Abstract
The breakthrough infection following COVID-19 vaccination has been a subject of concern recently. Evidence suggests that COVID-19 vaccine efficacy diminishes over time due to multiple factors related to the host, and vaccine. Coinfection with other pathogens was claimed earlier as a contributing cause for this phenomenon. Hence, we aimed to stratify the association of post-COVID-19 vaccination breakthrough coinfection with Toxoplasma gondii (T. gondii) and its impact on disease severity. This cross-sectional study included 330 COVID-19-vaccinated patients confirmed by RT-PCR. They were also screened for anti- T. gondii antibodies using ELISA. Toxoplasma seropositive cases’ whole blood was screened for DNA using PCR to correlate results with COVID-19 severity. Out of 330 COVID-19 vaccinated patients with breakthrough infection, 34.5% (114 patients) showed positivity for Toxoplasma IgG by ELISA, and none of the cases was IgM positive. Eleven patients (9.6%) of the IgG-positive cases were positive by PCR. Positive PCR cases correlated positively with the Toxoplasma IgG titer (P < 0.001), and the Cutoff point was 191.5. Molecular analysis of Toxoplasma and COVID-19 severity showed that 8 (72.7%), 1 (9.1%), and 2 cases (18.2%) had mild, moderate, and severe courses of the disease, respectively, with no significant correlation. Our study reported a heightened prevalence of latent toxoplasmosis among mild cases of COVID-19 breakthrough infection. Nevertheless, a discernible correlation between latent toxoplasmosis and COVID-19 severity is lacking. Hence, implementing studies on a larger scale could provide a more comprehensive comprehension of this association.
Similar content being viewed by others
Introduction
Toxoplasmosis, brought on by the obligatory intracellular parasite T. gondii, is one of the most prevalent foodborne diseases concerning hospitalization and mortality, making it a serious global public health concern1. Toxoplasma is widely recognized as a highly adaptable protozoan organism; having the capacity to endure within host cells throughout the host's lifespan2.
Among different parasitic infections, toxoplasmosis is a suitable infection model to test the mutual influence of post-COVID-19 vaccine breakthrough coinfection with protozoa owing to two factors. First, it is global prevalence infecting around one-third of the global population. The Spread of this protozoan has several routes, including consuming raw or undercooked meat, ingesting sporulated oocytes excreted by cats, and receiving blood transfusions. However, it cannot be passed from person to person; its prevalence can be used as a general indicator of group cleanliness2. Second, this eukaryotic intracellular coccidian protozoan is recognized to have at least some antiviral effects3.
Infection with T. gondii may pass asymptomatic in healthy people; nevertheless it can be fatal in patients with weakened immune systems (AIDS, bone marrow transplant, and neoplasia) due to either the parasite's reactivation or a recent acute infection4. The severe lethal consequences of Toxoplasma infection in immunocompromised make it challenging to diagnose, which has been enhanced lately by integrating serology and molecular techniques. Some authors observed that high IgG anti-T. gondii antibodies titer is linked with positive PCR results in AIDS patients; others, likewise, demonstrated no correlation between IgG anti-T. gondii antibody titer and positive PCR among healthy blood donors5,6.
The global health emergency created by coronavirus (COVID-19), which was induced by the severe acute respiratory syndrome coronavirus-2 pathogen (SARS-CoV-2), caused the severe acute respiratory syndrome that killed Thousands of people7.
COVID-19 primarily immune response involves activating innate cells and virus-specific T cells and B cells; meanwhile, extensive inflammatory syndrome is characterized by the unregulated synthesis of pro-inflammatory cytokines and chemokines, and the progression of this condition can result in pulmonary injury, subsequently leading to respiratory failure and ultimately culminating in mortality8,9. Respiratory viral illnesses, in turn, increase the vulnerability of individuals to subsequent parasitic infections, as recognized in earlier research. These secondary infections typically result in a more unfavorable prognosis for the affected patients10.
With the swift and detrimental proliferation of COVID-19 contagion and the lack of targeted antiviral therapy, global cooperation became imperative to pursue vaccine development. Over five billion individuals globally were immunized with COVID-19 vaccines till 2022 end11. However, the trend of unexplained reduction in the effectiveness of commonly administered vaccines and the occurrence of breakthrough infections is a worrisome development in regions where diseases are prevalent. This trend also significantly challenges global efforts to control infections6,12.
