Abstract
Although nonsteroidal antiinflammatory drugs (NSAIDs) are frequently used for fever and pain during pregnancy, their possible interaction with perinatal renal injury induced by preeclampsia (PE) has not been addressed. Here, studies were undertaken in the N(gamma)-nitro-l-arginine methyl ester (l-NAME) PE model to assess the influence of gestational NSAIDs on renal damage in weaning dams. PE-evoked increments and decrements in urine protein and creatinine clearance, respectively, were intensified by celecoxib and weakened by diclofenac or naproxen. Naproxen also improved renal cloudy swelling, necrosis, and reduced glomerular area evoked by PE. The concomitant rises in renal expression of markers of oxidative stress (NOX2/4), extracellular matrix metaloproteinase deposition (MMP9), and prostanoids (PGE2, PGF2α, TXA2) were all more effectively reduced by naproxen compared with celecoxib or diclofenac. Western blotting showed tripled expression of mitogen-activated protein kinases (MAPKs; p-p38, p-JNK1, p-ERK1, p-ERK2) in PE kidneys that was overturned by all NSAIDs, with naproxen producing the largest drop in p-ERK2 expression. The PE-provoked elevation in renal expression of autophagic marker LC3 was reduced by naproxen and diclofenac, but not celecoxib. The data suggests superior effect for naproxen over other NSAIDs in rectifying preeclamptic renal injury and predisposing inflammatory, oxidative, autophagic, and fibrotic signals.
Similar content being viewed by others
Introduction
Preeclampsia (PE) is a pregnancy-related syndrome characterized by a widespread endothelial dysfunction and vasospasm that negatively influences remote organs, including the kidney. The pathogenic trails of PE begin with defective placental cytotrophoblast invasion and spiral arteries remodeling that progresses to placental hypoxia and consequent unfavorable milieus of PE1,2,3. Proinflammatory cytokines4, mitogen-activated protein kinases (MAPKs)5, reactive oxygen species, matrix metalloproteinases (MMPs)6, apoptotic enzymes, and autophagosomes7 are offending pathways that critically contribute to PE onset and progression. The renal injurious action of PE is associated with proteinuria, glomerular endotheliosis, and tubular and vascular damage8,9. The unraveling of the bidirectional rapport of PE and kidney diseases is puzzling. Renal dysfunction caused by PE has been accounted for by directly damaging the glycocalyx and podocytes10 or indirectly via increasing the incidence of hypertension, diabetes mellitus, and dyslipidemia11,12.
Disturbances in cyclooxygenase (COX) pathway and arachidonate end products have been implicated in the pathophysiology and complications of PE. For instance, imbalances in prostacyclin and thromboxane, two COX metabolites of arachidonic acid, account for principal clinical symptoms associated with PE like hypertension, platelet aggregation, and compromised uteroplacental blood flow13. COX-1 and COX-2 have also been shown to contribute to the development of angiogenesis inhibitor-induced preeclamptic manifestation of hypertension and renal injury14,15. COX inhibition by aspirin rectifies the preeclamptic prostacyclin/thromboxane ratio16, blunt oxidative stress17, and reduce the incidence of PE in high-risk patients17. Furthermore, COX inhibition effectively improves preeclamptic myocardial damage, but had no effect or even exaggerated the concomitant rise in blood pressure18. Case reports showed that indomethacin exacerbates the PE-evoked hypertension19,20 and weaken the antihypertensive efficacy of β-adrenergic receptors blockers against preeclamptic hypertension21. NSAIDs are often prescribed during pregnancy to treat fever, pain and inflammation22. Notably, the COX inhibitory effects and reduced generation of renal vasodilator prostanoids have been implicated in the NSAID-induced nephrotoxicity. The latter is a recognized adverse effect of NSAIDs that manifest as acute or chronic kidney injury, electrolyte and acid–base disorders, and interstitial nephritis23,24. Notably, reported findings on the use of NSAIDs during pregnancy are inconsistent. Some authors argued against the use of COX-2 inhibitors during the last semester, but no data was provided to support such a claim25. Others failed to demonstrate significant maternal or neonatal adverse events and revealed a safer short-term profile for celecoxib compared with indomethacin26.
Despite the reported isolated renal damaging effects of PE8,9 and NSAIDs23, we are not aware of any study that assessed renal consequences of the exposure to the two interventions simultaneously. This was accomplished in the current investigation by evaluating functional and histopathological indices of renal function in PE rats treated during the last third of pregnancy with naproxen, celecoxib, or diclofenac. The latter compounds have been shown to exhibit variable COX-1/2 selectivity, with celecoxib being a selective COX-2 inhibitor while the other two NSAIDs elicits nonselective COX-1/2 inhibition27. Gene and protein expression studies were also undertaken to determine the roles of arachidonate end products, MAPK signaling, and downstream oxidative, fibrotic, and autophagic effectors in the interaction.
Results
Effects of gestational NSAIDs on renal function and architecture
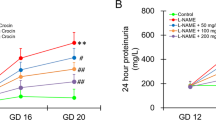
As depicted in Fig. 1a, the protein contents in the 24-h urine samples collected from PE dams at GD20 and weaning time were significantly higher than respective values seen in control (non-PE) rats. The rises in urine protein evoked by PE were intensified after gestational administration of celecoxib (10 mg/kg) and attenuated by gestational diclofenac (0.5 mg/kg) and naproxen (1 mg/kg). Further, the induction of PE was accompanied by dram/atic and significant decrements in creatinine clearance that were partially alleviated by concurrently administered NSAIDs (Fig. 1b). Naproxen was more effective than celecoxib or diclofenac in restoring the altered urine protein and CrCl back to non-PE levels (Fig. 1).
Effect of prenatal administration of celecoxib, diclofenac or naproxen on urine protein (a) and creatinine clearance (CrCl, b) in PE rats at gestational day 20 (GD20) and weaning time. Values are means ± S.D. of 3–5 rats. *P < 0.05 vs. non-PE group (a), PE (b), PE + celecoxib (c), and PE + diclofenac (d) according to two-way ANOVA (F-statistics, p > 0.0001).
