Abstract
Nemonoxacin is a novel non-fluorinated quinolone with strong antibacterial efficacy, but data of its effect on acute exacerbations of chronic obstructive pulmonary disease (AECOPD) is rare. This study was conducted to compare the efficacy of oral nemonoxacin with moxifloxacin in AECOPD outpatients. In this retrospective observational study, a total of 101 AECOPD outpatients initially treated with nemonoxacin or moxifloxacin from July 2021 to March 2022 were enrolled. We collected COPD assessment test (CAT), Transition Dyspnea Indices (TDI) scores, and exacerbations information during 24 weeks follow-up from the electronic medical records. Kaplan–Meier curve was used to analyze the time to the next moderate/severe exacerbation. Compared to the moxifloxacin group, changes in CAT scores and TDI scores were significantly higher in the nemonoxacin group, and the nemonoxacin group also had a greater probability to reach the minimal clinically important difference of CAT (71.40% vs. 97.80%, p < 0.01) and TDI (40.50% vs. 60.00%, p < 0.05) at week 4. Despite no significant difference in the incidence of exacerbations between two groups, patients treated with nemonoxacin had a significantly prolonged time to next moderate/severe exacerbation than those with moxifloxacin (p < 0.05). Nemonoxacin achieved a better symptomatic improvement and a prolonged interval to next moderate/severe exacerbation for AECOPD outpatients.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) places a significant health burden to China1, and acute exacerbations of COPD (AECOPD) cause substantial morbidity, mortality and marked reduction in lung function and quality of life2. Bacterial infection is a common reason for AECOPD, and possible causative pathogens varied from Hemophilus influenza to Pseudomonas species2,3,4,5. Thus, antibiotics are recommended in AECOPD patients with at least two cardinal symptoms, including increased purulence of sputum2,3. There was an abundance of evidence that antibiotic treatments could improve symptoms, reduce short-term mortality and the recurrence of exacerbations in AECOPD patients6,7,8. In addition, antibiotics could decrease the utilization of in-hospital health care for AECOPD outpatients2.
It is widely known that moxifloxacin and levofloxacin have been recommended to be used in the treatment of AECOPD3,9,10. Nemonoxacin is another innovative C8-methoxy non-fluorinated quinolone, which inhibits DNA synthesis through bacterial DNA gyrase, and is currently being used in clinical practice for treatments of common infectious diseases including community-acquired pneumonia (CAP), Clostridium difficile infections (CDIs) and so on11,12. In vitro activity studies demonstrated that nemonoxacin had a higher antibacterial activity against Gram-positive cocci and atypical pathogens than other quinolones (such as moxifloxacin and levofloxacin), while its activity against Gram-negative bacilli was similar to that of moxifloxacin and levofloxacin13,14. In addition, nemonoxacin also has a potent antimicrobial efficacy against antibiotic-resistant organisms like methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant Streptococcus pneumoniae13,14,15. Based on its non-inferiority antibacterial activity compared to other quinolones, nemonoxacin might be a preferable option for pulmonary infections.
Although lack of reliable evidence in randomized controlled trials (RCTs), Chinese consensus on AECOPD has recommended that nemonoxacin could be empirically used in the antimicrobial treatment of AECOPD4, owing to its broad antibacterial spectrum and potent antibacterial efficacy15. Moxifloxacin has been demonstrated in RCTs with good efficacy in AECOPD patients10,16. However, little is known about whether nemonoxacin could achieve the same clinical improvement in AECOPD patients as moxifloxacin. Therefore, this study was conducted to compare the efficacy of oral nemonoxacin with that of moxifloxacin for the treatment of outpatients with AECOPD.
Methods
Study design and population
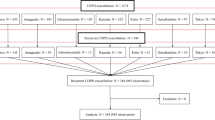
We performed a retrospective observational study using the database of outpatients with COPD in the Second Xiangya Hospital of Central South University. Details of these patients’ populations have been presented elsewhere17. To compare the efficacy of oral nemonoxacin with moxifloxacin in outpatients with AECOPD, we enrolled patients from the database of outpatients with COPD in the Second Xiangya Hospital of Central South University between July 1, 2021 and March 30, 2022. The inclusion criteria were as follows: (1) AECOPD with more symptoms including increased in sputum purulence; (2) age ≥ 40 years; (3) patients received nemonoxacin (500 mg once daily for 6–9 days) or moxifloxacin (400 mg once daily for 6–9 days) as initial antimicrobial treatment regimens. Diagnosis and severity of COPD were confirmed by spirometry according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)2. Patients with other chronic respiratory diseases such as asthma, pulmonary fibrosis, or lung cancer were excluded; and patients with pneumonia, other infection diseases, or who had been treated with antibiotics within 4 weeks before enrollment were also excluded. All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki.
