Abstract
To evaluate the association of intracranial non-stenotic atherosclerotic plaque with cerebral small vessel disease (CSVD) imaging markers in a CSVD population using 3.0 T high-resolution magnetic resonance imaging (HRMRI), which was validated in embolic stroke of undetermined source (ESUS) cohort. We retrospectively recruited consecutive patients who were diagnosed with CSVD or ESUS from January 2015 to December 2019. All patients underwent intracranial HRMRI to assess intracranial non-stenotic atherosclerotic plaques. Baseline and imaging data were collected and were measured among all patients. Among 153 patients with CSVD, there were 59 with intracranial atherosclerotic plaque (IAP) and 94 with non-IAP, including 36 with intracranial atherosclerotic complicated plaque (IACP). Among 227 ESUS patients, there were 155 with IAP and 72 with non-IAP, including 127 with IACP. In the CSVD population, we found that: (1) CSVD burden was associated with IAP (p = 0.036) and IACP (p = 0.008); (2) IAP was associated with white matter hyperintensity (51% vs. 34%; P = 0.039), and IACP was associated with lacunes (69% vs. 35%; P = 0.009) and enlarge perivascular space (69% vs. 39%; P = 0.022). A similar association of CSVD imaging markers with IAP or IACP was found in the ESUS population. Furthermore, the association of unilateral IAP or IACP with CSVD imaging markers of ipsilateral hemisphere was identified in the two cohorts. This is the first report that intracranial non-stenotic atherosclerotic plaque, especially complicated plaque, is closely associated with CSVD imaging markers, which provide further evidence for the association of large artery atherosclerosis with CSVD.
Similar content being viewed by others
Introduction
The association of carotid atherosclerotic plaque and its vulnerability with cerebral small vessel disease (CSVD) has been widely investigated1,2,3,4. For example, asymptomatic CSVD was found to be often accompanied by severe carotid artery stenosis5; The characteristics of vulnerable carotid plaques coexisted or were associated with CSVD6. In addition to carotid artery, some studies also investigated the association of intracranial artery atherosclerosis (either intracranial portion of the carotids or the circle of Willis) with CSVD, but inconsistent results occurred, which may be attributed to the difference and drawback of intracranial artery atherosclerosis measurement methods including intracranial arterial calcification on CT or the number of vessel wall lesions or blood-flow velocity pulse index or arterial distensibility7,8,9,10,11,12,13,14,15,16. Given the intracranial artery as the close upstream vessel of CSVD, and these inconsistent results, the relationship between intracranial artery atherosclerosis and CSVD deserves further investigation, especially in Asia population who have a higher prevalence of intracranial artery atherosclerosis17,18,19,20.
With the development of high-resolution magnetic resonance imaging (HRMRI) technology, the intracranial atherosclerotic plaque can be well identified and more information can be obtained compared with traditional techniques. In this context, the current study aimed to assess the relationship between intracranial non-stenotic atherosclerotic plaque and CSVD imaging markers by 3.0 T HRMRI in the CSVD cohort, which was further validated in embolic stroke of undetermined source (ESUS) cohort to confirm the reliability and generalizability of their association given the different etiologies between two cohorts.
Methods
Study population
We used the same ESUS and CSVD cohorts, which have been reported in detail in our recent study21. Briefly, eligible patients were adults presenting with acute stroke symptom who were diagnosed with unilateral anterior circulation ischemic by DWI, received high resolution magnetic resonance examination of the head within 1 week of onset and met the diagnostic criteria of ESUS and CSVD. In addition, all CSVD patients were diagnosed with arteriolosclerosis origin rather than cerebral amyloid angiopathy. The main exclusion criteria included non-stenosis carotid plaque with ≥ 3 mm thickness; aortic arch atherosclerotic plaque thickness ≥ 4 mm or ulcerative plaque, or history of balloon dilation or stent implantation or bilateral infarction, etc. Patient informed consent was waived given the retrospective nature of the analysis and minimal risk to subjects by the institutional review board of General Hospital of Northern Theater Command. Briefly, between January 2015 and December 2019, we enrolled all consecutive CSVD and ESUS patients at the Department of Neurology of General Hospital of Northern Theater Command. All enrolled patients underwent intracranial HRMRI measurements (including 3D T1-weighted and 2D T2-weighted imaging sequences) to assess the presence and vulnerability of plaques. We collected demographic data, clinical characteristics, a range of laboratory indicators, and neuroimaging data (including Flair T2, etc.). The detailed inclusion/exclusion criteria were shown in supplemental Table S1.
