Abstract
Worldwide, salinity severely affects agricultural production of crops such as mung bean in arid and semi-arid regions. In saline conditions, various species of Rhizobium can be used to enhance nodulation and induce salinity tolerance in maize. The present study conducted a pot experiment to determine the efficiency of three rhizobial isolates under different salinity conditions, such as 1.41, 4 and 6 dS m−1, on mung bean growth parameters, antioxidant status and yield. Results revealed that salt stress imparted adverse effects on the growth, antioxidants, yield and nodulation of mung bean. Under high salt stress conditions, fresh weights were reduced for roots (78.24%), shoots (64.52%), pods (58.26%) and height (32.33%) as compared to un-inoculated control plants. However, an increase in proline content (46.14%) was observed in high salt stressed plants. Three Rhizobium isolates (Mg1, Mg2, and Mg3), on the other hand, mitigated the negative effects of salt stress after inoculation. However, effects of Mg3 inoculation were prominent at 6 dS m−1 and it enhanced the plant height (45.10%), fresh weight of shoot (58.68%), root (63.64%), pods fresh weight (34.10%), pods number per plant (92.04%), and grain nitrogen concentration (21%) than un-inoculated control. Rhizobium strains Mg1, and Mg2 expressed splendid results at 1.41 and 4 dS m−1 salinity stress. The growth promotion effects might be due to improvement in mineral uptake and ionic balance that minimized the inhibitory effects caused by salinity stress. Thus, inoculating with these strains may boost mung bean growth and yield under salinity stress.
Similar content being viewed by others
Introduction
Globally, salinity severely affects agricultural production, mainly in arid to semi-arid regions. Over 800 million ha area has been estimated to be under salt stress with an annual increase of nearly 1–2%1. About 33% of irrigated areas worldwide and 20% of cultivated areas are under salt stress and deteriorated. Pakistan falls under arid and semi-arid regions where shortage and irregular rainfall patterns cause sodicity and salinity in fertile lands. Out of 23.80 million ha of cultivated land in Pakistan, about 6.8 million ha is adversely affected by salt stress2,3. Increasing salt-stressed land concerns food security by reducing crop yields by up to 50 percent annually in salt-affected agricultural zones4. Pulse crops are more susceptible to salinity stress than cereals and other crops.
Pulses are leguminous crops that have Rhizobium in their root nodules which contributes to atmospheric nitrogen (N2) fixation. Pulses serve as an important part of the human diet and are nitrogen fixers to improve soil fertility. Mung bean is a commonly grown pulse in Pakistan and has two growing periods in a year due to its short life span. Seeds of mung bean have high nutritional value, such as 20–25% protein and 55–65% carbohydrates, primarily starch5. In Pakistan, mung bean cultivation spans across approximately 215 thousand hectares, yielding a total of 141.2 thousand tons of grains annually. However, the calculated average yield of 1.1 tons per hectare falls below that of certain other countries. For instance, China and Uzbekistan achieve higher average yields of 1.41 tons/ha and 1.92 tons/ha, respective6,7. Due to its semi-arid location, it is quite sensitive to salinity8.
Salt stress influences the metabolic activities of plants to a great extent9,10. Salinity stress may affect growth and physiology of the plant in different ways that ultimately reduce crop yields. Presence of increased salt concentrations around root zone imposes osmotic stress leading to ion toxicity11,12,13. Osmotic stress, in turn, interferes with water uptake, cell elongation, seed germination, lateral branching, leaf development, photosynthetic rate, nutrient uptake, and subsequent translocation towards above ground plant parts, increased supply of photosynthates to meristematic regions, and ultimately, exerts adverse impact on overall plant growth14,15,16. Toxic concentrations of Na+ and Cl¯ interrupts the uptake of essential nutrients such as Ca2+ and K+ and thereby, cause nutrient imbalance in plants17,18,19. There is a dire need to find the ecofriendly methods to enhance the growth and productivity of crops under salt stressed conditions.
Several physiochemical and biological approaches can be employed to minimize salinity's impacts20. Among physical strategies are gypsum application, irrigation water management, and scraping of surface salts, but all are expensive and less effective techniques21. Nowadays, numerous salinity-tolerant Rhizobium species are well-known22. These species have the potential to regulate the ethylene synthesis in plants23 and yield improvement of leguminous crops in both arid and semi-arid regions24.
Because of rhizobia's environmental and economic benefits, its application may be useful to achieve sustainability in cropping systems25. Rhizobia improves soil fertility under salinity stress and helps reintroduce crops specifically to nitrogen-deficient areas26,27. A recent study found that Rhizobium inoculation in mung bean under normal conditions increased the nodulation, root and shoot length, photosynthetic activity, leaf area, plant biomass and plant height28. Rhizobia can either directly or indirectly promote plant growth. Many plant growth hormones, such as cytokinins, gibberellins, auxins, and abscisic acid, are synthesized directly by rhizobial isolates, and many other chemicals beneficial to plant growth, such as exopolysaccharides, siderophores, ACC-deaminase, and others, are secreted29,30,31,32,33. Rhizobia also improves plant nutrient availability by mobilizing nutrients in the soil and improving soil structure34. Rhizobia indirectly increase plant health by increasing plant self-defense through the induction of systemic resistance35 against damaging insects, infections, diseases, and viruses36. Rhizobia has also been shown to boost legume growth and production under salt stress conditions37,38. There have been numerous reports of rhizobia being used to increase the number of primary roots, root proliferation, and plant growth stimulation even when the salinity level is high39.
As limited work has been conducted to explore rhizobia potential in enhancing the growth of legumes and biological nitrogen fixation under salinity stress, so, current work was done to find out the efficacy of Rhizobium in growth improvement and yield enhancement of crop mung bean under saline soil.
Materials and methods
The effect of salt-tolerant rhizobial strains on mung bean growth, nodulation, antioxidant status, and yield under salt-stressed conditions was tested in a pot experiment. Soil was taken from the farm area of the Institute of Soil and Environmental Sciences (ISES), University of Agriculture in Faisalabad (UAF). Before filling the soil in the pots, it was dried, sieved, and analyzed for its physicochemical properties (Table 1).