Extracted information included: age; sex; body mass index (BMI); educational level; residence; the smoking history; acute exacerbations (AEs) in the previous year; inhalers; COPD Assessment Test (CAT) scores at baseline and follow-up; Baseline Dyspnea Index (BDI) at baseline; Transition dyspnea index (TDI) at follow-up; spirometry obtained in the year prior to exacerbation ascertainment; laboratory results; incidence of exacerbations during 24 weeks follow-up and underlying comorbidities.
Measurements
The CAT has been developed to provide a simple and reliable measure of disease-specific health status. The CAT consists of eight items (cough, phlegm, chest tightness, breathlessness, limited activities, confidence in leaving home, sleeplessness and energy) defined with contrasting adjectives. Item scores range from 0 to 5 points resulting in a CAT total score ranging from 0 to 40 points. The minimum clinically important difference (MCID) for CAT has been reported as a 2-point reduction18,19.
The Baseline and Transition Dyspnea Indices (BDI/TDI) provide measurements of breathlessness and of its impact on activities of daily living and 1-point increase is considered as the MCID for TDI20,21.
Outcomes
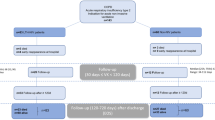
Patients’ clinical notes were reviewed three times: at baseline, at Week 4 post-therapy (follow-up visit 1), and at Week 24 post-therapy (follow-up visit 2) to require details about: (1) changes from baseline in CAT scores and changes in TDI scores at Week 4 and Week 24; (2) the effectiveness of antibiotics based on the response rate of the MCID of CAT and TDI during 4 weeks and 24 weeks follow-up; (3) the incidence of exacerbations during 4 weeks and 24 weeks follow-up; (4) the time to first moderate/severe exacerbation during 24 weeks follow-up.
Statistical analysis
Data are expressed as median values with interquartile ranges or means ± standard deviations. Statistical significance was reported using the Pearson’s χ2 test or Fisher’s exact test for categorical variables, and the Student’s t test, ANOVA or the Mann–Whitney U test for continuous variables. Kaplan–Meier curve with log-rank test was used to analyze the time to the first moderate/severe exacerbation during 24 weeks follow-up. A p value of < 0.05 was considered significant. All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University with reference number 2018-040. All participants provided written informed consent.
Results
Demographics and clinical characteristics
This study included 101 AECOPD patients with mean age of 64.12 ± 7.51 years (range 52–86 years). The total number of male patients was 97 (96.0%) (Table 1). Of the 101 patients, 50 (49.5%) were treated with nemonoxacin and 51 (50.5%) were treated with moxifloxacin. The median number of exacerbations in the previous year was 1.00 (IQR: 0.00–2.00) for all patients. The median duration of initial antibiotic therapy was 6.00 (IQR: 6.00–9.00) days for all patients and the median FEV1% predicted was 47.60 (IQR: 37.17–58.40) (Table 1). No significant difference was found in the proportion of GOLD stages 3 and 4 between the nemonoxacin group and the moxifloxacin group (66.0% vs. 45.1%, p = 0.083). There was no significant difference in inhalers and other medicines use. No differences were found at baseline characteristics and laboratory results [blood routine, serum C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)] between the two groups (Tables 1, 2).
Improvement of CAT
Overall, CAT total scores at week 4 and week 24 decreased significantly when compared with the scores at baseline in both the nemonoxacin and moxifloxacin groups (Table S1). However, the decline of CAT total scores in the nemonoxacin group was significantly greater than that in the moxifloxacin group either at week 4 or week 24 [− 12.00 (IQR: − 16.00 to − 6.00) vs. − 9.00 (IQR: − 16.00 to 0.00), p < 0.05; − 13.50 (IQR: − 20.75 to − 8.75) vs. − 10.50 (IQR: − 15.25 to − 6.50), p < 0.05, respectively, Table 3].