The objective of this study was to examine the reciprocal relationship between COVID-19 vaccination and Toxoplasma infection, as well as” the subsequent influence of this association on the prevalence of Toxoplasma infection and COVID-19 severity by molecular diagnosis in Menoufia Governorate, Egypt. Moreover, we will investigate the theory that T. gondii parasitemia is correlated with high serum levels of anti-T. gondii antibodies titer.
Methods
Sample size calculation
According to Geraili et al.13, who found that the seroprevalence (IgG) of T. gondii among COVID-19 positive cases was 26.1%, at a marginal error of 0.05, the power of the study was 95%, the estimated sample size was a minimum number of 296 and after addition 10% (30 cases) for any suspected defect in the patient's records or lab analysis. The final minimal sample was 326 COVID-positive cases.
The variable n represents the sample size, while the variable z denotes the standard error associated with the selected degree of confidence, which is 1.96., p = proportion detected in the reference study (26.1%), q = 1−p, and e = acceptable sample error (0.05).
Study setting, site, and subjects
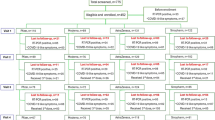
This cross-sectional observational study was conducted at various healthcare facilities, namely the ICU, hospitalized ward, outpatient care clinic, post-COVID-19 medical centers, and Vaccination Unit, The chest Division, of Menoufia University hospitals. The study was carried out from October 2021 with the start of ethical committee approval and ended with collection of calculated sample size. We recruited 330 patients vaccinated against COVID-19 in the study after getting their approval to participate. Recruited patients fulfilled the inclusion criteria of having concurrent coronavirus, screened positive by Reverse Transcription polymerase chain reaction (RT-PCR) in nasopharyngeal swabs, and T. gondii infection confirmed by ELISA. Patients with negative RT-PCR results and those with confirmed other acute illnesses were excluded from the study. Patient demographics, including age, gender, isolation location of cases, survival, and comorbidities such as hypertension, diabetes mellitus, and other chronic diseases, were available through a questionnaire, doctors' notes, and records of the medical exams, which provided pertinent information. All patients had thorough physical examinations, and the severity of COVID-19 was assessed following Egyptian MOH guidelines14. Mild cases with minor clinical signs, no dyspnea or shortness of breath, and normal chest imaging. Moderate cases called for intensive monitoring and home isolation. Moderate cases with lower respiratory infection symptoms and oxygen saturation (SpO2) of less than 92% in room air at sea level. They were received at the hospital in isolation. Severe cases that met any of the following requirements: During 24 to 48 h, the chest radiological findings had progressed by more than 50%, the respiratory rate was greater than 30 breaths per minute, and the oxygen saturation (SpO2) was less than 92%.
Blood sampling and procedures
Each participant in the study donated six mL of venous blood collected from their cubital vein divided into two parts. Three ml were slowly transferred into an EDTA vacutainer tube frozen at − 20 °C for subsequent DNA extraction, and three ml were transferred into a plain tube, then centrifuged to separate the serum and stored at − 20 °C for subsequent biochemical analysis.
The immunoassay enzyme approach was employed to assess the seropositivity and the titer of serum antibodies against T. gondii. Using the manufacturer's recommended procedures, we used commercial kits to measure the serum concentration of IgG and IgM antibodies (Cat No. 201-12-1847, 201–-12-1845 Shanghai Sunred Biological Technology Co., Ltd).
Genomic DNA Extraction was carried out from EDTA samples using Gene JET Whole Blood Genomic DNA Purification Mini Kit according to the manufacturer's instructions (Catalog No K0721, Thermo Scientific). Positive controls (RH and ME 49) were gifted from the Zoonotic Diseases Department, National Research Centre (Cairo, Egypt), after DNA extraction for RH and ME 49 strains of T. gondii. The isolated DNA was kept at 20 °C.