Imaging protocol
All MRI scans were performed on 3.0 Tesla MRI scanners (GE discovery MR750, Milwaukee, WI) using an 8-channel head coil with the standardized acquisition protocols (including 3-dimensional T1-weighted and 2-dimensional T2-weighted imaging sequences) for multidimensional evaluation of plaque. A fat suppression technique was used to reduce fat signals from the scalp. Zero-filled Fourier transform (ZIP 512, ZIP2) was used to reduce pixel size, and the final display resolution was 0.3 to 0.4 mm3, with a scan range of > 480 mm covering the internal carotid artery, middle cerebral artery, anterior cerebral artery, vertebral artery, basilar artery, and posterior cerebral artery. MRI scanning took 20 to 25 min, which is described in detail elsewhere21. In addition, two trained raters (Z.Y.S. and D.W.) with > 2 years of experience reviewing intracranial magnetic resonance images who were blinded to the clinical data analyzed magnetic resonance image quality by consensus, using a previously developed 4-point scale (1 = poor quality, 2 = acceptable, 3 = good quality, 4 = excellent), in which images of poor quality were excluded from our final result. Qualitative and quantitative analyses were performed using ImageJ version 1.49 (National Institutes of Health, Bethesda, Maryland) and RadiAnt DICOM Viewer version 5.0.2 (Medixant, Poznan, Poland) for 3-dimensional volume rendering using the appropriate magnification. The window width and level were adjusted to optimize the conspicuity of vessel contour.
MRI examination and CSVD burden assessment
Imaging markers of CSVD mainly include lacune presumed to be of vascular origin after excluding other possible reasons, white matter hyperintensity (WMH) presumed to be of vascular origin after excluding other possible reasons, enlarged perivascular space (EPVS), and cerebral microbleed (CMB)22. We calculated the number of lacunes, EPVS (EPVSs in the centrum of the oval and the basal ganglia respectively), and CMBs (lobar and deep/ infratentorial CMBs respectively) in both hemispheres of the brain. The EPVS was scored on a 5-grade semi-quantitative scale (0–4: 0 = no EPVS; 1 = 1–10 EPVS; 2 = 11–20 EPVS; 3 = 21–30 EPVS; 4 = 40–EPVS)23. The Fazekas scale (range from 0 to 3) was used to rate periventricular and deep WMH24. Finally, we calculated the presence of these four neuroimaging markers to determine the total CSVD burden based on an ordinal “CSVD score” (range from 0 to 4) according to previous study25. One point was awarded for any of the following definitions: ≥ 1 lacunes, ≥ 1 cerebral microbleeds, moderate to severe (grade 2–4) EPVS in basal ganglia or the centrum of the oval, periventricular WMH Fazekas 3 (extending into deep white matter) and/or deep WMH 2–3 (early confluent).
Definition of plaque on HRMRI
The signal characteristics of intracranial non-stenotic atherosclerosis plaque (IAP) components were determined as described previously21. The intracranial atherosclerosis plaque (IAP) was identified as markedly eccentric or focal wall thickening, with the thickest wall ≥ 50% of the thinnest part on HRMRI26. According to the American Heart Association (AHA) definition of type VI plaque, intracranial atherosclerosis complicated plaque (IACP) is defined as any or both discontinuity of plaque surface (DPS) or intraplaque hemorrhage (IPH). IPH is defined as T1 hypersignal greater than 150% of the adjacent muscle or pons. DPS is defined as an irregular luminal surface the of plaque, such as fibrous cap rupture, ulcerated plaque, or mural thrombus27. Based on the presence or absence of non-stenotic IAP or IACP (regardless of ipsilateral, contralateral, or bilateral), the patients were divided into IAP vs non-IAP group, and IACP vs non-IACP group, respectively.
