 (2020).
Wong, W. S. et al. The importance of phytohormones and microbes in biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem. Sustainable Development and Biodiversity (ed. Maheshwari, D.) 105–158 (Springer, 2015).
Dineshkumar, R., Kumaravel, R., Gopalsamy, J., Sikder, M. N. A. & Sampathkumar, P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valorization 9, 793–800 (2018).
Basu, A. et al. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 13, 1140 (2021).
Sun, B. O. et al. Application of biofertilizer containing Bacillus subtilis reduced the nitrogen loss in agricultural soil. Soil Biol. Biochem. 148, 107911 (2020).
Mitra, S. et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. 34, 101865 (2022).
Alengebawy, A., Abdelkhalek, S. T., Qureshi, S. R. & Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 9, 42 (2021).
Briffa, J., Sinagra, E. & Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6, e04691 (2020).
Jadia, Chhotu D., M. H. Fulekar. Phytoremediation of heavy metals: Recent techniques. African J. Biotechnol. 8(6), 921–928 (2009).
Ma, Y., Rajkumar, M., Zhang, C. & Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manage. 174, 14–25 (2016).
Banerjee, Aishiki, S. K. Barik, and S. R. Joshi. "Bacilli and Sustainable Jhum Agrobiotechnology." In Bacilli in Agrobiotechnology: Plant Stress Tolerance, Bioremediation, and Bioprospecting, pp. 231–254. Cham: Springer International Publishing, 2022. https://doi.org/10.1007/978-3-030-85465-2.
Glick, B. R. Introduction to Plant Growth-Promoting Bacteria BT - Beneficial Plant-Bacterial Interactions. in (ed. Glick, B. R.) 1–37 (Springer International Publishing, 2020) https://doi.org/10.1007/978-3-030-44368-9_1.
Aguilar, N. C. et al. Isolation and characterization of bacteria from a brazilian gold mining area with a capacity of arsenic bioaccumulation. Chemosphere 240, 124871 (2020).
Sharma, J. Advantages and limitations of in situ methods of bioremediation. Recent Adv. Biol. Med. 5, 10941 (2019).
Radhakrishnan, R., Hashem, A. & Abd Allah, E. F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 8, 667 (2017).
Özer, A. & Özer, D. Comparative study of the biosorption of Pb (II), Ni (II) and Cr (VI) ions onto S. cerevisiae: determination of biosorption heats. J. Hazard. Mater. 100, 219–229 (2003).
Ravindran, B., Mupambwa, H. A., Silwana, S. & Mnkeni, P. N. S. Assessment of nutrient quality, heavy metals and phytotoxic properties of chicken manure on selected commercial vegetable crops. Heliyon 3, e00493 (2017).
Wang, Y. et al. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 67, 75–81 (2007).
Smorkalov, I. A. & Vorobeichik, E. L. Does long-term industrial pollution affect the fine and coarse root mass in forests? Preliminary investigation of two copper smelter contaminated areas. Water, Air, Soil Pollut. 233, 1–17 (2022).
Ahmad, I. et al. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 21, 11054–11065 (2014).
Wuana, R. A. & Okieimen, F. E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 402647 (2011).
Igiri, B. E., et al. "Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review." J. Toxicol. 2018, 2568038 (2018).
Abbas, S. H., Ismail, I. M., Mostafa, T. M. & Sulaymon, A. H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 3, 74–102 (2014).
Abedinzadeh, M., Etesami, H. & Alikhani, H. A. Characterization of rhizosphere and endophytic bacteria from roots of maize (Zea mays L.) plant irrigated with wastewater with biotechnological potential in agriculture. Biotechnol. Rep. 21, e00305 (2019).
Jin, Y., Luan, Y., Ning, Y. & Wang, L. Effects and mechanisms of microbial remediation of heavy metals in soil: A critical review. Appl. Sci. 8, 1336 (2018).