The nemonoxacin group had a higher proportion of patients reaching the MCID of CAT than moxifloxacin at Week 4 (97.80% vs. 71.40%, p < 0.01, Fig. 1A). However, there was no significant difference in the proportion of patients reaching the MCID of CAT between groups at Week 24 (95.10% vs. 92.10%, Fig. 1C).
Comparison of the MCID response rate in CAT scores and TDI scores between the moxifloxacin group and the nemonoxacin group. (A) MCID response rate in CAT scores after 4 weeks; (B) MCID response rate in TDI scores after 4 weeks; (C) MCID response rate in CAT scores after 24 weeks; (D) MCID response rate in TDI scores after 24 weeks. CAT COPD assessment test, TDI transition dyspnea indices, MCID minimal clinically important differences, RR risk ratio.
Individual item scores for respiratory items were significantly improved in the nemonoxacin group than that in the moxifloxacin group at week 4, such as ‘phlegm’ [− 2.00 (IQR: − 2.00 to − 1.00) vs. − 1.00 (IQR: − 2.00 to 0.00), p < 0.05, Table 3] and ‘chest tightness’ [− 2.00 (IQR: − 3.00 to − 1.00) vs. − 1.00 (IQR: − 2.00 to 0.00), p < 0.01, Table 3]. Moreover, individual item scores for items ‘cough’ [− 2.00 (IQR: − 3.00 to − 1.00) vs. − 1.00 (IQR: − 2.00 to 0.00), p < 0.05, Table 3] and ‘phlegm’ [− 2.00 (IQR: − 3.00 to − 1.00) vs. − 1.00 (IQR: − 2.00 to 0.00), p < 0.05, Table 3] were also significantly improved in the nemonoxacin than moxifloxacin groups at week 24.
Accordingly, the percentages of patients reporting an improvement (≤ − 1 point) in eight CAT items also showed significant differences after moxifloxacin and nemonoxacin therapy. Compared with moxifloxacin, the percentages of an improvement in CAT items ‘phlegm’, ‘chest tightness’, ‘breathlessness’ and ‘confidence in leaving home’ were higher in the nemonoxacin group at week 4 (Table S2, Fig. 2A). At week 24, the percentages of reporting an improvement in CAT items ‘cough’, ‘chest tightness’ and ‘confidence in leaving home’ were higher after nemonoxacin treatment (Table S2, Fig. 2B).
Improvement of TDI
Dyspnea, evaluated using TDI total scores, improved significantly in the nemonoxacin group than that in the moxifloxacin group at week 4 [3.00 (IQR: 0.00–5.25) vs. 1.00 (IQR: − 5.00 to 6.00), p < 0.05, Table 4]. And the TDI effort scores were also significantly higher in the nemonoxacin group [0.00 (IQR: 0.00–2.00) vs. 0.00 (IQR: − 3.00 to 2.00), p < 0.05, Table 4] at week 4. However, no significant between-group differences in TDI scores were observed at week 24.
Compared to the moxifloxacin group, the nemonoxacin group was more likely to reach the MCID of TDI (40.50% vs. 60.00%, p < 0.05, Fig. 1B) at Week 4. While the proportion of patients reaching the MCID of TDI was similar between groups at Week 24 (55.30% vs. 61.00%, Fig. 1D).
Acute exacerbations of COPD
There were no deaths during 24 weeks follow-up. The median number of exacerbations during 24 weeks follow-up was 0.00 (IQR: 0.00–1.00). The incidence rate of exacerbations was 5.7% during 4 weeks follow-up, and was 36.7% during 24 weeks follow-up, indicating that the study population had a high probability to experience next exacerbation. Between the nemonoxacin and the moxifloxacin groups, the incidence rates of total exacerbations (2.2% vs. 9.5% at week 4, 31.7% vs. 42.1% at week 24, Table 5) and moderate/severe exacerbations (2.2% vs. 9.5% at week 4, 24.4% vs. 42.1% at week 24, Table 5) were similar.
Besides that, the median time to the next exacerbation of the whole population was 96.50 days and there was no significant difference in the median time to next exacerbation between the nemonoxacin and moxifloxacin groups (116.00 days vs. 86.00 days). Interestingly, the Kaplan–Meier curve found that patients treated with nemonoxacin had a significantly prolonged time to next moderate/severe exacerbation (p < 0.05, Fig. 3), compared to moxifloxacin.