Statistical analysis
Continuous variables were described as mean ± SD (if normally distributed), or median and IQR (if not normally distributed), while categorical variables are expressed as absolute and relative frequencies. We used Student's T test or Wilcoxon's rank sum-test for continuous data, and χ2 or Fisher’s exact test for categorical data. Demographic, clinical data and serological markers were compared between ESUS and CSVD patients with vs without IAP and with vs without IACP. Multifactorial analyses were performed to adjust for confounding factors such as age, sex, and other vascular risk factors to explore the association between IAP or IACP with CSVD neuroimaging markers. In order to adjust the confounding factors, these variables of vascular risk factors with P < 0.1 in binary univariate logistic regression analysis were included in binary multivariate logistic regression analysis. In addition, CSVD neuroimaging markers were rated by 2 researchers, who were blinded to clinical information. Interrater reliability and plaque characteristics was measured by the intraclass correlation coefficient (ICC), which was interpreted as followed: < 0.5: poor; 0.5–0.75: moderate; 0.75–0.9: good; > 0.9: excellent. Analyses were performed with SPSS software (version 26.0; IBM), when P < 0.05, the difference was statistically significant.
Ethics approval
This retrospective study was approved by the Institutional Review Board of General Hospital of Northern Theater Command (IRB: k2019-57).
Results
Study population characteristics
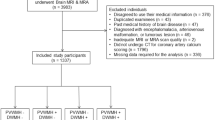
From the initial ESUS and CSVD cohorts, we excluded 16 out of 243 and 7 out of 160 patients, respectively, due to poor image quality or incomplete information. Therefore, a total of 380 patients were eventually included in the study including 227 ESUS patients and 153 CSVD patients. Supplemental Table S2 summarizes detailed baseline demographic characteristics and laboratory examination in patients with and without non-stenotic IAP. In the CSVD cohort, older age ([62.78 ± 9.38] vs [59.30 ± 9.45], P = 0.038), lower female incidence (20% vs 26%), higher incidence of diabetes and prior stroke or transient ischemic attack, higher glycated hemoglobin, more WMHs, and higher CSVD burden were found in patients with IAP compared to patients without. In the ESUS cohort, older age (62 [56–69] vs 58 [51–66], p = 0.014), and lower female incidence (30% vs. 42%), higher incidence of hypertension, higher glycated hemoglobin and cystatin c, more WMHs, lacunae and EPVSs, and higher CSVD burden were found in IAP compared to patients without. Similar demographic and laboratory data, as well as CSVD imaging markers, were found in patients with and without IACP in the two cohorts (Supplemental Table S3).
Logistic regression analyses for the association between intracranial non-stenotic atherosclerosis plaque and CSVD imaging markers
In CSVD patients, WMHs (2.00 [1.03–3.90], P = 0.041) and CSVD burden (1.57 [1.10–2.23], P = 0.012) were associated with IAP, while lacunes (4.26 [1.4–12.97], P = 0.011), EPVSs (3.54 [1.18–10.60], P = 0.024), and CSVD burden (2.49 [1.28–4.83], P = 0.007) were associated with IACP. No relationship between CMBs and plaques was found in the CSVD cohort (Table 1). Similar results were found in the ESUS cohort: lacunes (1.83 [1.04–3.21], P = 0.037), WMHs (1.88 [1.02–3.44], P = 0.042), EPVSs (2.89 [1.55–5.36], P = 0.001) and CSVD burden (1.67 [1.27–2.18], P < 0.001) were correlated with IAP, while lacunes (3.28 [1.40–7.71], P = 0.006), EPVSs (2.97 [1.22–7.25], P = 0.017), and CSVD burden (1.78 [1.15–2.75], P = 0.010) were associated with IACP (Table 1). After adjusting for gender, age, smoking, alcohol consumption, hypertension, diabetes, there was still an obvious correlation between CSVD neuroimaging markers and IAP or IACP in the two cohorts (Fig. 1, Table 1). Furthermore, to eliminate potential confounding factors between individuals, we further determined the association of unilateral IAP with ipsilateral hemisphere CSVD imaging markers. The results showed that unilateral IAP was associated with ipsilateral hemisphere CSVD imaging markers (Table 2, Fig. 2). Similar results about the association of CSVD imaging markers with unilateral IACP were identified in the two cohorts (Table 2, Fig. 2).