Determination of soil texture, pH and electrical conductivity (ECe)
Determination of soil texture was made by using the method of Moodie et al.40. One hundred and fifty milliliters of distilled water along with forty milliliters of 1% sodium hexametaphosphate solution were added in a 400 g soil sample. The mixture was placed overnight. The soil was stirred using a mechanical stirrer, followed by recording the readings with the help of a Bouyoucos hydrometer after the soil stirring with a mechanical stirrer. In addition, a soil textural triangle was used for assessing the textural class.
Soil pH was determined from the saturated paste. For this, 250 g of soil was used, and saturated paste was prepared using distilled water. After staying for one hour, its pH was determined through a pH meter. The soil paste was extracted using a vacuum pump to determine ECe. The electrical conductivity of the extract was recorded with a digital Jenway conductivity meter.
Soil organic matter
The organic matter content of soil was determined using the method outlined by Moodie et al.40 One gram of soil was well mixed with ten milliliters of 1N potassium dichromate solution and twenty milliliters of concentrated sulfuric acid. The excess was titrated against 0.1N potassium permanganate to pink end point using 150 mL of distilled water and 25 mL of 0.5 N ferrous sulphate solution.
Determination of soil nitrogen, phosphorus and potassium (NPK)
Soil samples in triplicate were digested using Ginning and Hibbard's H2SO4 technique to estimate the N. Digestion was followed by distillation using macro Kjeldhal’s apparatus (UDK-126D, Velp-Scientifica, Italy). While Watanabe and Olsen41 method was used for determining available P with the help of spectrophotometer (e-300, Thermo Electron Corporation, USA). Extractable K was detected using a flame photometer (Model FP-410, Sherwood, UK) following Ryan et al.42 method.
Selection of Rhizobium strains and preparation of inocula
Pre-isolated and pre-characterized three rhizobium isolates (Mg1, Mg2, and Mg3) were obtained from the Soil Microbiology and Biochemistry Laboratory, ISES, UAF. To prepare fresh inocula of all the rhizobial isolates, yeast extract mannitol (YEM) broth was used in 250 mL flasks. For growing selected isolates, 100 mL of YEM broth was inoculated with respective rhizobial strains and placed in a shaking incubator (100 rpm and 72 h). Optical density (OD) was measured after the incubation period using OD-meter, and a uniform population (OD540 = 0.45; 107–108 cfu mL−1) was achieved by dilution prior to seed inoculation.
Seed inoculation
The suspension was prepared for each rhizobial isolate (100 mL), and 250 g of sterilized peat was mixed in it. For inoculation purposes, mung bean seeds were coated with this inoculated peat mixed with 50 mL of sugar-sterilized solution (10%). Autoclaved sugar and broth solution were used for the control treatment.
Pot trial
The pot experiment was carried out in the wirehouse of the ISES, UAF. Each pot was filled with 12 kg of dried and sieved soil. There were three different levels of salinity i.e., original (1.41), 4 and 6 dS m−1. The measured quantity of NaCl salt was added to each pot, and after that, salt was properly mixed in order to achieve the desired level of salinity. In each pot, ten mung bean seeds that had been infected were planted. First treatment served as control (1.41 dS m−1) whereas, second, third and fourth treatments involved rhizobial strains (Mg1, Mg2, and Mg3) without salt stress. Similarly, fifth and sixth treatments comprised of two different salinity levels, 4 and 6 dS m−1 without any inoculation. While in the subsequent treatments, these rhizobial strains were tested individually against different salinity levels (1.41, 4 and 6 levels dS m−1). Each treatment had six replications. Pots were arranged in the wirehouse in accordance with a completely randomized design (CRD) at the ambient light and temperature. In each pot, the recommended amount of nitrogen, phosphorus, and potassium fertilizers (20: 60: 60 kg ha−1), which were applied as urea, diammonium phosphate, and sulphate of potash, respectively, were used. All fertilizers were applied as basal dose at the time of sowing. Pots were irrigated using good quality canal water. Thinning was done after almost fifteen days of germination. At the flowering stage, plants from three replicates were uprooted to evaluate the contribution of different strains to nodulation. After a period of sixty days, triplicate fresh leaf samples were obtained and examined to determine the relative water content, as well as the proline, salt, and potassium levels in the leaves. When the plants had reached their full maturity, three replicates were harvested, and data regarding growth and yield metrics were collected. Besides, chemical analysis was used to assess the amounts of nitrogen (N), phosphorus (P), potassium (K+), and sodium (Na+) present in various plant samples.
Determination of relative water content (RWC)
The formula given by Gonzalez and Gonzalez-Vilar43 was used to determine RWC:
Determination of proline content in plant samples
Bates et al.44 method was used to determine free proline contents. A leaf sample of one gram was homogenized in sulfosalicylic acid at a concentration of 3 percent before being filtered using Whatman filter paper No. 2. Following the addition of glacial acetic acid and acid ninhydrin, the mixture was placed in a water bath and heated to a temperature of 100 °C for 1 h. After that, the reaction was stopped by placing the mixing in an ice bath. The mixture was extracted with toluene, and its absorbance at 520 nm was determined. The amount of proline was calculated using a standard curve and given as mol g−1.
Antioxidant defense
Antioxidant enzymes, including catalase (CAT) and ascorbate peroxidase (APX), were measured in leaves using spectrophotometry. The activity of the CAT enzyme was assessed using the method of Aebi45. Furthermore, APX activity was measured by following the method of Nakano and Asada46. In addition, superoxide dismutase (SOD) activity was measured using nitro blue tetrazolium (NBT) method47 and was based on the photoreduction of NBT. Malondialdehyde (MDA) contents were measured using Heath and Packer's48 approach for the thio-barbituric Acid (TBA) reaction.
Determination of NPK in leaves
Following harvest, 0.1 g of oven dried and crushed plant sample in triplicate was digested using Wolf's Method49. Nitrogen was measured using Kjeldhal's method50; potassium was measured with a flame photometer (Mason, 1963); and phosphorus was measured with a spectrophotometer using the standard protocols51.
Statistical analysis
The recorded data were analyzed statistically by two-way analysis of variance technique (ANOVA) using a computer-based statistical software Statistix 8.1 (Statistix, USA)52, and the difference between the data means was determined by using Duncan’s Multiple Range post-hoc test (p ≤ 0.05)53. Means and standard errors were calculated with the help of Microsoft Excel.
Plant guidelines
All the plant experiments were performed by relevant institutional, national, and international guidelines and legislations.