The conventional PCR reaction was performed in a 25 μl total volume using five μl of genomic DNA, 0.5 μl of each primer, and 12.5 µl of master mix [Dream Taq Green PCR Master Mix (2X), Catalog no. K1081; Thermo Scientific] and 6.5 μl of H2O. The primers of the B1 gene were as follows: forward primer 5′-CGACAGCCGCGGTCATTCTC -3′ and reverse primer 5′-GCAACCAGTCAGCGTCGTCC 3′. were used according to the protocol previously applied. The length of the expected target is 96 bp. A ladder of 50 bp was used15 (Fig. 1).
Agarose gel electrophoresis of PCR results. Agarose gel electrophoresis showing T. gondii DNA detection results by conventional PCR. From left to right the first two lanes correspond to positive control samples of Rh and ME49 strains respectively; Lanes 3–13 represent positive samples at 96 bp. M refers to Molecular band size. The image was taken using the gel documentation system directly.
Statistical analysis
SPSS (statistical software for social science) version 20.0 was used on an IBM-compatible computer to gather and analyze the data (SPSS Inc., Chicago, IL, USA). (See “Diagnostic usefulness of GeneXpert MTB/RIF assay versus traditional…”) Spearman correlation was used to assess the relationship between IgG concentration and other quantitative parameters, and ROC curve analysis was performed. Qualitative data were described as frequency and percentage and analyzed using the chi-square test. In contrast, quantitative data were described as mean, standard deviation, and range. They were compared between 2 subgroups using the Mann–Whitney U test and between categories of comorbidities using the Kruskal–Wallis’s test. The P value was considered significant when < 0.05.
Ethical approval
Research Ethics Committee from the Institute of National Liver Disease (NLI IRB protocol N. 00334/2022) approved this investigation. The research adhered to pertinent guidelines and regulations outlined in the Declaration of Helsinki.
Consent to participate
Everyone who took part in this study was told of the study's goals and potential puncture side effects and provided their informed permission was obtained from all subjects and/or their legal guardian(s).
Results
Three hundred thirty vaccinated patients against COVID-19 whose PCR tests had given the COVID-19 positive diagnosis were screened for T. gondii antibodies IgG & IgM. 114/330 showed positivity for T. gondii IgG by ELISA, and none of the screened cases was IgM positive. Only positive cases for the two selected parameters, COVID-19, and positive toxoplasmosis, were included in this study representing 34.5% of screened cases (114) (Fig. 2).
Patients’ characteristics and outcome concerning the titer of Toxoplasma IgG
Table 1 lists patients’ characteristics and outcomes of the patients who took part in the trial. The patients ranged in age from 14 to 78, with a mean age of 47.69 years, 36.8% (42) of whom were men, and 63.2% (72) were women. 15.8% of patients had hypertension (HTN), 9.6% had diabetes mellites, respiratory diseases (asthma, chronic obstructive pulmonary disease (COPD) and others), and heart disease was reported at 1.8 & 4.4%, respectively, and 68.4% of those patients were without comorbidities. COVID-19 had a modest severity; nonetheless, 17.5% of patients needed hospitalization due to a severe condition, and mortality was recorded as 2.6%.
Demographic and clinical parameters of positive Toxoplasma cases by PCR
One hundred and fourteen COVID-19 patients with positive toxoplasmosis results were assessed for B1 gene detection using PCR. Eleven patients out of the 114 Toxoplasma IgG-positive cases had positive PCR results; eight were women, and three were men. (Table 2). The results showed no significant difference in the mean age of participants and gender regarding PCR positivity (P > 0.05). 9.1% of positive cases had HTN. Respiratory and heart diseases were reported at a rate of 9.1% for each comorbidity, and 27.7% of those patients were without comorbidities. Eight cases had mild COVID-19 severity at 72.7%, while two needed hospital admission due to a severe condition, and mortality was recorded as 9.1% with no significant difference regarding severity recorded.
Association between the ELISA concentration of Toxoplasma IgG antibodies and PCR
T. gondii's B1 gene underwent conventional PCR amplification. The results revealed a 9.6% positive rate among IgG seropositive cases. Anti-Toxoplasma IgG antibody concentration was significantly higher value in PCR positive group than those with negative PCR results (274.0 ± 53.69 vs. 68.54 ± 78.41), respectively (P < 0.001) (Table 3). (Fig. 3).