The reprogramming effect of gestational NSAIDs on modifications caused by PE in kidney architecture is shown in Fig. 2. The glomeruli in kidneys of PE dams showed mesangial cell proliferation with glomerular capillary endotheliosis. Few of the glomerular tufts displayed shrinkage, atrophy, and necrosis. The tubular epithelial lining showed severe degree of degeneration represented by cloudy swelling intermingled with areas of necrosis (Fig. 2i). Gestational treatment with celecoxib or diclofenac did not improve the distorted histological picture caused by PE. Meanwhile, naproxen treatment effectively preserved the glomerular space and diminished mesangial cell proliferation. Additionally, naproxen reduced the preeclamptic interstitial inflammation and restored the standard architecture of the tubular epithelial lining (Fig. 2i). Morphometrically, kidneys of PE rats showed similar glomerular counts to those of respective non-PE values, but significantly smaller glomerular areas. The reductions in glomerular area induced by PE were reversed by naproxen and not by celecoxib or diclofenac (Fig. 2ii).
Representative kidney photomicrographs demonstrating the effect of prenatal administration of celecoxib, diclofenac, or naproxen in PE rats. (i) H&E-stained renal photomicrographs (×200; scale bar = 100 μm and ×400; scale bar = 50 μm) of non-PE rats showed normal glomeruli cellularity with opened capillary loops (black arrowheads), standard tubular epithelial lining (black arrow) and intact Bowman’s space (green arrows). PE kidneys sjowed signs of glomerular shrinkage (blue arrowhead), cloudy swelling (C), and fatty degeneration of tubular epithelial lining and necrosis (N). PE + cele and PE + Diclo kidneys display similar renal histological alterations to the PE model in terms of narrow mesangial cell proliferation (red arrowheads) with glomerular capillary endotheliosis, in addition to cloudy swelling (C) of the tubular epithelial lining, and necrosis (N). The PE + Napro kidney reveals closer architecture to the kidney of control non-PE rat in terms of intact Bowman’s space (green arrows), normal tubular epithelial lining (black arrows). (ii) The scatter plot of the assessed histomorphometric parameters (glomerular number and surface area). Values are means ± S.D. of 4–5 rats. Non-PE group (a), PE (b), PE + celecoxib (c), and PE + diclofenac (d) according to one-way-ANOVA (F-statistics, p > 0.0001).
Effect of gestational NSAIDs on renal prostanoids
Changes in renal contents of the cyclooxygenase products of arachidonic acid caused by PE in the absence and presence of NSAIDs are shown in Fig. 4. ELISA determinations showed significantly higher levels of PGE2, PGF2α and TXA2 in weaning PE dams compared with respective control values (Fig. 3). The prenatal treatment with NSAIDs significantly reduced the PE-evoked elevations in renal prostanoids, with an order of potency of naproxen > celecoxib > diclofenac (Fig. 3).
Effect of prenatal administration of celecoxib, diclofenac or naproxen on renal PGE2 (a), PGF2α (b) and thromboxane A2 (c) in weaning PE rats. Values are means ± S.D. of 3 rats. *P < 0.05 vs. non-PE (a), PE (b), PE + celecoxib (c), and PE + diclofenac (d) according to one-way-ANOVA (F-statistics, p > 0.0001).
Effects of gestational NSAIDs on renal MAPKs
Western blotting showed that the protein expressions of all MAPK isoforms, p38, p-JNK, p-Erk1, and p-ERK2, were significantly increased in kidneys of weaning PE dams. In fact, the renal expression of all MAPKs was at least tripled in PE compared with non-PE rats. Except for p-ERK2, the heightened expression MAPKs expression was mostly and indiscriminately eliminated by gestational administration of the 3 NSAIDs (Fig. 4). Notably, the PE-mediated increase in renal p-ERK2 was also suppressed by all NSAIDs, but the magnitude of this effect depended on the NSAID type. The efficacy order of p-ERK2 downregulation was naproxen > diclofenac > celecoxib (Fig. 4d).
Effect of prenatal administration of celecoxib, diclofenac, or naproxen on renal protein expression of p-p38 MAPK (a), p-JNK (b), p-Erk1 (c) and p-Erk2 (d) in weaning PE rats. Values are means ± S.D. of 3 rats. *P < 0.05 vs. non-PE group (a), PE (b), PE + celecoxib (c), and PE + diclofenac (d) according to one-way-ANOVA (F-statistics, p > 0.0001).
Effects of gestational NSAIDs on renal oxidative, fibrotic, and autophagic markers
PCR studies demonstrated significant increases in gene expressions of the NADPH oxidases, NOX2 and NOX4 (Fig. 5a, b), matrix metalloproteinases (MMP2 and MMP9, Fig. 5c, d), and autophagic markers (Beclin-1 and LC3, Fig. 6A, B) in renal tissues of weaning PE compared with non-PE rats. The deteriorated renal oxidative potential evidenced by the upregulated NOX isozymes was significantly relieved by all NSAID therapies, with naproxen being the most effective NSAID (Fig. 5a, b). On the other hand, while the PE-associated rises in renal expression of MMP2 were comparably reduced by all NSAIDs (Fig. 5c), the significant rise in renal MMP9 expression by PE disappeared in dams receiving diclofenac or naproxen (Fig. 5D). Finally, none of the tested NSAIDs affected the surge caused by PE in the renal expression of the autophagy marker Beclin-1 (Fig. 6a). However, the simultaneous elevation in renal LC3 expression in PE kidneys was significantly diminished by naproxen or diclofenac, but not celecoxib (Fig. 6b).