Results
Salinity stress negatively impacted mung bean growth, antioxidant defense, yield and ionic parameters under salinity stress in pots. Rhizobial inoculation improved all the parameters significantly at multiple salinity levels. However, inoculation caused more improvement in parameters at higher levels of salinity when compared with respective uninoculated controls.
Growth parameters
Data showed that NaCl salinity adversely affected mung bean growth parameters more than its respective control (Table 2). At 1.41 dS m−1 salt level, the most remarkable increase in the shoot fresh (17.11%) as well as dry weight (20.94%), fresh weight (32.68%) and dry weight of root (46.01%) was recorded in Mg1 isolate than respective uninoculated control. However, Mg2 and Mg3 behaved differently for different parameters when compared with uninoculated control. At 4 dS m−1 salinity level, plant maximum height (37.20%), shoot fresh (25.48%) and dry weight (54.24%), root length (29.63%), and dry weight of root (59.28%) compared to uninoculated control was recorded in mung bean plants which were inoculated with Mg1. Similarly, at a salinity level of 6 dS m−1, Mg3 proved best due to the increased height of the plant (82.17%), fresh (142%) and dry weight of shoot (258%), root length (57.25%), fresh (168%) and dry weight of root (294%) than un-inoculated control. Strains Mg1 and Mg2 responded differently at different parameters. However, in the case of root fresh weight, Mg2 performed best and showed an increase of 58.17% than the respective uninoculated control.
Nodulation
Adverse effects of salt stress on the number of nodules and their fresh weights were observed, pronounced with elevation in salt concentration (Table 3). Negative impacts of salt stress on nodule number and their fresh weight were recorded, pronounced with the elevation in salt concentration (Table 3). In non-saline control, nodules had the highest number due to Mg3 isolate inoculation, which was 69.33% higher than the nodules obtained due to the inoculation of Mg1 isolate. Mg1 increased 6.42 folds compared to the respective uninoculated unstressed plants at par with isolate Mg2. Likewise, at 4 dS m−1, the highest nodules number (ten nodules/plant) were observed in Mg3 inoculated plants, with a significant increase of 32% over the plants inoculated with Mg3 isolate. The remaining strains (Mg3 and Mg1) produced 7 and 9 nodules per plant, respectively. However, salt stress inhibited nodulation in control plants. At the highest level of salts, i.e., 6 dS m−1, uninoculated plants failed to develop nodules again. While the plants inoculated with Mg1 produced 50% more nodules than the Mg2-inoculated plants.
Similarly, at original (1.41 dS m−1) salinity, in the case of nodule fresh weight, the highest nodule fresh weight was noted due to the strain Mg3, which was 122% more than the respective uninoculated control followed by Mg2 isolate that statistically enhanced the fresh weight of nodule per plant by 98.8% than uninoculated control. At 4 dS m−1 salinity level (medium), uninoculated control plants could not develop nodules. While Mg1 isolate develop nodules with maximum fresh weight (0.13 g) followed by Mg3 (0.12 g). At 6 dS m−1 salinity level (highest), Mg2 was the best isolate regarding the nodule fresh weight per plant (0.54 g), followed by Mg1 with 0.053 g per plant nodules fresh weight.
Yield parameters
Data exhibited the effect of inoculation with rhizobia on pod number per plant, pod fresh weight and grain yield per mung bean plant under stressed salt conditions (Table 3). At low salinity (1.41 dS m−1), Mg2, Mg1 and Mg3 inoculation significantly increased pods number per plant by 36.43, 31.92 and 13.64%, respectively, compared to uninoculated control. On the other hand, at 4 dS m−1 (medium salinity level), Mg2 and Mg3 strains caused 31.60 and 5.31% increase in the number of pods per plant, respectively, than uninoculated control. However, at 6 dS m−1, an increase in pod number/plant by rhizobial strains Mg3, Mg2 and Mg1 strains was estimated to be increased by 130, 100 and 60%, respectively, than respective uninoculated control. While in the case of pods' fresh weight, a maximum increase was noted for Mg1 (173%) at 6 dS m−1, followed by the same strain (51.72%) at the original (1.41 dS m−1) salinity level than the respective uninoculated control. However, a non-significant increase in pods' fresh weight was noted at 4 dS m−1 due to rhizobial inoculation. While in the case of grain yield, inoculation with Mg1, Mg3 and Mg2 resulted in 46.97, 40.91 and 9.6% increase, respectively, with respect to respective uninoculated control, whereas Mg2 strain caused a maximum increase (38.98% than respective uninoculated control) in yield of mung bean grain at 4 dS m−1. At 6 dS m−1 (highest salinity level), uninoculated control plants could not develop grains. While Mg3 inoculated plants produced the maximum amount of grains (1.60 g plant−1), followed by plants inoculated with Mg1 isolate (1.26 g plant−1), which was 27.24% lower than the produce of Mg3 isolate and 8.93% higher than the produce of Mg2 isolate.
Ionic contents
Data showed the accumulation of ionic and protein contents of mung bean under salt-stressed conditions (Table 3). An increase in salt concentration significantly decreased the grain concentration of nitrogen and phosphorous, leaves potassium concentration, K+/Na+ ratio in leaves, and grains protein content of uninoculated plants. However, the Na concentration of leaves of uninoculated plants increased with the increase in salt concentration. The maximum increase in the concentration of nitrogen (60.47%), phosphorus (36.36%) and protein (60.47%) in grain than respective uninoculated control was recorded due to Mg2 strain at original (1.41 dS m−1) salinity. In comparison, Mg3 caused a maximum increase in potassium concentration (32.57%) and K+/Na+ ratio (63.57%) in leaves as compared to respective uninoculated control at this same salinity level. At 4 dS m−1, the greatest increase in the concentration of nitrogen, phosphorus, protein in grains, and potassium and K+/Na+ ratio in leaves was recorded due to Mg2 strain, which was 73.57, 133.3, 73.57, 45.45 and 110.5% than uninoculated control, respectively. Almost similar results trend was observed by Mg2 strain at 6 dS m−1 (highest salinity level). In the case of sodium concentration in leaves of mung bean plant, Mg isolates were efficient in reducing the accumulation of Na+ in plants which was 20% lower than respective uninoculated plants at the original (1.41 dS m−1) salinity level, i.e. 1.41 dS m−1 and statistically similar to the rest of the treatments. At 4 dS m−1, Mg3 and Mg2 gave similar results and reduced Na+ accumulation by 35% each when compared with the respective uninoculated control. Both these treatments were similar statistically to Mg1 but different from the respective uninoculated control. At 6 dS m−1, Mg3 isolate caused a 50% decrease in the accumulation of Na+ concentration of leaves than uninoculated control plants, followed by Mg1 and Mg2 that caused a concentration of 35% each over a respective uninoculated control.