We used ROC curve in current work to assess the sensitivities and specificities for various values. The best cut-off was determined to be an ELISA IgG abs concentration of 191.5 IU/mL, with diagnostic specificity, sensitivity, and accuracy being 92.2%, 100%, and 93.0%, respectively. ROC analysis showed an area underneath the curve (AUC) of 0.95 in relation to the IgG levels (Fig. 4).
Discussion
The present study recorded chronic infection with toxoplasmosis among 34.5% of patients with coronavirus breakthrough infection following vaccination with the COVID-19 vaccine. At the same time, no active cases were recorded by ELISA. Humans are frequently co-infected with viruses and protozoa. The immunosuppression effects of infections with protozoa can reduce the effectiveness of immunizations, decrease the establishment of immunological memory, and reduce protection against co-infecting pathogens12.
In other earlier studies, the seroprevalence of T. gondii among COVID-19 patients was recorded in Egypt, where the prevalence was 22.4%16. In Iran, Ghaffari et al.6, found that 84% of the patients with COVID-19 had anti-T. gondii antibodies (IgG). In contrast Geraili et al.13, reported a 26.1% prevalence rate in Northern Iran. This variation in toxoplasmosis prevalence between studies may have been influenced by local environmental factors such as humidity, geography, and age distribution of the research population. According to earlier reports, infected people are more likely to get toxoplasmosis in humid and warmer climates17. We also refer to the variation of the infection of toxoplasmosis reported before vaccine development to the restriction system applied by different countries to coronavirus.
This work found T. gondii DNA in eleven cases with positive IgG and negative IgM antibodies and high IgG titers by conventional PCR amplification of the B1 gene, constituting 9.6% of the positive results by ELISA. Fallahi et al.18 involved children with cancer in their study. They found that molecular studies provide superior sensitivity and specificity for diagnosis, especially using B1 genes utilized often in the T. gondii DNA detection. Ibrahim et al.19 also determined the prevalence based on B1 amplification in Egypt with similar results.
One of the main insights of the current work was the significant correlation between Toxoplasma antibodies titer and PCR results where T. gondii positive cases were correlated with higher antibodies IgG titer in serum with a significant difference. To our knowledge, this is the first study in Egypt to correlate T. gondii parasitemia in COVID-19 breakthrough infection following vaccination using a molecular approach. This finding is supported by Halleyantoro et al.20, who found that positive PCR samples had greater anti-Toxoplasma IgG titer in HIV encephalitis patients in Iran using the B1 gene. Also, nine women with high IgG titer of ≥ 200 IU had T. gondii DNA in their blood samples, indicating that they may have contracted a recent infection21. The data presented here are not supported by other reports that observed a significant linkage between toxoplasmosis and IgM anti-T. gondii antibodies, despite reporting the potentiality of infection to IgG antibodies carriers20,21.
SARS-CoV-2 severity and mortality are impacted by innate and adaptive immune responses to two primary immune dysregulations. One is cytokine storm with overproduction of pro-inflammatory cytokines, which leads to immune exhaustion, which was also reported experimentally following vaccination22. The other is a dysregulation of lymphocytes that leads to B-cell lymphopenia and is characterized by CD4 + T cells23. Due to the cellular immune system's deficiency in these individuals, the infection by T. gondii may be reactivated. The parasite may be found in the blood as tachyzoites, revealing positive cases by the molecular test. In the latest study, CD8 T dysfunction brought on by CD4 T cell depletion was considered a contributing factor to the infection's reactivation in persistently infected hosts24. Subjects infected with Toxoplasma were more likely to develop Covid-19 and have a more severe disease course; they were also more likely to require hospitalization and intensive care unit treatment25.
The Association between Toxoplasma DNA and COVID severity post-vaccine was explored in the present work. Mild cases were more associated with the Toxoplasma DNA-positive cases; however, the association was non-significant. Hamad & Garedaghi26 hypothesized that the influenza virus parasitizes T. gondii. This symbiotic relationship lessens T. gondii's pathogenicity while increasing the survival of the influenza virus. As a result, patients experience the flu longer than is typical. In contrast, others found that toxoplasmosis is prevalent in COVID patients as a part of the general population and is not considered a risk factor for disease severity13.