Effect of prenatal administration of celecoxib, diclofenac, or naproxen on renal gene expression of NOX2 (a), NOX4 (b), MMP2 (c) and MMP9 (d) in weaning PE rats. Values are means ± S.D. of 3 rats. *P < 0.05 vs. non-PE group (a), PE (b), PE + celecoxib (c), and PE + diclofenac (d) according to one-way-ANOVA (F-statistics, p = 0.0013–0.0001).
Discussion
Despite the increased risk of perinatal renal injury in preeclamptic mothers8,9 and the routine use of NSAIDs during pregnancy for the control of pain and inflammatory disorders28, we are not aware of any study that has determined if renal programming incited by PE could be modified by gestationally administered NSAIDs. The current study establishes a comparative investigation of the effect of prenatal administration of 3 prominent NSAIDs on renal damage provoked by PE in weaning mothers. Compared with celecoxib or diclofenac, naproxen appeared to be more advantageous in relieving functional and morphological manifestations of renal injury. Molecularly, the suppression of the upregulated offending signals of inflammatory (arachidonate-derived prostanoids, p-ERK2), oxidative (NOX2/4), fibrotic (MMP9), and autophagic (LC3) cascades provides a mechanistic basis for the privileged action of naproxen.
Consistent with reported clinical and experimental studies of exacerbated renal damage and morbidity and mortality in PE dams29,30, the present study demonstrated distinct functional and structural manifestations of renal injury in the L-NAME rodent model of PE. Opposite changes in urine protein (increases) and CrCl (decreases), positive hallmarks of renal damage31,32, were demonstrated in PE dams at GD20 compared with respective control rats and remained manifest till weaning (3 weeks post-labor). The excessive proteinuria has been attributed to the L-NAME-induced renal vascular wall thickening and elevated glomerular pressure33. Others suggested that the L-NAME-induced glomerular hypertension creates sheer stress that facilitates the urinary loss of protein34. Additionally, like in previous reports29, the disturbed kidney function in PE rats was accompanied by a number of pathological lesions such endotheliosis, cloudy swelling of the tubular epithelial lining, necrosis and reduced glomerular area.
The principal objective of the current study was to assess if and how the deteriorated renal profile in PE dams would be influenced by gestational supplementation of NSAIDs. The data showed that the net renal effect of individual antiinflammatory therapies was proportional to the magnitude of suppression of prostanoid biosynthesis. In this context, naproxen was more effective than the other two NSAIDs (celecoxib and diclofenac) in (i) rectifying biochemical and glomerular and tubular signs of renal injury caused by PE programming, and (ii) diminishing the upregulated renal levels of the arachidonate end products PGE2, PGF2α and TXA2. It is widely recognized that TXA2 and PGF2α are vitally implicated in the preeclamptic inflammatory response and consequent tissue remodeling and stiffness31,35. Moreover, a pathogenic role for PGE2 has been reported in oxidative and inflammatory damage associated with PE Moreover, a pathogenic role for PGE2 has been reported in oxidative and inflammatory damage associated with PE36,37. The MAPK family modulates diverse cellular processes including inflammation, cell differentiation, cell growth, and cell death38,39. The phosphorylation of p38 and JNK is increased in human placental explants after exposure to various PE-associated stresses such as angiotensin II, hypoxia and inflammatory cytokines, suggesting a causal relationship between MAPKs and PE pathophysiology40. Moreover, the inflammatory response provoked by p38 and ERK remodels spiral artery, interrupts trophoblast invasion, and consequently PE-like symptoms induced by lipopolysaccharides41,42. Western blotting data of the current study revealed that the expressions of all MAPK isoforms (p-p38, p-JNK, and p-ERK1/2) were upregulated in renal tissues of PE dams and this effect subsided after antenatal administration of NSAIDs. Notably, the depression of MAPK expression caused by all 3 NSAIDs was of similar magnitude, with the only exception that the reduced availability of renal p-ERK2 was more evident with naproxen than with celecoxib or diclofenac. Although ERK1 and ERK2 are co-expressed and regulated similarly in virtually all tissues and phosphorylate common subset of substrates in the cytosol and nucleus, ERK2 appears to be the predominant isoform in brain and hematopoietic cells43,44,45. Recent evidence reveals dynamic differences in the function and interplay between ERK1 and ERK2 in the regulation of cell signaling and proliferation46. Together, our data specifically implicate the reduced abundance of ERK2 in the distinct counterbalancing action of gestational naproxen against renal complications induced by PE. More studies are necessary to further clarify the correlation between the two ERK isoforms in this interaction.
The greater inhibitory effect of naproxen on renal ERK2 expression was paralleled with more noticeable suppression of the PE-induced upregulation of the gene expression of MMP9 as well as NADPH oxidases of the NOX2 and NOX4. Remarkably, the excessive generation of reactive oxygen species in placental and renal tissues of PE dams is primarily incited by NADPH oxidases47. Further, MMPs are a family of zinc-dependent proteases that are extensively distributed in tissues and involved in the degradation and turnover of extracellular matrix components in normal and complicated pregnancies48. It is widely recognized that oxidative and fibrotic bursts are key constituents that fine-tune and transduce the inflammatory response prompted by MAPK signaling. Recent data from studies on cardiorenal injuries suggests that the activation of the pro-oxidant NADPH oxidase following MAPKs phosphorylation results in the upregulation of pro-fibrotic transforming growth factor β1 and extracellular degradation signals regulated by MMPs49,50,51. The latter molecules are necessary for provoking tissue transition from the inflammatory phase to the fibrotic remodeling phase52,53. Thus, it is likely that the downregulation of consecutive signals of the ERK2/NOX2/NOX4/MMP9 cascade is causally related to naproxen renoprotection against preeclamptic renal injury.