Relative water content (RWC)
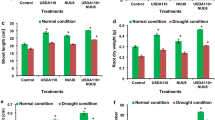
Data concerning the impact of rhizobial inoculation on the relative water contents of mung bean plants indicated a tremendous increase in the RWC at all the applied salinity levels (Fig. 1A). A maximum increment of 6.76% in the RWC at lower salinity levels, such as 1.41 dS m−1, was pragmatic by inoculation of Mg2 compared to control. However, inoculation of Mg3 and Mg1 enhanced RWC by 4.47 and 3.52%, respectively, than respective uninoculated control. Relative water contents (RWC) were also increased by 9.65% at a medium salinity level of 4 dS m−1 with the inoculation of Mg3 compared to the uninoculated control treatment. Similarly, at medium salinity level, Mg2 and Mg1 uplifted the RWC by 7.28 and 4.13%, respectively, than the control. Similarly, Mg3 provided tremendous results than other treatments at high salinity levels (6 dS m−1) and significantly improved the RWC of mung bean by 10.82% than the uninoculated control. However, inoculation of Mg2 and Mg1 promoted RWC by 10.04 and 8.34%, respectively, at a high salinity level of 6 dS m−1 compared to the control treatment (Fig. 1A).
Proline content
Reduction in proline content of inoculated plants ranging from 8.31 to 10.46% to uninoculated control was recorded due to the Mg3 isolate under saline conditions (Fig. 1B). At the original salinity level, i.e., 1.41 dS m−1, strain Mg3 resulted in 8.31% lower proline accumulation than uninoculated control. Mg1 and Mg2 accumulated 1.21 and 2.5% less proline contents than the respective uninoculated control. Similarly, Mg3 isolate resulted in 10.5 and 9% reductions in the proline content of mung bean plants under 4 and 6 dS m−1 salinity levels, respectively, followed by Mg2 isolate that caused a decrease of 7.06 and 6.9% in proline content at 4 and 6 dS m−1 level of salt concentration in comparison to uninoculated control respectively (Fig. 1B).
Antioxidants
Salinity stress causes oxidative stress in plants, which produce different antioxidants in their response. Regulation of other antioxidants under abiotic stresses indicates plants’ ability to tolerate oxidative stress. The findings of current study revealed that salt stress led to a substantial increment in the production of different antioxidants. However, the application of bacterial isolates (Mg1, Mg2, Mg3) boosted the levels of antioxidants such as catalase, ascorbate peroxidase (APX), and superoxide dismutase (SOD) under salt stress as compared to uninoculated control. Among different rhizobial isolates, strain ‘MG3’ caused maximum production of CAT (15, 40 and 48%) (Fig. 2A), SOD (39, 60 and 67%) (Fig. 2B), and APX (58, 64 and 74%) (Fig. 2C) and against salinity stress of 1.41, 4 and 8 dS m−1, respectively.
Effect of Rhizobium inoculation on (A) catalase (CAT), (B) superoxide dismutase (SOD), (C) ascorbate peroxidase (APX) activities and (D) malondialdehyde (MDA) contents in mung bean leaves under salt-stressed conditions (average of three replicates). Mg1 Rhizobial strain 1, Mg2 Rhizobial strain 2, Mg3 Rhizobial strain 3.
Malondialdehyde (MDA) contents
Salt stress also triggered lipid peroxidation in mung bean by producing malondialdehyde, where maximum MDA contents were observed under 6 dS m−1 salt concentration. Rhizobacterial isolates reduced the MDA concentration under all salinity levels. Among different rhizobial strains, inoculation of isolate ‘Mg3’ resulted in a maximum decrease of 40, 50 and 90% against salinity stress of 1.41, 4 and 8 dS m−1, respectively, in MDA content (Fig. 2D).
Discussion
Salinity stress hampers the growth and yield of crops by reducing the photosynthetic activity through reduced gaseous exchange, altering the morphological development, disrupting the membrane functions and affecting the activities of antioxidants54,55,56. In addition, it causes a reduction in the productivity of plants by increasing Na+/ K+ ratio, ionic imbalance and decreasing the N fixation, nutrient metabolism, ionic content of plant and leaf relative water content57,58,59. Nowadays, use of microorganisms has gained worldwide attention to improve leguminous crop production. There is a need to understand the mechanism behind the growth and yield promotion by Rhizobium, and all other factors that halt crop productivity under saline soil conditions. Therefore, current study evaluated different rhizobia strains for promoting mung bean growth, yield and ionic content under saline soil conditions.
Inhibition of nodule formation was observed at higher concentrations of salts which might be due to the collapse of root hair structure, leading to a decline in the growth of root hairs. Thus, it can be concluded that failure to form nodules due to higher salinity levels negatively affects the growth of plants60. Salinity affected nodule initiation, lowered the number, weight, and N-fixing efficiency of mungbean nodules69, resulting in a considerable fall in leghaemoglobin concentration, which declined with nodule ageing due to irreversible oxidation. Rhizobium inhibition of root colonization was the primary cause of inadequate nodulation70. Despite the presence of nodules, N fixation was largely reduced in affected plants growing at 6 dS m−1. High concentrations of Na+ and Cl– present in the root and shoots of mung bean under salt stress can also disturb osmotic pressure and metabolic pathways of plants, especially in leaves because shoots can accumulate a higher amount of Cl– and Na+ compared to roots61,62. Roots can sustain the tolerable limits of NaCl because of their ability to regulate the levels of NaCl through different mechanisms. In case of Na+ toxicity, Na+ replaces the K+ at binding sites, and disrupts cellular functions. There are more than 60 enzymes which require K+ ions for their activation. Na+ cannot substitute in this role62,63. Consequently, higher Na+ to K+ ratios or elevated concentrations of Na+ can interrupt the numerous enzymatic activities in cells. Furthermore, a high concentration of K+ ions is required to bind tRNA to the ribosome during translation process64,65. The negative effect on growth parameters and low yield of plants due to disorders in the protein synthesis process is caused by high Na+ concentration. These findings are confirmed by the conclusions of Hasanuzzaman et al.66. Similarly, Ahmad et al.67 examined that the production of dry mass of mung bean plants gradually decreased with an increase in NaCl level. Moreover, they observed that salinity stress adversely affected pods and nodule formation. Likewise, Panwar et al.68 found that salinity levels of 5 and 10 dS m−1 in soil caused a 17–26.8% decline in mung bean biomass, whereas seed weight was reduced by 18.6–26% at the same salinity levels. In contrast, pod number/plant and fresh weight under rhizobium inoculation increased significantly. Nyoki and Ndakidemi77 also found similar results while studying the effects of Rhizobium on cowpea. Similarly, Kyei-Boahen et al.78 observed a marked improvement in pods number plant−1 and seeds pod−1 in inoculated cowpea plants than control plants. Inoculation with rhizobial isolates improved the root length, fresh, dry weight of the shoot and root at multiple salinity levels. The study of Simon et al.79 revealed similar results. Ahmed et al.80 described a significant increment in seed-inoculated plant yield using inoculum.