Naveed, M. et al. Burkholderia phytofirmans PsJN and tree twigs derived biochar together retrieved Pb-induced growth, physiological and biochemical disturbances by minimizing its uptake and translocation in mung bean (Vigna radiata L.). J. Environ. Manage. 257, 109974 (2020).
Thomas, L. & Singh, I. "Microbial Biofertilizers: Types and Applications." Biofertilizers Sustain. Agric. Environ. 55, 1 (2019).
Rao, M. A., Scelza, R., Acevedo, F., Diez, M. C. & Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 107, 145–162 (2014).
Dale, V. H., and Beyeler, S. C. Challenges in the development and use of ecological indicators. Ecol. Indic. 1, 3–10 (2001).
Gianfreda, L., Rao, M. A., Piotrowska, A., Palumbo, G. & Colombo, C. Soil enzyme activities as affected by anthropogenic alterations: Intensive agricultural practices and organic pollution. Sci. Total Environ. 341, 265–279 (2005).
Renella, G. et al. Functional activity and microbial community structure in soils amended with bimetallic sludges. Soil Biol. Biochem. 37, 1498–1506 (2005).
Lee, S.-H., Kim, E.-Y., Hyun, S. & Kim, J.-G. Metal availability in heavy metal-contaminated open burning and open detonation soil: Assessment using soil enzymes, earthworms, and chemical extractions. J. Hazard. Mater. 170, 382–388 (2009).
Kirkland, K. et al. Validation and peer review of US environmental protection agency sampling methods for chemical and radiochemical parameters. 2007-02, 10–22 (2016).
Lee, S.-H., Kim, M.-S., Kim, J.-G. & Kim, S.-O. Use of soil enzymes as indicators for contaminated soil monitoring and sustainable management. Sustainability 12, 8209 (2020).
Anderson, B. A Study of Enhanced De-chlorination and Bio-Remediation: Molasses Injections into Groundwater. The Faculty of the Natural Resource Management and Environmental Science Department California Polytechnic State University, San Luis Obispo. A Senior Project. 6–11 (2012).
Scoma, A., Coma, M., Kerckhof, F.-M., Boon, N. & Rabaey, K. Efficient molasses fermentation under high salinity by inocula of marine and terrestrial origin. Biotechnol. Biofuels 10, 1–17 (2017).
Nishida, O., Kuwazaki, S., Suzuki, C. & Shima, J. Superior molasses assimilation, stress tolerance, and trehalose accumulation of baker’s yeast isolated from dried sweet potatoes (hoshi-imo). Biosci. Biotechnol. Biochem. 68, 1442–1448 (2004).
Teclu, D., Tivchev, G., Laing, M. & Wallis, M. Determination of the elemental composition of molasses and its suitability as carbon source for growth of sulphate-reducing bacteria. J. Hazard. Mater. 161, 1157–1165 (2009).
Bremner, J. M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55, 11–33 (1960).
OLSEN, SR. "Estimation of available phosphorus in soils by extraction with sodium bicarbonate." USDA Circ. 939 (1954).
Huang, X. et al. Study on the correlation between potassium dichromate external heating method and ASI for soil organic matter determination. Hubei Agric. Sci. 59, 122 (2020).
Mussa, S. A. B., Elferjani, H. S., Haroun, F. A. & Abdelnabi, F. F. Determination of available nitrate, phosphate and sulfate in soil samples. Int. J. PharmTech Res. 1, 598–604 (2009).
Shidan, B. Soil Agrochemical Analysis. Beijing: China Agricultural Press. 3rd Edition [M]. (2000).
Arunachalam, J., Mohl, C., Ostapczuk, P. & Emons, H. Multielement characterization of soil samples with ICP-MS for environmental studies. Fresenius. J. Anal. Chem. 352, 577–581 (1995).