Discussion
This retrospective observational study compared the efficacy of oral nemonoxacin with that of moxifloxacin for the treatment of AECOPD outpatients. Moxifloxacin is a well-accepted antibiotic in the treatment of AECOPD2,10, and nemonoxacin is also slightly recommended in Chinese consensus on AECOPD without abundance data4. However, litter is known about whether there are differences between the two quinolones in the treatment of AECOPD in real world. This study found that initial treatment with nemonoxacin in AECOPD outpatients could achieve a better symptomatic improvement assessed by CAT and TDI scales, and a longer interval to next moderate/severe exacerbation in comparison with moxifloxacin.
It is widely known that most incidences of exacerbations in COPD are associated with frequent bacterial infections5,22. Thus, antibiotics play a pivotal role in the treatment regimens. The MAESTRAL study found that moxifloxacin resulted in significantly high clinical success rates and high bacterial eradication for outpatients with moderate-to-severe AECOPD with a suspected bacterial etiology10. Llor et al.23 reported that amoxicillin/clavulanate is more effective and significantly prolongs the time to the next exacerbation in moderate exacerbations of mild-to-moderate COPD compared with placebo. These clinical trials confirmed clinical (such as symptomatic improvements, fewer exacerbations, etc.) or bacteriological (bacterial eradication) superiority of antibiotics in the treatment of AECOPD. The CAT and BDI/TDI scales are frequently used measurement tools to evaluate patients’ symptoms in clinical practice24. Several studies recently demonstrated that monitoring the changes of the CAT scores during an AECOPD could assess the treatment response25,26. This study evaluated the response to antibiotics in AECOPD outpatients by tracking CAT and TDI score changes. In line with previous studies, our results showed significantly improved symptom scores after nemonoxacin and moxifloxacin treatment. It indicated the good efficacy of both nemonoxacin and moxifloxacin in AECOPD outpatients, which may be explained by their effective antibacterial activity against common pathogens causing exacerbations13.
Moreover, this study observed more obvious improvements in total CAT and TDI scores of AECOPD outpatients treated with nemonoxacin rather than moxifloxacin during follow-up, indicating that nemonoxacin may be better than moxifloxacin in terms of symptom reduction during the treatment of exacerbations. Chinese consensus on AECOPD demonstrated that the three most common pathogens are H. influenzae, Moraxella catarrhalis, and S. pneumoniae respectively in AECOPD4. One study in Canada reported that nemonoxacin was at least fourfold more active than moxifloxacin against most Gram-positive cocci13. Liu et al.27 found that nemonoxacin had stronger antibacterial activity against S. pneumoniae, S. aureus (including MRSA), H. influenzae and Klebsiella pneumoniae strains than levofloxacin in the treatment of CAP. What’s more, in vivo and in vitro studies demonstrated that nemonoxacin had less minimum inhibitory concentrations (MIC) than moxifloxacin and levofloxacin, against some Gram-positive or negative bacteria11,28. These studies suggest that nemonoxacin might be a promising choice for bacterial infections with better antibacterial activity than other quinolones, and could explain a better clinical outcome from nemonoxacin than moxifloxacin in our results.
Although microbiological eradication rates are generally considered to be an important indicator for effective antibacterial therapy29, the effectiveness of antibiotics for outpatients cannot be determined only by this factor because sputum culture is sometimes limited in the outpatient setting due to time-consuming (at least 2 days) and technical reasons8,30. The evidence based on sputum culture results suggests that purulence of sputum is the most sensitive indicator to judge the elevated bacterial load of the lower respiratory tract4,30. Accordingly, the more significant improvement in individual CAT item ‘phlegm’ in nemonoxacin-treated patients could also confirm the better efficacy in symptomatic improvements and lower bacterial burden in the nemonoxacin group than the moxifloxacin group.
It should be pointed out that our study population was at high risk of experiencing next exacerbation, with 36.7% of the relapse rate and 96.50 days of the median time to next exacerbation during 24 weeks follow-up. Previous studies indicated that the recurrence is associated with persistent airway and systemic inflammation and thereby structural airway damage31. One possible explanation of this persistent inflammation is incomplete resolution of airway bacterial infections. Despite no significant differences in the relapse rates of exacerbations between two groups, nemonoxacin had a significantly longer time to the next moderate/severe exacerbation. Evidence from clinical trials indicated that COPD patients with improved health status or lower CAT scores had a lower likelihood of exacerbation24. Based on the potent strong efficacy against pathogens of nemonoxacin, this result may indicate more effective bacterial eradication and better health status in patients treated with nemonoxacin.