Plants in a salty environment acquire proline and glutamine while increasing the content of amino di-carboxylic acid71,72. In the present study, improved grain yield might be due to increased dry weight, nodules number plant−1 and photosynthetic activities73. Tena et al.74 obtained similar results while studying the symbiotic efficiency of Rhizobium inoculation in lentils, and recorded 59% increase in grain yield of inoculated plants. Lamptey et al.75 also reported an improvement in soya bean seed yield due to inoculation. Rhizobial inoculation in current study enhanced the nodulation, improving the atmospheric N2 fixation ability. Therefore, high N utilization might cause improved growth and ultimately, plant yield. Similarly, Mondal et al.76 found that Rhizobium enhanced N2 utilization and nodule formation resulting in a high seed yield. They used various Rhizobium strains to investigate their effects on nodule formation, and mung bean yield under salinity stress. There was a significant difference among both uninoculated and inoculated plants. High proline contents were accumulated in plants due to the adoption of different stress resistance mechanisms under salinity stress87,88. However, Rhizobium inoculation significantly reduced proline contents by regulating the concentration of K+, Na+, P and K+/Na+ ratio in different parts of plants, which resulted in the reduction of salinity adverse effects and lower accumulation of proline content87,88,92.
A decrease in nitrogen concentration and protein content due to salinity stress, and improvement in these parameters through inoculation was observed in the study. Many previous scientists have witnessed the drastic effects of salinity on the N concentration and protein content of plants81,82. Chakrabarti and Mukherjee83 reported that different NaCl concentrations caused significant reductions in total N, the concentration of N present in tissues, protein and amino acids contents, N2 fixation and overall growth of plants. However, improvement in the N concentration of leaves and protein content due to the rhizobium inoculation under saline conditions correlates with the finding of many other scientists84. High N concentration of leaves and protein content might be due to increased N fixation by rhizobium strains76. Moreover, salinity has an impact on the acquisition of N and P by plants; it reduces their uptake through the root system while increasing Na uptake. According to our findings, plants with high salt concentrations have lower N and P contents while salt-treated plants have extremely high Na contents. Increased Na concentrations in plants have been found to reduce the accumulation of other elements like N, P, and K, causing competition in uptake, passage, or dissemination, and altering cationic and anionic ratios, such as Na+/K+ and Cl/NO3. By competing with K+ at protein-binding sites, Na+ damages plants by impeding the function of enzymes85. The interaction between Na and NH3 or Cl− and NO3−, as well as the toxicity of certain ions like Na, S, and Cl, may be the cause of the decreased N absorption in plants under saline conditions. This, in turn, reduces the absorption and accretion of other crucial nutrients86. Additionally, salt stress raises the amount of Na in cell cytoplasm, which substitutes the cytosolic K, and causes Na+/K+ ratio to rise85.
Relative leaf water content indicates a plant’s capability to maintain water status. Therefore, it can be used as a criterion to examine salt stress effects on mung bean plants grown under salinity stress. Plants exposed to salt stress showed a decrease in relative water content. Several studies confirmed a decrease in RWC of salt-stressed plants87,88. Reduced relative water content of leaves in the present study might be due to the lower water uptake under higher salt concentrations89. It might also be due to the retarded flow of sap flux that results in reduced root hydraulic conductivity with a possibility of lower leaf RWC90. However, improvement in the relative water content of stressed plants due to inoculation of rhizobia has been recorded by many researchers87,88,91.
Antioxidant enzymes are crucial in detoxifying ROS, which are harmful and accumulate in plants under salt stress. We observed an increase in defensive antioxidant enzyme activity in inoculated plants compared to uninoculated plants under salt-stress conditions. These results are in line with Wu et al.93, who reported that PGPR inoculation raised SOD activity and improved resistance to salt stress in willows. Under salt stress, E. cloacae PM23-treated maize plants substantially enhanced the activities of APX (14–24%), SOD (23–36%) and POD (26–36%)94. The current study's findings support the idea that each isolate has a distinct enzymatic capability that may be enhanced in stressful and non-stressful circumstances. Antioxidant enzymes increase concurrently with a reduction in other biochemical parameters like MDA. Previous research showed that PGPR strains of Enterobacter cloacae HSNJ4 increased antioxidant systems of canola and sweet corn and Pseudomonas fluorescens against salt stress because MDA contents were reduced due to increasing antioxidant production95,96,97.
Conclusion
Salinity stress drastically affected mung bean plants' growth, yield and ionic parameters under salinity stress. However, rhizobial inoculation significantly improved all the parameters under all the salinity levels. Different strains performed differently under varied salt concentrations. Mg3 performed excellently in enhancing the growth, yield and ionic parameters under the highest salt concentration, i.e., 6 dS m−1. Mg1 and Mg2 performed better at lower salt stress, i.e., 1.41 and 4 dS m−1. The obtained results clearly demonstrate that tested rhizobacterial strains can uplift the growth and yield of mung bean grown in marginal saline lands.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hernández, J. A. Salinity tolerance in plants: Trends and perspectives. Int. J. Mol. Sci. 20, 2408 (2019).
Qureshi, A. S., McCornick, P. G., Qadir, M. & Aslam, Z. Managing salinity and waterlogging in the Indus Basin of Pakistan. Agric. Water Manag. 95, 1–10 (2008).