Considering recent studies of cases of breakthrough infection after the COVID vaccine the reported data about disease severity recorded 27% of asymptomatic cases, with the need for hospitalization in ten percent; it seems that toxoplasmosis in our research affected the severity of disease and death rate recorded27. However, other factors may be responsible for mild cases observed; one important factor is the emergence of the Omicron variant, characterized by its unparalleled transmission rate, heightened immune evasion, transmissibility, and reduced pathogenicity28,29. Breakthrough infections could be attributed to other factors, including characteristics of immunity, host determinants (such as age, comorbidities, immune status, and use of immunosuppressive drugs30. Hypertension, respiratory, and cardiac diseases exhibited a prevalence of comorbidity, with a rate of 9.1% for each. Our present study recorded no significant difference regarding different comorbidities and disease severity or coinfection which is in agreement with Montazeri et al.31.
Investigators considered the age of participants in the epidemiology of toxoplasmosis and correlated it with acquiring infection. They found that infection increases with age, reaching a peak in patients older than 5032,33. The COVID-19 patients' mean age (47.69 ± 16.53years) was lower than in the research listed above and similar to some extent to Abdel-Hamed et al.16 with a mean age of 44.72 ± 13.82years. Ghaffari et al.6 found that aging is not significantly associated with the prevalence of Toxoplasma Additionally, no correlation was found between gender and the prevalence of latent toxoplasmosis. These findings contradict earlier research that found a statistically significant relationship between Toxoplasma seropositivity and age, gender, and other factors34. Different study populations and study regions could be the cause of this discrepancy.
We know the drawbacks of small numbers of positive T. gondii DNA cases for concurrent infection with COVID and the difficulties of drawing any added conclusions about the relationships using molecular analysis. However, the sample size was calculated before the study. Nevertheless, because the current work demonstrates the effectiveness of molecular diagnostics and its correlation with serology titer, our data constitute a breakthrough in this research point. Its connection to ELISA will forecast COVID-19-potentially reactivated toxoplasmosis cases that should be considered in managing and relation to disease severity. The genotyping of T. gondii and the correlation between the severity of COVID will be considered a future next step.
Conclusion and recommendations
The current investigation represents an initial endeavor to examine the frequency of chronic T. gondii infection in individuals who have experienced breakthrough infection of SARS-CoV-2 after vaccination. The findings indicate a high prevalence of latent Toxoplasma infection among individuals infected with SARS-CoV-2. Moreover, there was no discernible correlation found between COVID-19 and latent toxoplasmosis. Additionally, our findings indicate the need for future investigations to detect the impact of acute to chronic protozoan infection on immune-mediated changes and vaccine efficacy in individuals coinfected with both pathogens.
Data availability
The datasets utilized and examined in the present investigation can be obtained from the corresponding author upon a reasonable request.
Abbreviations
- AUC:
-
The area under the ROC curve
- Bp:
-
Base pair
- COPD:
-
Chronic obstructive pulmonary diseases
- COVID-19:
-
Coronavirus disease 2019
- DM:
-
Diabetes mellitus
- DNA:
-
Deoxyribonucleic acid
- EDTA:
-
Ethylene diamine-tetra-acetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- HIV:
-
Human immunodeficiency virus
- HTN:
-
Hypertension
- IFA:
-
Immunofluorescence antibody assay techniques
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- MOH:
-
Egyptian Ministry of Health’s
- PB:
-
Peripheral blood
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse Transcription polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SPSS:
-
Statistical package for social science
- T. gondii :
-
Toxoplasma gondii
References
Innes, E. A., Hamilton, C., Garcia, J. L., Chryssafidis, A. & Smith, D. A one health approach to vaccines against Toxoplasma gondii. Food Waterborne Parasitol. 18(15), e00053. https://doi.org/10.1016/j.fawpar.2019.e00053 (2019).
Marín-García, P.-J., Planas, N. & Llobat, L. Toxoplasma gondii in foods: Prevalence, control, and safety. Foods (Basel, Switzerland) https://doi.org/10.3390/Foods11162542 (2022).
Weeratunga, P. et al. Dense Granule Protein-7 (GRA-7) of Toxoplasma gondii inhibits viral replication in vitro and in vivo. J. Microbiol. 55(11), 909–917. https://doi.org/10.1007/s12275-017-7392-5 (2017).