Logistic regression analyses for the association of intracranial atherosclerosis plaque with CSVD imaging markers in CSVD and ESUS patients. CSVD, cerebral small vessel disease; ESUS, embolic stroke of undetermined source; WMH, white matter hyperintensity; EPVS, enlarge perivascular space; CMB, cerebral microbleed; IAP, intracranial atherosclerosis plaque; IACP, intracranial atherosclerosis complicated plaque.
Logistic regression analyses for the association of unilateral intracranial atherosclerosis plaque with ipsilateral hemisphere CSVD imaging markers in CSVD and ESUS patients. Multivariate Logistic regression analyses after adjusted for age, gender, current smoker, alcohol use, hypertension and diabetes mellitus. CSVD, cerebral small vessel disease; ESUS, embolic stroke of undetermined source; WMH, white matter hyperintensity; EPVS, enlarge perivascular space; CMB, cerebral microbleed; IAP, intracranial atherosclerosis plaque; IACP, intracranial atherosclerosis complicated plaque.
To further confirm the relationship between intracranial atherosclerotic plaque and CSVD burden, we dichotomized the overall CSVD burden severity as none to mild (score 0–2) and moderate to severe (score 3–4). In the CSVD cohort, moderate-to-severe CSVD burden was more prevalent in the patients with IAP vs non-IAP (25.43% vs 8.51%, P = 0.004, Table 3) or IACP vs non-IACP (41.66% vs 13%, P = 0.02, Table 3). IACP was found to have the highest proportion of moderate-to-severe CSVD burden, followed by the patients with IAP and finally the patients with non-IAP (41.66% vs. 25.43% vs. 8.51%, Table 3). Similar results were also found in ESUS patients (Table 3).
Reproducibility
In addition, interrater reliability was good for the measurement of CSVD biomarkers: the ICC of lacunes, WMHs, CMBs, and EPVSs was calculated as 0.80, 0.85, 0.73, and 0.78, respectively. Similarly, the ICC of IAP and IACP was calculated as 0.85 and 0.87, respectively.
Discussion
In this HRMRI-based analysis, we assessed the association of non-stenotic IAP with CSVD imaging markers and identified a relationship between CSVD and IAP/IACP in both CSVD and ESUS patients. Furthermore, the reliability of this correlation is also supported by our finding of an association between unilateral IAP/IACP and ipsilateral hemisphere CSVD imaging markers in both cohorts, which significantly limits potential confounding factors between individuals.
CSVD burden can reflect brain microstructural damage, because the presence of CSVD often indicates impaired cerebrovascular reserve28,29. Given that cerebral large arterial segments like the carotid arteries represent the upstream of cerebral small vessel and they share common vascular risk factors30,31,32, the association between carotid artery atherosclerosis and CSVD has been widely investigated and well recognized1,2,3,4,5,6,33,34. Given that the intracranial arterial segments lie closer to the small cerebral vessels than the carotid artery, it could be hypothesized that intracranial atherosclerosis may be more closely associated with CSVD. In the current study, we found that non-stenotic IAP was closely associated with CSVD imaging markers in both CSVD and ESUS cohorts. The findings provided further evidence for the association of large artery atherosclerosis with CSVD. Previous studies also investigated the association of intracranial atherosclerosis with CSVD, but the results were conflicting, which may be attributed to imperfect measurements or other potential confounding factors. For example, some studies assessed intracranial atherosclerosis by calculating the volume and severity of intracranial arterial calcification, but one main limitation of this approach is that calcification on CT is only a fraction of IAP, and thus cannot accurately reflect IAP9,10,11,35,36. Some studies used intracranial atherosclerosis burden (defined as the number of vessel wall lesions) to determine the relationship between intracranial atherosclerosis and CSVD7,8. In addition, in some studies, blood-flow velocity pulse index and arterial distensibility were used as indicator to explore the correlation between intracranial atherosclerosis and CSVD12,37,38. In the current study, we used IAP, identified by HRMRI, as an imaging marker of intracranial artery atherosclerosis, which can provide more information of intracranial atherosclerosis compared with these previous methods. Previous studies showed that carotid vulnerable plaque is related to CSVD burden4,6,39,40. Given that complicated plaques may represent active artery atherosclerosis, there is reason to believe that such plaques may be more likely associated with the cerebral small vessel. Indeed, in the current study, we found a strong association of IACP with CSVD, which further supports the relationship between large artery atherosclerosis and CSVD. In addition, we found no relationship between CMBs and IAP, which is consistent with previous studies8,41. This seems in concordance with the previous view that microbleeds are generally caused by intrinsic small vessel disease, such as hypertensive vasculopathy and cerebral amyloid angiopathy42.