Economic Survey of Pakistan. Economic Survey of Pakistan 2012–13. Government of Pakistan, Finance Division. Economic Adviser Wing, Islamabad (2013).
Abd-Alla, M. H., Vuong, T. D. & Harper, J. E. Genotypic differences in dinitrogen fixation response to NaCl stress in intact and grafted soybean. Crop Sci. 38(1), 72–77 (1998).
Ganesan, K. & Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 7, 11–33 (2018).
Rani, S., Schreinemachers, P. & Kuziyev, B. Mung bean as a catch crop for dryland systems in Pakistan and Uzbekistan: A situational analysis. Cogent Food Agric. 4, 1499241 (2018).
Li, L. et al. Food legume production in China. Crop J. 5, 115–126 (2017).
Suresh, K. In vitro evaluation of mung bean (Vigna radiata L.) genotypes under salinity stress conditions. J. Pharm. Phytochem. 8, 3896–3900 (2019).
Abbas, A. et al. Investigating the dynamic responses of Aegilops tauschii Coss. to salinity, drought, and nitrogen stress: a comprehensive study of competitive growth and biochemical and molecular pathways. Front. Plant Sci. 14, 1238704 (2023).
Hussain, S., Ashraf, U., Ali, M. F., Zulfiqar, U. & Khaliq, A. Aquaporins: A potential weapon in plants for abiotic stress tolerance. In Transporters and Plant Osmotic Stress (pp. 63–76). (Academic Press, 2021).
Mishra, P., Mishra, J. & Arora, N. K. Plant growth promoting bacteria for combating salinity stress in plants–Recent developments and prospects: A review. Microbiol. Res. 252, 126861 (2021).
Shabaan, M. et al. Halotolerant rhizobacterial consortium confers salt tolerance to maize under naturally salt-affected soil. Soil Sci. Soc. Am. J. 86(5), 1264–1279 (2022).
Ahmed, S. R. et al. Potential role of silicon in plants against biotic and abiotic stresses. Silicon 15(7), 3283–3303 (2023).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 (2008).
Sheldon, A. R., Dalal, R. C., Kirchhof, G., Kopittke, P. M. & Menzies, N. W. The effect of salinity on plant-available water. Plant Soil 418, 477–491 (2017).
Van Zelm, E., Zhang, Y. & Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 71, 403–433 (2020).
Maqsood, M. F. et al. Enhancing wheat growth and yield through salicylic acid-mediated regulation of gas exchange, antioxidant defense, and osmoprotection under salt stress. Stresses 3(1), 372–386 (2023).
Sohail, S., Ali, M. F., Zulfiqar, U., Hussain, S. & Khosa, S. Influence of sewage sludge and heavy fertilization on nitrate leaching in soils: An overview. Soil Contam. Recent Adv. Future Perspect. 1, 1 (2022).
Shahid, S. et al. Proline-induced modifications in morpho-physiological, biochemical and yield attributes of pea (Pisum sativum L.) cultivars under salt stress. Sustainability 14(20), 13579 (2022).
Fatima, A. et al. Differential morphophysiological, biochemical, and molecular responses of maize hybrids to salinity and alkalinity stresses. Agronomy 11(6), 1150 (2021).
Shrivastava, P. & Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22, 123–131 (2015).
Ventorino, V. et al. Response to salinity stress of Rhizobium leguminosarum bv. viciae strains in the presence of different legume host plants. Ann. Microbiol. 62, 811–823 (2012).
Etesami, H. & Maheshwari, D. K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotox. Environ. Safe. 156, 225–246 (2018).
Karmakar, K., Rana, A., Rajwar, A., Sahgal, M., & Johri, B. N. Legume-rhizobia symbiosis under stress. In Plant microbes Symbiosis: Applied facets. pp. 241–258 (Springer, New Delhi, 2015).
Mehmood, N. et al. Multifaceted impacts of plant-beneficial pseudomonas spp. in managing various plant diseases and crop yield improvement. ACS Omega 1, 1 (2023).
Hussain, M. B., Mehboob, I., Zahir, Z. A., Naveed, M. & Asghar, H. N. Potential of Rhizobium spp. for improving growth and yield of rice (Oryza sativa L.). Soil Environ. 28, 49–55 (2009).
Ali, B., Hafeez, A., Afridi, M. S., Javed, M. A. & Sumaira, S. Bacterial-mediated salinity stress tolerance in maize (Zea mays L.): A fortunate way toward sustainable agriculture. ACS Omega 1, 1 (2023).
Diatta, A. A. et al. Inoculation and Soil texture effects on yield and yield components of mung bean. J. Agric. Sci. 10, 6–16 (2018).
Bellés-Sancho, P. et al. A novel function of the key nitrogen-fixation activator NifA in beta-rhizobia: Repression of bacterial auxin synthesis during symbiosis. Front. Plant Sci. 13, 991548 (2022).
Ambika, R., Kavitha, P. and Sengottaian, N., 2014. Production and extraction of Indole acetic acid by using efficient strain of Rhizobium isolated from maize. World Journal of Pharmaceutical Sciences, pp.294–297.
Ferguson, B. J. & Mathesius, U. Phytohormone regulation of legume-rhizobia interactions. J. Chem. Ecol. 40, 770–790 (2014).
Nascimento, F. X., Brígido, C., Glick, B. R. & Rossi, M. J. The role of rhizobial ACC deaminase in the nodulation process of leguminous plants. Int. J. Agron. 1, 1 (2016).
Ali, Q. et al. Synergistic effects of Rhizobacteria and salicylic acid on maize salt-stress tolerance. Plants 12(13), 2519 (2023).
Etesami, H. Root nodules of legumes: A suitable ecological niche for isolating non-rhizobial bacteria with biotechnological potential in agriculture. Curr. Res. Biotechnol. 4, 78–86 (2022).
Santoyo, G. et al. Plant growth stimulation by microbial consortia. Agronomy 11(2), 219 (2021).
Abdelkhalek, A. et al. Protective activity of Rhizobium leguminosarum bv. viciae strain 33504-Mat209 against Alfalfa Mosaic Virus Infection in Faba Bean Plants. Plants 12(14), 2658 (2023).
Ali, Q., Zahir, Z. A., Asghar, H. N. & Jamil, A. Inoculation with Rhizobial consortium for improving the growth, yield and quality of maize under salt-stressed conditions. Pak. J. Agric. Sci. 54(1), 1 (2017).