The study also has some limitations. First, this was a single-center, retrospective, observational study with a small sample in China, which limits its generalizability to other populations. Second, we lack bacteriological assessment, which precludes a correlation with the clinical outcomes. A further prospective clinical trial with larger sample is required.
Conclusion
This retrospective observational study indicated that oral nemonoxacin was superior to oral moxifloxacin in rapid symptom improvement for initial antimicrobial treatment of outpatients with AECOPD. As an antibiotic with a broad antibacterial spectrum, strong antibacterial efficacy, and less tendency to being resistant, nemonoxacin may be a useful option for the treatment of AECOPD outpatients.
Data availability
The datasets presented in this article are not readily available because “The raw data supporting the conclusions of this article will be made available with reasonable request.” Requests to access the datasets should be directed to “chenyan99727@csu.edu.cn”.
References
Wang, C. et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 391, 1706–1717. https://doi.org/10.1016/s0140-6736(18)30841-9 (2018).
Committee, G. E. (2020).
CTS, C. G. o. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021). Chin. J. Tuberc. Respir. Dis. 44, 170–205. https://doi.org/10.3760/cma.j.cn112147-20210109-00031 (2021).
Disease, E. G. O. o. M. o. A. E. o. C. O. P. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in China (Updated 2017). Int. J. Respir. 37, 1041–1057. https://doi.org/10.3760/cma.j.issn.1673-436X.2017.14.001 (2017).
White, A. J. et al. Resolution of bronchial inflammation is related to bacterial eradication following treatment of exacerbations of chronic bronchitis. Thorax 58, 680–685. https://doi.org/10.1136/thorax.58.8.680 (2003).
Miravitlles, M. et al. Sputum colour and bacteria in chronic bronchitis exacerbations: A pooled analysis. Eur. Respir. J. 39, 1354–1360. https://doi.org/10.1183/09031936.00042111 (2012).
Stockley, R. A., O’Brien, C., Pye, A. & Hill, S. L. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 117, 1638–1645. https://doi.org/10.1378/chest.117.6.1638 (2000).
Vollenweider, D. J., Frei, A., Steurer-Stey, C. A., Garcia-Aymerich, J. & Puhan, M. A. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 10, Cd010257. https://doi.org/10.1002/14651858.CD010257.pub2 (2018).
Petitpretz, P., Choné, C. & Trémolières, F. Levofloxacin 500 mg once daily versus cefuroxime 250 mg twice daily in patients with acute exacerbations of chronic obstructive bronchitis: Clinical efficacy and exacerbation-free interval. Int. J. Antimicrob. Agents 30, 52–59. https://doi.org/10.1016/j.ijantimicag.2006.11.033 (2007).
Wilson, R. et al. Moxifloxacin versus amoxicillin/clavulanic acid in outpatient acute exacerbations of COPD: MAESTRAL results. Eur. Respir. J. 40, 17–27. https://doi.org/10.1183/09031936.00090311 (2012).
Chen, Y. H., Liu, C. Y., Lu, J. J., King, C. H. & Hsueh, P. R. In vitro activity of nemonoxacin (TG-873870), a novel non-fluorinated quinolone, against clinical isolates of Staphylococcus aureus, enterococci and Streptococcus pneumoniae with various resistance phenotypes in Taiwan. J. Antimicrob. Chemother. 64, 1226–1229. https://doi.org/10.1093/jac/dkp370 (2009).
Huang, C. H., Lai, C. C., Chen, Y. H. & Hsueh, P. R. The potential role of nemonoxacin for treatment of common infections. Expert Opin. Pharmacother. 16, 263–270. https://doi.org/10.1517/14656566.2015.978288 (2015).
Adam, H. J. et al. In vitro activity of nemonoxacin, a novel nonfluorinated quinolone, against 2,440 clinical isolates. Antimicrob. Agents Chemother. 53, 4915–4920. https://doi.org/10.1128/aac.00078-09 (2009).
Lauderdale, T. L., Shiau, Y. R., Lai, J. F., Chen, H. C. & King, C. H. Comparative in vitro activities of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, and other quinolones against clinical isolates. Antimicrob. Agents Chemother. 54, 1338–1342. https://doi.org/10.1128/aac.01197-09 (2010).