The main strength of this study is that it is the first to investigate the association of non-stenotic IAP and IACP with CSVD imaging markers based on HRMRI, and provide evidence for their association. Second, for the first time, our study identified their relationship in non-stenotic intracranial arteries, in contrast to previous studies which explored the relationship between large artery atherosclerosis and CSVD in stenotic vessels (either the extracranial or the intracranial artery). In addition, we demonstrated the relationship of unilateral non-stenotic IAP or IACP to ipsilateral hemisphere CSVD imaging markers, which could limit the potential confounders, given that more prevalence of intracranial atherosclerotic plaque or complicated plaques ipsilateral to stroke21,43. Finally, the association of non-stenotic IAP or IACP with CSVD imaging markers was demonstrated in both cohorts, which further supports the reliability of the conclusion.
We acknowledge some limitations. First, this is a retrospective analysis, which might be subject to selection bias. To eliminate potential confounders, we assessed also the association of unilateral IAP or IACP with ipsilateral hemisphere CSVD imaging markers, and the results support their association. Second, the moderate sample size is another limitation, but our strict inclusion and exclusion criteria might enhance the reliability of the conclusion. Third, lack of histological validation on the plaque is a common limitation in HRMRI studies, although preliminary studies have demonstrated a high level of agreement between MRI-defined plaque signal features and histology8,21. Fourth, given the young age, relatively low percentages of risk factors, the exclusion of more than > 50% ICAS stenosis, and the relatively low mean CVSD burden in current population, these findings limits the implication in a higher-risk population. Fifth, there was no measurement of plaque volume or degree of stenosis in the current study. In addition, CSVD markers only including WMHs, EPVS, CMBs, and lacunes is incomplete due to inclusion of recent small subcortical infarcts and brain atrophy. Sixth, contrast enhancement was not used in the current cohort, given that contrast enhancement of atherosclerotic plaque is a strong, reliable marker of culprit plaques40. Seventh, we only investigated the association in CSVD and ESUS populations due to lack of HR-MRI data in other stroke subtypes. In addition, ESUS patients with bilateral infarctions and non-stenosis carotid plaque with ≥ 3 mm thickness were excluded, which cannot represent usual ESUS patients. These limitations may affect the generalization of this finding. Finally, given the high prevalence of intracranial atherosclerosis in Asia population17,18,19,20, the relationship between non-stenotic IAP and CSVD imaging markers needs to be confirmed also in other non-Chinese populations.
Conclusion
In this HRMRI-based analysis, we found that intracranial non-stenotic atherosclerotic plaque, especially complicated plaque, is closely associated with CSVD imaging markers. These findings provide evidence to support the association between cerebral large artery atherosclerosis and CSVD.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
Moroni, F. et al. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. Int. J. Cardiol. 223, 681–687 (2016).
Saba, L. et al. Volumetric analysis of carotid plaque components and cerebral microbleeds: A correlative study. J. Stroke Cerebrovasc. Dis. 26(3), 552–558 (2017).
Ye, H. et al. White matter hyperintensities and their subtypes in patients with carotid artery stenosis: a systematic review and meta-analysis. BMJ Open 8(5), e020830 (2018).