Sindhu, S., Dahiya, A., Gera, R. & Sindhu, S. S. Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agric. Res. 9, 444–459 (2020).
Ait Bessai, S. et al. The plant growth-promoting potential of halotolerant bacteria is not phylogenetically determined: Evidence from two bacillus megaterium strains isolated from saline soils used to grow wheat. Microorganisms 11(7), 1687 (2023).
Moodie, C.D., Smith, W., & MeCreery, R.A. Laboratory Manual for soil fertility. pp. 1–75. Dept. Agron, State College of Washington. Pullman, Washington (1959).
Watanabe, F. S. & Olsen, S. R. Test of an ascorbic acid method for determining phosphorus in water and sodium bicarbonate extracts from soil. Soil Sci. Soc. Proc. 29, 677–678 (1965).
Ryan, J., Estefan, G., & Rashid, A. Soil and plant analysis: laboratory mannual. In International Centre for Agricultural Research in Dry Areas (ICARDA) Aleppo. pp. 172 (2001).
Gonzalez, L. & Gonzalez-Vilar, M. Determination of relative water content. In: Handbook of plant ecophysiology techniques. pp. 207–212. In M.I. Reigosa Roger (eds.) (Kluwer Publishers, 2001).
Bates, L. S., Waldren, R. P. & Tear, I. D. Rapid determination of free proline for water stress studies. Plant Soil 39, 205–207 (1973).
Aebi, H. Catalase in vitro. Methods Enzymol. 105(C), 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3 (1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(5), 867–880. https://doi.org/10.1093/OXFORDJOURNALS.PCP.A076232 (1981).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Heath, R.L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem Biophy. 125, 189–198 (1968).
Wolf, B. The comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Comm. Soil Sci. Plant Anal. 13, 1035–1059 (1982).
Jackson, M. L. Soil chemical analysis (Prentice Hall. Inc., Englewood Cliffs, New York, USA, 1962).
Quinlan, K. P. & DeSesa, M. A. Spectrophotometric determination of phosphorus as molybdovanadophosphoric acid. Anal. Chem. 27, 1626–1629 (1955).
Steel, R. G. D. & Torrie, J. H. Principles and procedures of statistics 630 (A Biometrical Approach. McGraw Hill Book Co., Inc., 1984).
Duncan, D. B. Multiple range and multiple F test. Biometrics 11, 1–42 (1955).
Taiz, L. & Zeiger, E. Plant physiology 4th edn. (Sinauer Associates, 2006).
Machado, R. M. A. & Serralheiro, R. P. Soil salinity: Effect on vegetable crop growth management practices to prevent and mitigate soil salinization. Hortic. 3, 30 (2017).
Ahmed, J. et al. Screening and growth assessment of indigenous and exotic sesame genotypes under osmotic Stress. S. Afr. J. Bot. 158, 203–213 (2023).
Porcel, R., Aroca, R., Azcon, R. & Ruiz-Lozano, J. M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 26, 673–684 (2016).
Zhang, Y., J. Fang, X. Wu & L. Dong. Na+/K+ balance and transport regulatory mechanisms in weedy and cultivated rice (Oryza sativa L.) under salt stress. BMC Plant Biol. 18, 375 (2018).
Jiang, J. L. et al. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front. Plant Sci. 10, 678 (2019).
Egamberdieva, D., Jabborova, D. & Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth and nodulation of soybean under salt stress. Plant Soil 405, 35–45 (2016).
Chakraborty, K., Basak, N., Bhaduri, D., Ray, S., Vijayan, J., Chattopadhyay, K., & Sarkar, R. K. Ionic basis of salt tolerance in plants: nutrient homeostasis and oxidative stress tolerance. In: Plant nutrients and abiotic stress tolerance. pp. 325–362 (Springer, Singapore, 2018).
Hniličková, H., Hnilička, F., Orsák, M. & Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 65, 90–96 (2019).
Wakeel, A., Farooq, M., Qadir, M. & Schubert, S. Potassium substitution by sodium in plants. Crit. Rev. Plant Sci. 30, 401–413 (2011).
Sharma, A., Shankhdhar, D., & Shankhdhar, S. C. Potassium-solubilizing microorganisms: mechanism and their role in potassium solubilization and uptake. In Potassium solubilizing microorganisms for sustainable agriculture. pp. 203–219 (Springer, New Delhi, 2016).
Gralla, J. D. & Vargas, D. R. Potassium glutamate as a transcriptional inhibitor during bacterial osmoregulation. EMBO J. 25, 1515–1521 (2006).
Hasanuzzaman, M., Alam, M. M., Rahman, A., Hasanuzzaman, M., Nahar, K., & Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed. Res. Int. 757219 (2014).
Ahmad, M., Zahir, Z. A., Asghar, H. N. & Asghar, M. Inducing salt tolerance in mung bean through co-inoculation with rhizobia and plant-growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate-deaminase. Can. J. Microbiol. 57, 578–589 (2011).
Panwar, M., Tewari, R. & Nayyar, H. Native halo-tolerant plant growth promoting rhizobacteria Enterococcus and Pantoea sp. improve seed yield of Mung bean (Vigna radiata L.) under soil salinity by reducing sodium uptake and stress injury. Physiol. Mol. Biol. Plants 22, 445–459 (2016).
Balasubramanian, V. & Sinha, S. K. Effects of salt stress on growth, nodulation and nitrogen fixation in cowpea and mung beans. Physiol. Plant. 36(2), 197–200 (1976).
Mudgal, V., Madaan, N. & Mudgal, A. Biochemical mechanisms of salt tolerance in plants: A review. Int. J. Bot. 6(2), 136–143 (2010).
Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 25(2), 239–250 (2002).
Bhattacharya, A. Low-temperature stress and nitrogen metabolism in plants: A review. In Physiological processes in plants under low temperature stress, pp. 299–407 (2022).
Rani, B. P. & Kodandaramaiah, D. Response of soybean varieties to inoculation with different strains of Rhizobium japonicum in the black soils of Andhra Pradesh. J. Res. Acharya NG Ranga Agric. Univ. (India) (1997).
Tena, W., Wolde-Meskel, E. & Walley, F. Symbiotic efficiency of native and exotic Rhizobium strains nodulating lentil (Lens culinaris Medik.) in soils of Southern Ethiopia. Agron. 6, 11–15 (2016).