Autophagy is a conserved recycling process that regulates lysosomal degradation and removal of dysfunctional cellular organelles and invading pathogens and plays key roles in fertilization and embryonic development54. The question whether PE and associated inflammatory and oxidative insults are positively or negatively modulated by autophagy remains unresolved55,56,57. Here we report two novel observations regarding the role of autophagy in the renal PE/NSAID interaction. First, the gene expression of LC3 and beclin-1, two autophagy-related markers58, were potentiated in renal tissues of weaning PE dams, which is coherent with an offensive role for autophagy in the pathophysiological events leading to PE. The suppression of angiogenetic and vascular endothelial activities has often been incriminated in the disrupting effect of autophagy during PE56. We further show that while the elevated renal beclin-1 expression was altered by none of the NSAID therapies, the concomitant rise in renal LC3 expression was diminished by diclofenac and naproxen, but not celecoxib. The data emphasizes the importance of downregulation of the LC3-dependent autophagic pathway in the renoprotection brought in by gestational naproxen and diclofenac in PE dams.
The current observation that antiinflammatory therapies counterbalance the PE-associated renal damage appears to be at odds with previous clinical and experimental assessments that highlight a compromising effect for NSAIDs on renal function. Depending on the dose and duration of use, acute and chronic kidney damage and tubulointerstitial nephritis are common adverse effects for NSAIDs that limit their use in clinical practice23. The mechanism possibly relates to the NSAIDs-mediated inhibition of vasodilator prostanoids, e.g. PGE2 and prostacyclin, and consequent vasoconstriction and reduced renal blood flow24. That said, our current observation that NSAIDs restrain renal damage induced by PE would advocate for a conditioning effect for NSAIDs in the setting of PE. In a similar fashion, Talab et al. reported that the NSAID compound indomethacin potentiated the preconditioning effect of lithium against renal injury induced by ischemia/reperfusion injury59.
As the current study was performed in weaning preeclamptic rats, it is not clear whether NSAIDs would interact similarly with functional and morphometric renal consequences of PE when tested during early postpartum days. This is especially important as the PE-associated elevations in blood pressure and renal glomerular filtration rate and proteinuria peak immediately after delivery and begin to gradually decline over subsequent months60. More studies are also necessary to determine whether (i) the PE/NSAID interaction could be replicated with other experimental PE models such as the reduced uterine perfusion pressure model61, and (ii) gestational administration of NSAIDs could modulate PE-induced neonatal illnesses and reductions in neonatal number and weight is62,63. Finally, the electron microscopic visualization of podocytes and glomerular endothelial cells might provide important insights into the PE/NSAIDs renal interaction. The investigation of these issues provides a framework for future studies in our laboratory to assess the PE/NSAIDs interaction on disturbances in renal homeostasis and possibly other end organ damage.
In conclusion, the present functional, histopathological, and molecular evidence suggests a preferential favorable effect for naproxen when administered during the last week of pregnancy on PE programming of renal function in weaning dams. The diminution of PE-mediated inflammatory, oxidative, fibrotic, and autophagic offences underlies the renoprotective action of naproxen. More experimental and clinical studies are necessary to reinforce such renoprotective action of naproxen in PE and underlying cellular mechanisms.
Materials and methods
Materials
N(gamma)-nitro-l-arginine methyl ester (l-NAME; sigma-Aldrich, St. louis. MO, USA), naproxen (Sigma, St. Louis, MO, USA) and thiopental sodium (Biochemie GmbH, Vienna, Austria) were purchased from commercial suppliers. Celecoxib and diclofenac were supplied as gifts from PHARCO Corporation, Alexandria, Egypt and Amriya Pharmaceutical Industries, Alexandria, Egypt, respectively. All drugs were dissolved in saline. All ELISA kits were acquired from Chongqing Biospes Co. (Chongqing, China) or Abcam (Cambridge, UK). Kits for RNA isolation and reverse transcription were bought from Qiagen (USA). Western Blot technique using V3 Western Workfow™ Complete System was acquired from Bio-Rad® Hercules, CA, USA.
Experimental animals
The study was performed in the Animal House of Pharos University in Alexandria, Alexandria, Egypt. Thirty female Wistar rats weighing 170–200 g were kept at 23–25 °C in a 12/12 h light/dark cycle under optimum humidity with access to food and water ad libitum. The experiments were conducted in adherence to the guidelines of the Egyptian guide for the care and use of laboratory animals64 and in accordance with the Unit of Research Ethics Approval Committee, Pharos University in Alexandria, Egypt (Approval No.: PUA01202002233008) and with ARRIVE guidelines and the National Institutes of Health, USA, for the care and use of laboratory animals.
PE induction
Adult nulliparous female rats were housed with male rats (ratio 1:1) and mated overnight. The date of conception was determined by checking sperms in the vaginal smears or detecting a vaginal plug. PE was induced by oral administration of l-NAME using oral gavage at a dose of 50 mg/kg/day for 7 continuous days from gestational day14 (GD14)65,66,67. Non-PE rats received water by gavage to correct for the stress of manipulation.
Urine collection
Dams, at GD20 and weaning time, were housed in metabolic cages with stainless steel wire mesh bottom for 1 day prior to sacrifice, and 24-h urine samples were collected under light oil for measuring protein and creatinine. The collected urine was stored at −80 °C until processed.
Experimental groups and study design
Pregnant female rats were randomly assigned into five different groups; n = 5–6 rats each: (i) pregnant non-PE control rats, (ii) PE rats, (iii) PE/celecoxib (10 mg/kg/day)68, (iv) PE/diclofenac (0.5 mg/kg/day)69, and (v) PE/naproxen (1 mg/kg/day)70. NSAIDs were co-administered with l-NAME starting at GD14 till GD20. At weaning (3 weeks post-labor), dams were euthanized by i.p. injection of an overdose of thiopental (100 mg/kg) and blood was collected via cardiac puncture, spun (800×g, 4 °C, 20 min), and serum was stored at −80 °C until used for the measurement of creatinine levels. Portions of the left kidneys were dissected, immediately frozen in liquid nitrogen, and stored at − 80 °C for protein and gene expression studies. Right kidneys were fixed in 10% formaldehyde and embedded in paraffin blocks for histopathological examination and morphometric analysis.