Hori, S. et al. Correlation between cerebral microbleeds and vulnerable plaque in patients with severe carotid artery stenosis; comparative magnetic resonance imaging study. J. Stroke Cerebrovasc. Dis. 28(10), 104300 (2019).
Komura, S. et al. Asymptomatic cerebral findings on 3-Tesla MRI in patients with severe carotid artery stenoses. J. Clin. Neurosci. 101, 106–111 (2022).
Li, J., Wu, H., Hang, H., et al. Carotid vulnerable plaque coexisting with cerebral small vessel disease and acute ischemic stroke: a Chinese Atherosclerosis Risk Evaluation study. Eur. Radiol. 2022.
Del Brutto, O. H. et al. Cerebral small vessel disease score and atherosclerosis burden: A population study in community-dwelling older adults. Clin. Neurol. Neurosurg. 194, 105795 (2020).
Zwartbol, M. H. et al. Intracranial vessel wall lesions on 7T MRI and MRI features of cerebral small vessel disease: The SMART-MR study. J. Cerebral Blood Flow Metab. 41(6), 1219–1228 (2021).
Del Brutto, O. H. & Mera, R. M. Enlarged perivascular spaces in the basal ganglia are independently associated with intracranial atherosclerosis in the elderly. Atherosclerosis 267, 34–38 (2017).
Chen, Y. C. et al. Correlation between intracranial arterial calcification and imaging of cerebral small vessel disease. Front. Neurol. 10, 426 (2019).
Vinke, E. J. et al. Intracranial arteriosclerosis is related to cerebral small vessel disease: a prospective cohort study. Neurobiol. Aging 105, 16–24 (2021).
van Tuijl, R. J., Ruigrok, Y. M., Geurts, L. J., et al. Does the internal carotid artery attenuate blood-flow pulsatility in small vessel disease? A 7 T 4D-flow MRI study. J. Magn. Resonan. Imaging; 2022.
Wardlaw, J. M. et al. Little association between intracranial arterial stenosis and lacunar stroke. Cerebrovasc. Dis. (Basel, Switzerland) 31(1), 12–18 (2011).
Boulouis, G. et al. Intracranial atherosclerosis and cerebral small vessel disease in intracerebral hemorrhage patients. J. Neurological Sci. 369, 324–329 (2016).
Chen, H. et al. Cerebral small vessel disease or intracranial large vessel atherosclerosis may carry different risk for future strokes. Stroke Vasc. Neurol. 5(2), 128–137 (2020).
Shen, M., Wei, G., Cheng, M. & Jiang, H. Association between enlarged perivascular spaces and internal carotid artery stenosis: A study in patients diagnosed by digital subtraction angiography. J. Stroke Cerebrovasc. 29(4), 104635 (2020).
Mak, W., Cheng, T. S., Chan, K. H., Cheung, R. T. & Ho, S. L. A possible explanation for the racial difference in distribution of large-arterial cerebrovascular disease: ancestral European settlers evolved genetic resistance to atherosclerosis, but confined to the intracranial arteries. Med. Hypotheses 65(4), 637–648 (2005).
Boden-Albala, B. et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke 39(1), 30–35 (2008).
Bang, O. Y. et al. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J. Neurological Sci. 284(1–2), 40–45 (2009).
Bang, O. Y. Intracranial atherosclerosis: current understanding and perspectives. J. Stroke 16(1), 27–35 (2014).
Tao, L. et al. Intracranial atherosclerotic plaque as a potential cause of embolic stroke of undetermined source. J. Am. Coll. Cardiol. 77(6), 680–691 (2021).
Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12(8), 822–838 (2013).
Doubal, F. N., MacLullich, A. M., Ferguson, K. J., Dennis, M. S. & Wardlaw, J. M. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41(3), 450–454 (2010).
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I. & Zimmerman, R. A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149(2), 351–356 (1987).
Staals, J., Makin, S. D., Doubal, F. N., Dennis, M. S. & Wardlaw, J. M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83(14), 1228–1234 (2014).
Swartz, R. H. et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 72(7), 627–634 (2009).