Soltanpour, P. N., Khan, A. & Lindsay, W. L. Factors affecting DTPA-extractable Zn, Fe, Mn, and Cu from soils. Commun. Soil Sci. Plant Anal. 7, 797–821 (1976).
Kandeler, E. & Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 6, 68–72 (1988).
Von Mersi, W. & Schinner, F. An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. soils 11, 216–220 (1991).
Shaw, L. J. & Burns, R. G. 7.7 Enzyme Activity Profiles and Soil Quality. Methods Assess. Soil Qual. 158 https://doi.org/10.1079/9780851990989.0114 (2006).
Tabatabai, M. A. & Bremner, J. M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1, 301–307 (1969).
Eivazi, F. & Tabatabai, M. A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 20, 601–606 (1988).
Tabatabai, M. A. & Bremner, J. M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. J. 34, 225–229 (1970).
Hindersah, R., Setiawati, M. R., Asmiran, P. & Fitriatin, B. N. Formulation of Bacillus and Azotobacter consortia in liquid cultures: Preliminary research on microbes-coated urea. Int. J. Agric. Syst. 8, 1–10 (2020).
Jett, B. D., Hatter, K. L., Huycke, M. M. & Gilmore, M. S. Simplified agar plate method for quantifying viable bacteria. Biotechniques 23, 648–650 (1997).
Pro, O. Version 2021. Orig. Corp. Northampton, MA, USA (2021).
Kaushal, M. & Prasad, R. Microbial biotechnology in crop protection. Microbial Biotechnol. Crop Prot. https://doi.org/10.1007/978-981-16-0049-4 (2021).
Faye, A. et al. Evaluation of commercial arbuscular mycorrhizal inoculants. Can. J. Plant Sci. 93, 1201–1208 (2013).
Mahanty, T. et al. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 24, 3315–3335 (2017).
Hinojosa, M. B., García-Ruíz, R., Viñegla, B. & Carreira, J. A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 36, 1637–1644 (2004).
Vandana, U. K., Chopra, A., Bhattacharjee, S. & Mazumder, P. B. "Microbial Biofertilizer: A Potential Tool for Sustainable Agriculture." Microorganisms for Green Revolution: Microbes for Sustainable Crop Production. 1, 6–25 (2017).
Tak, H. I., Ahmad, F. & Babalola, O. O. Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev. Environ. Contam. Toxicol. 223, 33–52 (2013).
Adesemoye, A. O., Torbert, H. A. & Kloepper, J. W. Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can. J. Microbiol. 54, 876–886 (2008).
Egamberdiyeva, D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. soil Ecol. 36, 184–189 (2007).
Kizilkaya, R. Nitrogen fixation capacity of Azotobacter spp. strains isolated from soils in different ecosystems and relationship between them and the microbiological properties of soils. J. Environ. Biol 30, 73–82 (2009).
Berger, L. R. et al. Plant and soil characteristics affected by biofertilizers from rocks and organic matter inoculated with diazotrophic bacteria and fungi that produce chitosan. J. Soil Sci. plant Nutr. 13, 592–603 (2013).
Jnawali, A. D., Ojha, R. B. & Marahatta, S. Role of Azotobacter in soil fertility and sustainability–a review. Adv. Plants Agric. Res 2, 1–5 (2015).
Demir, Z. Effects of microbial bio-fertilizers on soil physicochemical properties under different soil water regimes in greenhouse grown eggplant (Solanum Melongena L.). Commun. Soil Sci. Plant Anal. 51, 1888–1903 (2020).
Meena, A. L. et al. Impact of 12-year-long rice based organic farming on soil quality in terms of soil physical properties, available micronutrients and rice yield in a typic Ustochrept soil of India. Commun. Soil Sci. Plant Anal. 51, 2331–2348 (2020).
Du, T.-Y. et al. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 175, 104457 (2022).
Tale, K. S. & Ingole, S. A review on role of physico-chemical properties in soil quality. Chem. Sci. Rev. Lett. 4, 57–66 (2015).