Biochemical measurements
The levels of serum and urine creatinine were assessed using Jaffe' reaction71, then creatinine clearance (CrCl) was calculated. Urine protein level was measured using pyrogallol red method72. Total protein levels in kidney tissues were measured using the Lowry method73.
Enzyme-linked immunosorbent assay (ELISA) determinations
The left kidney portions were homogenized in phosphate buffered saline in ratio of 1:9 and used to determine prostaglandin E2 (PGE2) and prostaglandin F2 alpha (PGF2α) (Enzo Life Sciences, NY, USA) and thromboxane A2 (TXA2) (LifeSpan BioSciences, Inc, Seattle, USA) using commercially available ELISA kits.
Real time reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from kidney tissue using the miRNeasy kit according to the manufacturer's instructions. The isolated RNA was reverse transcribed into complementary DNA (cDNA) using reverse transcriptase, amplified, and detected by qRT-PCR using specific primers. The primer sequences of the genes examined; beclin-1, microtubule-associated protein 1A/1B-light chain 3 (LC3), MMP2, MMP9, NADPH oxidase 2 (NOX2), NADPH oxidase 4 (NOX4) and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are shown in Table 1. Reverse transcription of all RNA species into cDNA was performed using the miScript II RT Kit (Qiagen, Germany) according to the manufacturer's instructions. The cDNA was used to quantify kidney expression of the investigated genes by Rotor-Gene Q qPCR using the QuantiTect SYBR Green PCR Master Mix. PCR amplification was initiated with an initial denaturation at 95 °C for 10 min and subsequent amplification by 40 PCR cycles as follows: Denaturation at 95 °C for 15 s, annealing at 58 °C for 15 s and extension at 60 °C for 15 s. Threshold cycle (Ct) values were determined using Rotor-Gene Q-Pure Detection version 2.1.0. (build 9). For each gene, changes in sample mRNA levels were determined using the 2−ΔΔCt method and normalized to the reference gene31.
Western blotting
The ReadyPrepTM protein extraction kit (total protein) provided by Bio-Rad Inc (Catalog #163-2086) was employed according to manufacturer instructions was added to each sample of the homogenized tissues of all different groups. A Bradford assay was performed to determine protein concentration in each sample (Bio basic Inc., Markham Ontario, Canada). A 20 μg protein concentration of each sample was then loaded with an equal volume of 2× Laemmli sample buffer containing 4% SDS, 10% 2-mercaptoehtanol, 20% glycerol, 0.004% bromophenol blue and 0.125 M Tris HCl. The pH was checked and brought to 6.8. Each mixture was boiled at 95 °C for 5 min to ensure denaturation of protein before loading on polyacrylamide gel electrophoresis. Polyacrylamide gels were performed using TGX Stain-Free™ FastCast™ Acrylamide Kit (SDS-PAGE), which was provided by Bio-Rad Laboratories Inc (Cat # 161-0181). The gel was assembled in transfer sandwich as following from below to above (filter paper, PVDF membrane, gel and filter paper). The sandwich was placed in the transfer tank with 1× transfer buffer, which was composed of 25 mM Tris and 190 mM glycine and 20% methanol. Then, the blot was run for 7 min at 25 V to allow protein bands transfer from gel to membrane using BioRad Trans-Blot Turbo. The membrane was blocked in tris-buffered saline with Tween 20 (TBST) buffer and 3% bovine serum albumin at room temperature for 1 h. The components of blocking buffer were as follow; 20 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween 20 and 3% bovine serum albumin. Primary antibodies of phospho-p38 mitogen-activated protein kinases (p-p38 MAPK), phospho-c-Jun N-terminal kinase (p-JNK), extracellular signal-regulated protein kinase (ERK) p-Erk1, and p-ERK2 were diluted in TBST according to manufactured instructions. Incubation was done overnight in each primary antibody solution, against the blotted target protein at 4 °C. Blots were cut prior to hybridization with antibodies during blotting. The blot was rinsed 3–5 times for 5 min with TBST. Incubation was done in the HRP-conjugated secondary antibody (Goat anti-rabbit IgG- HRP-1mg Goat mab -Novus Biologicals) solution against the blotted target protein for 1 h at room temperature. The chemiluminescent substrate (Clarity TM Western ECL substrate Bio-Rad cat#170-5060) was applied to the blot according to the manufacturer’s recommendation. Briefly, equal volumes were added from solution A (Clarity western luminal/enhancer solution) and solution B (peroxidase solution). The chemiluminescent signals were captured using a CCD camera-based imager. The densities of phosphorylated protein bands were expressed as ratios of respective β-actin bands. Image analysis software was used to read the band intensity of the target proteins against control sample beta actin (housekeeping protein) by protein normalization on the ChemiDoc MP imager74,75.
Histological and histomorphometric analysis
Kidneys were fixed in 10% neutral buffered formalin for 48 h, embedded in paraffin, and slices of 4–5 μm were stained with hematoxylin & eosin (H&E) and examined for structural defects at 200× and 400×. Morphometric determinations of the glomerular count and mean area percentage were performed in 20 random fields per rat and averaged. Areas of the glomerular capillary tufts were circumferenced on images captured at 200×. The ImageJ software (1.52p software 32, NIH, USA) was employed to measure the circumferenced areas using the fit ellipse parameter and presented as a percentage to the whole image76,77.
Statistical analysis
The findings are presented as mean ± standard deviation (SD) and analyzed using one-way or two-way ANOVA and F-statistics as appropriate, followed by Tukey post-hoc test with a probability level (P) ≤ 0.05 taken as the limit for significance. The GraphPad Prism v7.0 (GraphPad Prism Inc., La Jolla, CA, USA) was utilized for these analyses (Supplementary Information).
Data availability
Raw data are publicly available in the Mendeley repository, as part of this record: https://data.mendeley.com/datasets/hg7h7883gz/178.