Cai, J. M. et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 106(11), 1368–1373 (2002).
Evans, N. R. et al. Carotid atheroinflammation is associated with cerebral small vessel disease severity. Front. Neurol. 12, 90935 (2021).
Wardlaw, J. M., Smith, C. & Dichgans, M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18(7), 684–696 (2019).
Xu, W. H. Large artery: an important target for cerebral small vessel diseases. Ann. Transl. Med. 2(8), 78 (2014).
Jung, K. W., Shon, Y. M., Yang, D. W., Kim, B. S. & Cho, A. H. Coexisting carotid atherosclerosis in patients with intracranial small- or large-vessel disease. J. Clin. Neurol. (Seoul, Korea) 8(2), 104–108 (2012).
Zhu, H., Li, Z., Lv, J. & Zhao, R. Effects of cerebral small vessel disease on the outcome of patients with ischemic stroke caused by large artery atherosclerosis. Neurol. Res. 40(5), 381–390 (2018).
Kandiah, N., Goh, O., Mak, E., Marmin, M. & Ng, A. Carotid stenosis: a risk factor for cerebral white-matter disease. J. Stroke Cerebrovasc. Dis. 23(1), 136–139 (2014).
Saba, L. et al. Association between the volume of carotid artery plaque and its subcomponents and the volume of white matter lesions in patients selected for endarterectomy. AJR Am. J. Roentgenol. 201(5), W747–W752 (2013).
Hong, N. R. et al. The correlation between carotid siphon calcification and lacunar infarction. Neuroradiology 53(9), 643–649 (2011).
Del Brutto, O. H., Mera, R. M., Gillman, J., Ha, J. E. & Zambrano, M. Calcifications in the carotid siphon correlate with silent cerebral small vessel disease in community-dwelling older adults: A population-based study in rural Ecuador. Geriatr. Gerontol. Int. 16(9), 1063–1067 (2016).
Webb, A. J. et al. Increased cerebral arterial pulsatility in patients with leukoaraiosis: Arterial stiffness enhances transmission of aortic pulsatility. Stroke 43(10), 2631–2636 (2012).
Mok, V. et al. Transcranial Doppler ultrasound for screening cerebral small vessel disease: A community study. Stroke 43(10), 2791–2793 (2012).
Ammirati, E. et al. Progression of brain white matter hyperintensities in asymptomatic patients with carotid atherosclerotic plaques and no indication for revascularization. Atherosclerosis 287, 171–178 (2019).
Fan, X. et al. Cerebral small vessel disease burden related to carotid intraplaque hemorrhage serves as an imaging marker for clinical symptoms in carotid stenosis. Front. Neurol. 12, 731237 (2021).
Bos, D. et al. Calcification in major vessel beds relates to vascular brain disease. Arteriosclerosis Thromb. Vasc. Biol. 31(10), 2331–2337 (2011).
Greenberg, S. M. et al. Cerebral microbleeds: A guide to detection and interpretation. Lancet Neurol. 8(2), 165–174 (2009).
Xu, W. H. et al. Intracranial artery atherosclerosis and lumen dilation in cerebral small-vessel diseases: A high-resolution MRI Study. CNS Neurosci. Therap. 20(4), 364–367 (2014).
Funding
The work was supported by grants from the Science and Technology Project Plan of Liao Ning Province (2022JH2/101500020).
Author information
Authors and Affiliations
Contributions
K.L.Z., Z.Y.S., B.J.L., Y.W. and J.L. retrospectively enrolled patients, acquired the data and did the literature search. K.L.Z. wrote the paper. K.L.Z. and Z.Y.S. did statistical analysis. B.Q.Y acquired the imaging data. H.S.C. designed the study and critically revised the manuscript. G.N. critically revised the manuscript. All authors approved the content of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, KL., Shang, ZY., Liu, Bj. et al. The association of intracranial atherosclerosis with cerebral small vessel disease imaging markers: a high-resolution magnetic resonance imaging study. Sci Rep 13, 17017 (2023). https://doi.org/10.1038/s41598-023-44240-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44240-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.