Lima, T. S. & Lodoen, M. B. Mechanisms of human innate immune evasion by Toxoplasma gondii. Front. Cell. Infect. Microbiol. 9, 103. https://doi.org/10.3389/fcimb.2019.00103 (2019).
Nakashima, F. et al. Serum IgG anti-Toxoplasma gondii antibody concentrations do not correlate nested PCR results in blood donors. Front. Cell. Infect. Microbiol. 9, 461. https://doi.org/10.3389/fcimb.2019.00461 (2020).
Ghaffari, S. et al. Is COVID-19 associated with latent toxoplasmosis?. Environ. Sci. Pollut. Res. Int. 28(47), 67886–67890. https://doi.org/10.1007/s11356-021-17126-w (2021).
Nicola, M. et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. (London, England) 78, 185–193. https://doi.org/10.1016/j.ijsu.2020.04.018 (2020).
Sinha, P., Matthay, M. A. & Calfee, C. S. Is a “cytokine storm” relevant to COVID-19?. JAMA Intern. Med. 180(9), 1152–1154. https://doi.org/10.1001/jamainternmed.2020.3313 (2020).
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 20(6), 363–374. https://doi.org/10.1038/s41577-020-0311-8 (2020).
Arnold, F. W. & Fuqua, J. L. Viral respiratory infections: A cause of community-acquired pneumonia or a predisposing factor?. Curr. Opin. Pulm. Med. 26(3), 208–214. https://doi.org/10.1097/MCP.0000000000000666 (2020).
Sadoff, J. et al. Safety and efficacy of single-dose Ad2.6COV2.S vaccine against Covid-19. N. Engl. J. Med. 384(23), 2187–2201. https://doi.org/10.1056/NEJMoa2101544 (2021).
Akoolo, L., Rocha, S. C. & Parveen, N. Protozoan co-infections and parasite influence on the efficacy of vaccines against bacterial and viral pathogens. Front. Microbiol. 25(13), 1020029. https://doi.org/10.3389/fmicb.2022.1020029 (2022).
Geraili, A. et al. Toxoplasmosis and symptoms severity in patients with COVID-19 in referral centers in Northern Iran. J. Parasit. Dis. https://doi.org/10.1007/s12639-022-01556-5 (2023).
Abdelmoty, A. A., Abdeldayem, W. A., Metwally, R. H. & Elshamy, A. M. Evaluation of laboratory parameters and their correlation with Covid-19 severity. Egypt. J. Hosp. Med. 87(1), 1602–1607. https://doi.org/10.21608/ejhm.2022.227005 (2022).
Burg, J. L., Grover, C. M., Pouletty, P. & Boothroyd, J. C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27(8), 1787–1792. https://doi.org/10.1128/jcm.27.8.1787-1792.1989 (1989).
Abdel-Hamed, E. F. et al. Role of interferon gamma in SARS-CoV-2-positive patients with parasitic infections. Gut Pathog. 13(1), 29. https://doi.org/10.1186/s13099-021-00427-3 (2021).
Astrid, M. T., Anja, R. H. & Louis, M. W. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 30(12–13), 1217–1258. https://doi.org/10.1016/s0020-7519(00)00124-7 (2000).
Fallahi, S. et al. Comparison of the RE and B1 gene for detection of Toxoplasma gondii infection in children with cancer. Parasitol. Int. 63(1), 37–41. https://doi.org/10.1016/j.parint.2013.08.005 (2014).
Ibrahim, H. M., Mohamed, A. H., El-Sharaawy, A. A. & El-Shqanqery, H. E. Molecular and serological prevalence of Toxoplasma gondii in pregnant women and sheep in Egypt. Asian Pac. J. Trop. Med. 10(10), 996–1001. https://doi.org/10.1016/j.apjtm.2017.09.012 (2017).
Halleyantoro, R., Andriyani, Y., Sari, I. P. & Kurniawan, A. Nested PCR methods for detection of Toxoplasma gondii B1 gene in Cerebrospinal Fluid of HIV patients. J. Biomed. Transl. Res. 5(2), 62–66. https://doi.org/10.14710/jbtr.v5i2.4840 (2019).