References
Guerby, P. et al. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 40, 101861. https://doi.org/10.1016/j.redox.2021.101861 (2021).
Venkatesha, S. et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649. https://doi.org/10.1038/nm1429 (2006).
Burton, G. J. et al. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30, 473–482 (2009).
Burton, G. J., Yung, H. W. & Murray, A. J. Mitochondrial–endoplasmic reticulum interactions in the trophoblast: Stress and senescence. Placenta 52, 146–155. https://doi.org/10.1016/j.placenta.2016.04.001 (2017).
Tian, J. et al. Angiotensin-(1–7) attenuates damage to podocytes induced by preeclamptic serum through MAPK pathways. Int. J. Mol. Med. 34, 1057–1064. https://doi.org/10.3892/ijmm.2014.1870 (2014).
Possomato-Vieira, J. S. & Khalil, R. A. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv. Pharmacol. 77, 361–431. https://doi.org/10.1016/bs.apha.2016.04.008 (2016).
Hu, H. et al. Cyclosporin A alleviates trophoblast apoptosis and senescence by promoting autophagy in preeclampsia. Placenta 117, 95–108. https://doi.org/10.1016/j.placenta.2021.11.003 (2022).
Kattah, A. Preeclampsia and kidney disease: Deciphering cause and effect. Curr. Hypertens. Rep. 22, 91. https://doi.org/10.1007/s11906-020-01099-1 (2020).
Stillman, I. E. & Karumanchi, S. A. The glomerular injury of preeclampsia. J. Am. Soc. Nephrol. 18, 2281–2284. https://doi.org/10.1681/asn.2007020255 (2007).
Garovic, V. D. et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol. Dial. Transplant 22, 1136–1143. https://doi.org/10.1093/ndt/gfl711 (2007).
Heida, K. Y. et al. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension 66, 1116–1122. https://doi.org/10.1161/hypertensionaha.115.06005 (2015).
Stuart, J. J. et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: An observational cohort study. Ann. Intern. Med. 169, 224–232. https://doi.org/10.7326/m17-2740 (2018).
Szczuko, M. et al. The role of arachidonic and linoleic acid derivatives in pathological pregnancies and the human reproduction process. Int. J. Mol. Sci. 21, 9628. https://doi.org/10.3390/ijms21249628 (2020).
Mirabito Colafella, K. M. et al. Differential effects of cyclo-oxygenase 1 and 2 inhibition on angiogenesis inhibitor-induced hypertension and kidney damage. Clin. Sci. (Lond). 136, 675–694. https://doi.org/10.1042/cs20220182 (2022).
Tai, Y. et al. Celecoxib reduces hepatic vascular resistance in portal hypertension by amelioration of endothelial oxidative stress. J. Cell Mol. Med. 25, 10389–10402. https://doi.org/10.1111/jcmm.16968 (2021).
Majed, B. H. & Khalil, R. A. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol. Rev. 64, 540–582. https://doi.org/10.1124/pr.111.004770 (2012).
Mirabito Colafella, K. M. et al. Aspirin for the prevention and treatment of pre-eclampsia: A matter of COX-1 and/or COX-2 inhibition?. Basic Clin. Pharmacol. Toxicol. 127, 132–141. https://doi.org/10.1111/bcpt.13308 (2020).
Ali, M. A. et al. Gestational NSAIDs distinctly reprogram cardiac injury in preeclamptic rats: Roles of cyclooxygenase, apoptotic and autophagic trails. Life Sci. 310, 121130. https://doi.org/10.1016/j.lfs.2022.121130 (2022).
Mousavy, S. M. Indomethacin induces hypertensive crisis in preeclampsia irrespective of prior antihypertensive drug therapy. Am. J. Obstet. Gynecol. 165, 1577. https://doi.org/10.1016/0002-9378(91)90414-m (1991).
Ruoff, G. E. The impact of nonsteroidal anti-inflammatory drugs on hypertension: Alternative analgesics for patients at risk. Clin. Ther. 20, 376–387. https://doi.org/10.1016/s0149-2918(98)80049-0 (1998).
Schoenfeld, A. et al. Antagonism of antihypertensive drug therapy in pregnancy by indomethacin?. Am. J. Obstet. Gynecol. 161, 1204–1205 (1989).
Antonucci, R. et al. Use of non-steroidal anti-inflammatory drugs in pregnancy: Impact on the fetus and newborn. Curr. Drug Metab. 1, 474–490. https://doi.org/10.2174/138920012800166607 (2012).
Drożdżal, S. et al. Kidney damage from nonsteroidal anti-inflammatory drugs-Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 9, e00817. https://doi.org/10.1002/prp2.817 (2021).
Chang, R. W., Tompkins, D. M. & Cohn, S. M. Are NSAIDs safe? Assessing the risk-benefit profile of nonsteroidal anti-inflammatory drug use in postoperative pain management. Am. Surg. 87, 872–879. https://doi.org/10.1177/0003134820952834 (2021).
Marhofer, D. et al. Schmerztherapie in der Schwangerschaft : Eine expertInnenbasierte interdisziplinäre Konsensus-Empfehlung [Pain management during pregnancy : An expert-based interdisciplinary consensus recommendation]. Schmerz 35, 382–390 (2021).
Stika, C. S. et al. A prospective randomized safety trial of celecoxib for treatment of preterm labor. Am. J. Obstet. Gynecol. 187, 653–660 (2002).
Díaz-González, F. & Sánchez-Madrid, F. NSAIDs: Learning new tricks from old drugs. Eur. J. Immunol. 45, 679–686 (2015).
Ying, X. H. et al. Maternal non-steroidal anti-inflammatory drug exposure during pregnancy and risk of miscarriage: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 78, 171–180. https://doi.org/10.1007/s00228-021-03222-w (2022).