Singh, J. S., Kumar, A., Rai, A. N. & Singh, D. P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 7, 529 (2016).
Sinha, S. K., Kumar, V. & Jha, C. K. Effect of integrated use of bio-compost and nitrogen on productivity and soil properties of sugarcane plant–ratoon system in calcareous soil. Sugar Tech 19, 485–491 (2017).
Rahman, Z. & Singh, V. P. Bioremediation of toxic heavy metals (THMs) contaminated sites: Concepts, applications and challenges. Environ. Sci. Pollut. Res. 27, 27563–27581 (2020).
Riyanto, D., Afriani, R. & Srihartanto, E. The effect of biological fertilizer application on soil fertility, heavy metals reduction and eggplant yield on the rice field of Bantul regency. IOP Conf. Ser. Earth Environ. Sci. 672, 12093 (2021).
Mesa-Marín, J. et al. Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils. Front. Microbiol. 11, 553018 (2020).
Abdel-Azis, O. A., El-Ghandour, I. A., Galal, Y. G. M. & El-Sheikh, H. H. Effect of Bioremediation on growth of wheat plant cultivated in contaminated soil with heavy metals. EG0800365, 13759 (2008).
Yang, X. et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 23, 974–984 (2016).
Oleszczuk, P. et al. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 214, 10–18 (2014).
Acosta-Martínez, V., Cruz, L., Sotomayor-Ramírez, D. & Pérez-Alegría, L. Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl. Soil Ecol. 35, 35–45 (2007).
Zhou, X., Yu, G. & Wu, F. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur. J. Soil Biol. 47, 279–287 (2011).
Aseri, G. K., Jain, N., Panwar, J., Rao, A. V. & Meghwal, P. R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar Desert. Sci. Hortic. (Amsterdam) 117, 130–135 (2008).
Yang, L.-Y., Lin, C.-S., Huang, X.-R., Neilson, R. & Yang, X.-R. Effects of biofertilizer on soil microbial diversity and antibiotic resistance genes. Sci. Total Environ. 820, 153170 (2022).
Karaca, A., Cetin, S. C., Turgay, O. C. & Kizilkaya, R. Effects of heavy metals on soil enzyme activities. Soil Heavy Met. 16, 237–262 (2010).
Datt, N. & Singh, D. Enzymes in Relation to Soil Biological Properties and Sustainability BT - Sustainable Management of Soil and Environment. in (eds. Meena, R. S., Kumar, S., Bohra, J. S. & Jat, M. L.) 383–406 (Springer Singapore, 2019). https://doi.org/10.1007/978-981-13-8832-3_11.
Acknowledgements
The authors would like to thank all those who helped us through this work for their valuable assistance and technical support. The authors wish to thank Muhi Eldeen Hussein and Nimir Ahmed for their support in the proofreading and the review of the article.
Funding
This study was funded via Key Research and Development Projects (social development) in Yangzhou China, funding number (“YZ2022060”) and Ministry of Agriculture and Rural Affairs, Key Laboratory of Arable Land Quality Monitoring and Evaluation (Yangzhou University), Yangzhou Jiangsu, China “225127”.
Author information
Authors and Affiliations
Contributions
For Conceptualization, X.Q. and S.X.; methodology, M.H.; software, J.W.; validation, X.Q., J.W. and M.H.; formal analysis, W.A.; investigation, M.H.; writing—original draft preparation, M.H.; writing—Article and editing, M.H.; visualization, J.W.; supervision, X.Q.; project administration, X.Q.; funding acquisition, X.Q. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haroun, M., Xie, S., Awadelkareem, W. et al. Influence of biofertilizer on heavy metal bioremediation and enzyme activities in the soil to revealing the potential for sustainable soil restoration. Sci Rep 13, 20684 (2023). https://doi.org/10.1038/s41598-023-44986-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44986-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.