Berredjem, H., Aouras, H., Benlaifa, M., Becheker, I. & Djebar, M. R. Contribution of IgG avidity and PCR for the early diagnosis of toxoplasmosis in pregnant women from the North-Eastern region of Algeria. Afr Health Sci. 17(3), 647–656. https://doi.org/10.4314/ahs.v17i3.7 (2017).
Farshi, E. Cytokine storm response to COVID-19 vaccinations. J. Cytokine Biol. 5(1000125), 2 (2020).
Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27(6), 992–1000. https://doi.org/10.1016/j.chom.2020.04.009 (2020).
Khan, I. A., Hwang, S. & Moretto, M. Toxoplasma gondii: CD8 T cells cry for CD4 help. Front. Cell. Infect. Microbiol. 9, 136. https://doi.org/10.3389/fcimb.2019.00136 (2019).
Flegr, J. Toxoplasmosis: An important risk factor for acquiring SARS-CoV-2 infection and a severe course of Covid-19 disease. Parasit Vectors https://doi.org/10.1186/s13071-021-05021-9 (2021).
Hamad, M. N. M. & Garedaghi, Y. Metals explain the relationship between Toxoplasma gondii, influenza virus, and COVID-19: A Hypothesis. Int. J. Med. Parasitol. Epidemiol. Sci. 2(2), 33–34. https://doi.org/10.34172/ijmpes.2021.10 (2021).
COVID-19 Vaccine Breakthrough Infections Reported to CDC - United States, January 1-April 30, 2021. (2021). MMWR. Morbidity and Mortality Weekly Report, 70(21), 792–793. Doi: https://doi.org/10.15585/mmwr.mm7021e3.
Galván-Ramírez, M., Salas-Lais, A. G., Muñoz-Medina, J. E., Fernandes-Matano, L. & la Pérezde, L. L. R. R. Association of toxoplasmosis and COVID-19 in a Mexican population. Microorganisms 11, 1441. https://doi.org/10.3390/microorganisms11061441 (2023).
Menni, C. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet (London, England) 399, 1618–1624 (2022).
Amanatidou, E. et al. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metab. Open 14, 100180. https://doi.org/10.1016/j.metop.2022.100180 (2022).
Montazeri, M. et al. Exploring the association between latent Toxoplasma gondii Infection and COVID-19 in hospitalized patients: First registry-based study. Acta Parasitol. 67(3), 1172–1179. https://doi.org/10.1007/s11686-022-00559-9 (2022).
Kalantari, N. et al. Preliminary study on association between toxoplasmosis and breast cancer in Iran. Asian Pac. J. Trop. Biomed. 5(1), 44–47. https://doi.org/10.1016/j.fawpar.2019.e00053 (2015).
Nasiru Wana, M. et al. A review on the prevalence of Toxoplasma gondii in humans and animals reported in Malaysia from 2008–2018. Int. J. Environ. Res. Public Health 17, 4809. https://doi.org/10.3390/ijerph17134809 (2020).
Yılmaz, A., Yazıcı, E. & Turk, C. Assessment of seroprevalence of Toxoplasma gondii in blood donors applied to the blood center of Gazi University Hospital. Iran. J. Microbiol. 13(2), 243–247. https://doi.org/10.18502/ijm.v13i2.5986 (2021).
Acknowledgements
The authors wish to extend their sincere appreciation to Dr. Reda Abdel-Latif, Associate Professor, Department of Public Health, Faculty of Medicine-Menoufia University, for her contribution to the data analysis and review of current work. We express our gratitude to the individuals who took part in this study and agreed to donate their blood and some of their valuable time.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The initiation and formulation of the study incorporated contributions from all authors. The materials were created, and the data was gathered and evaluated by M.A.G., H.S.A., A.S.A., A.S., K.A.A., Y.E., A.A.A., and Y.H. The initial draft of the manuscript was composed by M.A.G., while the remaining authors contributed their thoughts on preceding drafts. All authors thoroughly reviewed and unanimously endorsed the work’s ultimate version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gouda, M.A., AboShabaan, H.S., Abdelgawad, A.S. et al. Association between breakthrough infection with COVID-19 and Toxoplasma gondii: a cross-sectional study. Sci Rep 13, 17636 (2023). https://doi.org/10.1038/s41598-023-44616-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44616-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.