Hamza, R. Z. et al. Ameliorative effect of apelin-13 against renal complications in l-NAME-induced preeclampsia in rats. PeerJ 9, e11110. https://doi.org/10.7717/peerj.11110 (2021).
Trakarnvanich, T., Ngamvichchukorn, T. & Susantitaphong, P. Incidence of acute kidney injury during pregnancy and its prognostic value for adverse clinical outcomes: A systematic review and meta-analysis. Medicine (Baltimore) 101, e29563. https://doi.org/10.1097/md.0000000000029563 (2021).
Habib, Y. H. et al. Prenatal endothelin or thromboxane receptor antagonism surpasses sympathoinhibition in improving cardiorenal malfunctions in preeclamptic rats. Toxicol. Appl. Pharmacol. 426, 115615. https://doi.org/10.1016/j.taap.2021.115615 (2021).
Abuiessa, S. A. et al. Dysregulated ACE/Ang II/Ang1–7 signaling provokes cardiovascular and inflammatory sequelae of endotoxemia in weaning preeclamptic rats. Eur. J. Pharmacol. 936, 175344. https://doi.org/10.1016/j.ejphar.2022.175344 (2022).
Yoneyama, T. et al. The contribution of nitric oxide to renal vascular wall thickening in rats with l-NAME-induced hypertension. Virchows Arch. 433, 549–557. https://doi.org/10.1007/s004280050288 (1998).
Hladunewich, M., Karumanchi, S. A. & Lafayette, R. Pathophysiology of the clinical manifestations of preeclampsia. Clin. J. Am. Soc. Nephrol. 2, 543–549. https://doi.org/10.2215/cjn.03761106 (2007).
Qu, H. & Khalil, R. A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 319, H661–H681. https://doi.org/10.1152/ajpheart.00202.2020 (2020).
Cao, Y. et al. Vitamin D stimulates miR-26b-5p to inhibit placental COX-2 expression in preeclampsia. Sci. Rep. 11, 11168. https://doi.org/10.1038/s41598-021-90605-9 (2021).
Vural, P., Akgül, C. & Canbaz, M. Urinary PGE2 and PGF2alpha levels and renal functions in preeclampsia. Gynecol. Obstet. Invest. 45, 237–241. https://doi.org/10.1159/000009975 (1998).
Sargent, I. L. et al. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J. Reprod. Immunol. 59, 153–160. https://doi.org/10.1016/s0165-0378(03)00044-5 (2003).
Arthur, J. S. & Ley, S. C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692. https://doi.org/10.1038/nri3495 (2013).
Noyola-Martínez, N. et al. Calcitriol downregulates TNF-α and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine 61, 245–250. https://doi.org/10.1016/j.cyto.2012.10.001 (2013).
Fan, M. et al. LPS induces preeclampsia-like phenotype in rats and HTR8/SVneo cells dysfunction through TLR4/p38 MAPK pathway. Front. Physiol. https://doi.org/10.3389/fphys.2019.01030 (2019).
Liao, L. et al. The long noncoding RNA TARID regulates the CXCL3/ERK/MAPK pathway in trophoblasts and is associated with preeclampsia. Reprod. Biol. Endocrinol. 20, 159. https://doi.org/10.1186/s12958-022-01036-8 (2022).
Adams, J. P. & Sweatt, J. D. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu. Rev. Pharmacol. Toxicol. 42, 135–163. https://doi.org/10.1146/annurev.pharmtox.42.082701.145401 (2002).
Milella, M., Kornblau, S.M. & Andreeff, M. The mitogen-activated protein kinase signaling module as a therapeutic target in hematologic malignancies. Rev. Clin. Exp. Hematol. 7, 160–190 https://pubmed.ncbi.nlm.nih.gov/14763161/ (2003).
Pages, G. & Pouyssegur, J. Study of MAPK signaling using knockout mice. Methods Mol. Biol. 250, 155–166. https://doi.org/10.1385/1-59259-671-1:155 (2004).
Lavoie, H., Gagnon, J. & Therrien, M. ERK signaling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 21, 607–632. https://doi.org/10.1038/s41580-020-0255-7 (2020).
Santana-Garrido, Á. et al. Oxidative and inflammatory imbalance in placenta and kidney of sFlt1-induced early-onset preeclampsia rat model. Antioxidants (Basel) 11, 1608. https://doi.org/10.3390/antiox11081608 (2022).
Chen, J. & Khalil, R. A. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 148, 87–165. https://doi.org/10.1016/bs.pmbts.2017.04.001 (2017).
Arab, H. H. et al. Camel milk mitigates cyclosporine-induced renal damage in rats: Targeting p38/ERK/JNK MAPKs, NF-κB, and matrix metalloproteinases. Biology (Basel) 10, 442. https://doi.org/10.3390/biology10050442 (2021).
Garlapati, V. et al. Targeting myeloid cell coagulation signaling blocks MAP kinase/TGF-β1 driven fibrotic remodeling in ischemic heart failure. J. Clin. Invest. https://doi.org/10.1172/JCI156436 (2023).
Guo, L., Liu, M. & Duan, T. Hydrogen suppresses oxidative stress by inhibiting the p38 MAPK signaling pathway in preeclampsia. Adv. Clin. Exp. Med. https://doi.org/10.17219/acem/154623 (2022).
Deten, A. et al. Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J. Mol. Cell Cardiol. 33, 1191–1207. https://doi.org/10.1006/jmcc.2001.1383 (2001).
Frangogiannis, N. G. The role of transforming growth factor (TGF)-β in the infarcted myocardium. J. Thorac. Dis. 9, S52–S63. https://doi.org/10.21037/jtd.2016.11.19 (2017).
Qin, X. Y. et al. Insight of autophagy in spontaneous miscarriage. Int. J. Biol. Sci. 18, 1150–1170. https://doi.org/10.7150/ijbs.68335 (2022).
Nakashima, A. et al. Current understanding of autophagy in pregnancy. Int. J. Mol. Sci. 20, 2342. https://doi.org/10.3390/ijms20092342 (2019).