Lamptey, S., Ahiabor, B. D. K., Yeboah, S. & Asamoah, C. Response of soybean (Glycine max) to rhizobial inoculation and phosphorus application. J. Exp. Biol. Agric. Sci. 2, 73–77 (2014).
Mondal, H. K., Mehta, S., Kaur, H. & Gera, R. Characterization of stress tolerant mung bean rhizobia as PGPR and plant growth promotion under abiotic stress. Indian Ecol. Soc 44, 38 (2017).
Nyoki, D. & Ndakidemi, P. A. Economic benefits of Bradyrhizobium japonicum inoculation and phosphorus supplementation in cowpea (Vigna unguiculata (L.) Walp) grown in northern Tanzania. Am. J. Res. Commun. 1, 173–189 (2013).
Kyei-Boahen, S., Savala, C. E., Chikoye, D. & Abaidoo, R. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front. Plant Sci. 8, 646 (2017).
Simon, T. S., Kalalova, A. & Sindlarova, M. Study of nodulation and yield parameters of soyabean after application of two types of inoculants in different conditions of irrigation. Sci. Agric. Bohemoslovaca 24, 215–229 (1992).
Ahmed, Z. I., Anjum, S. M. & Rauf, C. A. Effect of Rhizobium inoculation on growth and nodule formation of green gram. Int. J. Agric. Biol. 2, 235–237 (2006).
HanumanthaRao, B., Nair, R. M. & Nayyar, H. Salinity and high temperature tolerance in mung bean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front. Plant Sci. 7, 957 (2016).
Srivastava, M. & Shahi, S. Biochemical response of salt-tolerant and salt-sensitive wheat cultivars to salinity. Res. Crop 18, 605–611 (2017).
Chakrabarti, N. & Mukherji, S. Effect of phytohormone pretreatment on DNA, RNA, amino acid and protein content in different plant parts of Vigna radiata under salt stress. Indian Agric. 47, 57–78 (2003).
Getachew, Z., Abera, G. & Beyene, S. Rhizobium inoculation and sulphur fertilizer improved yield, nutrients uptake and protein quality of soybean (Glysine max L.) varieties on Nitisols of Assosa area. Western Ethiopia. Afr. J. Plant Sci. 11, 123–132 (2017).
Mushtaq, Z., Faizan, S., Gulzar, B. & Hakeem, K. R. Inoculation of Rhizobium alleviates salinity stress through modulation of growth characteristics, physiological and biochemical attributes, stomatal activities and antioxidant defence in Cicer arietinum L.. J. Plant Growth Regul. 40, 2148–2163 (2021).
Jouyban, Z. The effects of salt stress on plant growth. Tech. J. Eng. Appl. Sci. 2(1), 7–10 (2012).
Ahmad, M., Zahir, Z. A., Asghar, H. N. & Arshad, M. The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vigna radiata L.) under salt-stressed conditions. Ann. Microbiol. 62, 1321–1330 (2012).
Ali, Q., Zahir, Z. A., Asghar, H. N. & Jamil, A. Inoculation with Rhizobial consortium for improving the growth, yield and quality of maize under salt-stressed conditions. Pak. J. Agric. Sci. 54, 1 (2017).
Amirjani, M. R. Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int. J. Bot. 7, 73–81 (2011).
Vysotskaya, L., Hedley, P. E., Sharipova, G., Veselov, D., Kudoyarova, G., Morris, J., & Jones, H.G. Effect of salinity on water relations of wild barley plants differing in salt tolerance. AoB Plants (2010).
Bano, A. & Fatima, M. Salt tolerance in Zea mays L. following inoculation with rhizobium and pseudomonas. Biol. Fertil. Soils. 45, 405–413 (2009).
Iqbal, M. A., Khalid, M., Zahir, Z. A. & Ahmad, R. Auxin producing plant growth promoting rhizobacteria improve growth, physiology and yield of maize under saline field conditions. Int. J. Agric. Biol. 18, 37–45 (2016).
Wu, T. Y., Wang, Y. H., Wu, F. & Wu, X. Q. Dual inoculation with rhizosphere-promoting bacterium Bacillus cereus and beneficial fungus Peniophora cinerea improves salt stress tolerance and productivity in willow. Microbiol. Res. 268, 127280 (2023).
Babar, P. et al. Low-overpotential overall water splitting by a cooperative interface of cobalt-iron hydroxide and iron oxyhydroxide. Cell Rep. Phys. Sci. 3, 100762 (2022).
Zarei, T., Moradi, A., Kazemeini, S. A., Akhgar, A. & Rahi, A. A. The role of ACC deaminase producing bacteria in improving sweet corn (Zea mays L. var saccharata) productivity under limited availability of irrigation water. Sci. Rep. 10, 20361 (2020).
Li, H. et al. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 119, 26–34 (2017).
Ali, B. et al. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 11, 345 (2022).
Acknowledgements
The authors extend their appreciation to the Researchers supporting project number (RSP2023R306), King Saud University, Riyadh, Saudi Arabia.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization, Q.A., M.S., and S.A.; methodology, Q.A, and M.K..; software, Q.A., and U.Z., validation and formal analysis, U.Z., M.A., and Z.A.Z.; resources, Z.A.Z.; data curation, Q.A., M.J.S., and R.I.; writing—original draft preparation, Q.A., and M.S., writing—review and editing, U.Z., R.I., B.A., M.A.A., M.S.E., and M.A., supervision, Z.A.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, Q., Shabaan, M., Ashraf, S. et al. Comparative efficacy of different salt tolerant rhizobial inoculants in improving growth and productivity of Vigna radiata L. under salt stress. Sci Rep 13, 17442 (2023). https://doi.org/10.1038/s41598-023-44433-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44433-8
This article is cited by
-
Mitigation effect of alpha-tocopherol and thermo-priming in Brassica napus L. under induced mercuric chloride stress
BMC Plant Biology (2024)
-
Exogenous ascorbic acid as a potent regulator of antioxidants, osmo-protectants, and lipid peroxidation in pea under salt stress
BMC Plant Biology (2024)
-
Investigating the growth promotion potential of biochar on pea (Pisum sativum) plants under saline conditions
Scientific Reports (2024)
-
Mycorrhiza in Improving Morpho-Physiological and Biochemical Parameters of Chickpea Genotypes (Cicer arietinum L.) Under Salinity Stress
Journal of Crop Health (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.