Zhao, H. et al. The inhibition of protein kinase C β contributes to the pathogenesis of preeclampsia by activating autophagy. EBioMedicine. 56, 102813. https://doi.org/10.1016/j.ebiom.2020.102813 (2020).
Nakashima, A. et al. Placental autophagy failure: A risk factor for preeclampsia. J. Obstet. Gynaecol. Res. https://doi.org/10.1111/jog.14489 (2020).
Lokeswara, A. W. et al. Preeclampsia: From cellular wellness to inappropriate cell death, and the roles of nutrition. Front. Cell Dev. Biol. 9, 726513. https://doi.org/10.3389/fcell.2021.726513 (2021).
Talab, S. S. et al. Protective effects of acute lithium preconditioning against renal ischemia/reperfusion injury in rat: Role of nitric oxide and cyclooxygenase systems. Eur. J. Pharmacol. 681, 94–99. https://doi.org/10.1016/j.ejphar.2012.01.042 (2012).
Kaleta, T. et al. Predictors of impaired postpartum renal function in women after preeclampsia: Results of a prospective single center study. Dis Mark. 2016, 7861919 (2016).
Colson, A. et al. Specific HIF-2α (hypoxia-inducible factor-2) inhibitor PT2385 mitigates placental dysfunction in vitro and in a rat model of preeclampsia (RUPP). Hypertension 80, 1011–1023 (2023).
Amaral, L. M. et al. Preeclampsia: Long-term consequences for vascular health. Vasc Health Risk Manag. 11, 403–415 (2015).
von Dadelszen, P. et al. Preterm and term pre-eclampsia: Relative burdens of maternal and perinatal complications. BJOG 130, 524–530 (2023).
Fahmy, S. R. & Gaafar, K. Establishing the first institutional animal care and use committee in Egypt. Philos. Ethics Hum. Med. https://doi.org/10.1186/s13010-016-0035-3 (2016).
Abuiessa, S. A. et al. Pre-eclamptic fetal programming alters neuroinflammatory and cardiovascular consequences of endotoxemia in sex-specific manners. J. Pharmacol. Exp. Ther. 373, 325–336. https://doi.org/10.1124/jpet.119.264192 (2020).
Habib, Y. H. et al. Modulation by antenatal therapies of cardiovascular and renal programming in male and female offspring of preeclamptic rats. Naunyn Schmiedebergs Arch. Pharmacol. 394, 2273–2287. https://doi.org/10.1007/s00210-021-02146-7 (2021).
Molnár, M. et al. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am. J. Obstet. Gynecol. 170, 1458–1466 (1994).
El-Gowelli, H. M. et al. Celecoxib offsets the negative renal influences of cyclosporine via modulation of the TGF-β1/IL-2/COX-2/endothelin ET(B) receptor cascade. Toxicol. Appl. Pharmacol. 275, 88–95. https://doi.org/10.1016/j.taap.2014.01.008 (2014).
Kushima, K. et al. Effect of prenatal administration of NSAIDs on the immune response in juvenile and adult rats. Toxicology. 232, 257–267. https://doi.org/10.1016/j.tox.2007.01.012 (2007).
Wong, S. et al. Antiarthritic profile of BF-389—A novel anti-inflammatory agent with low ulcerogenic liability. Agents Actions 37, 90–98. https://doi.org/10.1007/bf01987895 (1992).
Toora, B.D. & Rajagopal, G. Measurement of creatinine by Jaffe's reaction—Determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J. Exp. Biol. 40, 352–354. https://pubmed.ncbi.nlm.nih.gov/12635710/ (2002).
Jia, J. et al. Urine protein quantification in stacking gel by SDS-PAGE. Electrophoresis. 40, 487–490. https://doi.org/10.1002/elps.201800379 (2019).
Taquini, C. M. et al. Improvement of cardiac contractile response to beta-adrenergic stimulation in normal and two-kidney, one-clip hypertensive rats treated with nitrendipine. J. Cardiovasc. Pharmacol. Supp 2, S175–S178. https://doi.org/10.1097/00005344-199117002-00045 (1991).
El-Mas, M. M. et al. Estrogen dependence of the renal vasodilatory effect of nicotine in rats: Role of α7 nicotinic cholinergic receptor/eNOS signaling. Life Sci. 88, 187–193. https://doi.org/10.1016/j.lfs.2010.11.009 (2011).
Zhang, J., El-Mas, M. M. & Abdel-Rahman, A. A. Imidazoline I(1) receptor-induced activation of phosphatidylcholine-specific phospholipase C elicits mitogen-activated protein kinase phosphorylation in PC12 cells. Eur. J. Pharmacol. 415, 117–125. https://doi.org/10.1016/s0014-2999(01)00834-2 (2001).
Nakagawa, T. et al. Hyperuricemia causes glomerular hypertrophy in the rat. Am. J. Nephrol. 23, 2–7 (2003).
Kashif, A. W. et al. Utility of glomerular morphometry in diagnosing pediatric renal disease. Med. J. Armed Forces India 77, 194–199 (2021).
El-Mas, M. et al. The Suppression of MAPK/NOX/MMP Signaling Prompts Renoprotection Conferred by Prenatal Naproxen in Weaning Preeclamptic Rats [Internet]. https://data.mendeley.com/datasets/hg7h7883gz/1 (2023).
Acknowledgements
This study received no funds, grants, or other support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the research. S.A. and M.A. conducted experiments, analyzed the data, and wrote the first draft of the manuscript. M.E. supervised and administered the research. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelhady, S.A., Ali, M.A., Yacout, D.M. et al. The suppression of MAPK/NOX/MMP signaling prompts renoprotection conferred by prenatal naproxen in weaning preeclamptic rats. Sci Rep 13, 17498 (2023). https://doi.org/10.1038/s41598-023-44617-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44617-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.