Li, C. R. et al. In vivo antibacterial activity of nemonoxacin, a novel non-fluorinated quinolone. J. Antimicrob. Chemother. 65, 2411–2415. https://doi.org/10.1093/jac/dkq341 (2010).
Chuchalin, A. et al. Efficacy and safety of moxifloxacin in acute exacerbations of chronic bronchitis: A prospective, multicenter, observational study (AVANTI). BMC Pulm. Med. 13, 5. https://doi.org/10.1186/1471-2466-13-5 (2013).
Meng, W. W. et al. Reliability and validity of the Chinese version of the test of the adherence to inhalers (TAI). Zhonghua Jie He He Hu Xi Za Zhi 45, 423–430. https://doi.org/10.3760/cma.j.cn112147-20211108-00783 (2022).
Houben-Wilke, S. et al. Contribution of individual COPD assessment test (CAT) items to CAT total score and effects of pulmonary rehabilitation on CAT scores. Health Qual. Life Outcomes 16, 205. https://doi.org/10.1186/s12955-018-1034-4 (2018).
Kon, S. S. et al. Minimum clinically important difference for the COPD Assessment Test: A prospective analysis. Lancet Respir. Med. 2, 195–203. https://doi.org/10.1016/s2213-2600(14)70001-3 (2014).
Mahler, D. A. & Witek, T. J. Jr. The MCID of the transition dyspnea index is a total score of one unit. COPD 2, 99–103. https://doi.org/10.1081/copd-200050666 (2005).
Rodrigo, G. J. & Neffen, H. Comparison of indacaterol with tiotropium or twice-daily long-acting β -agonists for stable COPD: A systematic review. Chest 142, 1104–1110. https://doi.org/10.1378/chest.11-2252 (2012).
Sethi, S. & Murphy, T. F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 359, 2355–2365. https://doi.org/10.1056/NEJMra0800353 (2008).
Llor, C., Moragas, A., Hernández, S., Bayona, C. & Miravitlles, M. Efficacy of antibiotic therapy for acute exacerbations of mild to moderate chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 186, 716–723. https://doi.org/10.1164/rccm.201206-0996OC (2012).
Gupta, N., Pinto, L. M., Morogan, A. & Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 44, 873–884. https://doi.org/10.1183/09031936.00025214 (2014).
Tu, Y. H., Zhang, Y. & Fei, G. H. Utility of the CAT in the therapy assessment of COPD exacerbations in China. BMC Pulm. Med. 14, 42. https://doi.org/10.1186/1471-2466-14-42 (2014).
Zhou, A. et al. The role of CAT in evaluating the response to treatment of patients with AECOPD. Int. J. Chronic Obstr. Pulm. Dis. 13, 2849–2858. https://doi.org/10.2147/copd.S175085 (2018).
Liu, Y. et al. A randomized, double-blind, multicenter Phase II study comparing the efficacy and safety of oral nemonoxacin with oral levofloxacin in the treatment of community-acquired pneumonia. J. Microbiol. Immunol. Infect. 50, 811–820. https://doi.org/10.1016/j.jmii.2015.09.005 (2017).
Lai, C. C. et al. Comparative in vitro activities of nemonoxacin, doripenem, tigecycline and 16 other antimicrobials against Nocardia brasiliensis, Nocardia asteroides and unusual Nocardia species. J. Antimicrob. Chemother. 64, 73–78. https://doi.org/10.1093/jac/dkp144 (2009).
Rhee, C. K. et al. Zabofloxacin versus moxifloxacin in patients with COPD exacerbation: A multicenter, double-blind, double-dummy, randomized, controlled, Phase III, non-inferiority trial. Int. J. Chronic Obstr. Pulm. Dis. 10, 2265–2275. https://doi.org/10.2147/copd.S90948 (2015).
Wilson, R., Sethi, S., Anzueto, A. & Miravitlles, M. Antibiotics for treatment and prevention of exacerbations of chronic obstructive pulmonary disease. J. Infect. 67, 497–515. https://doi.org/10.1016/j.jinf.2013.08.010 (2013).
Sethi, S. & Aaron, S. D. Antibiotic retreatment for acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 202, 481–482. https://doi.org/10.1164/rccm.202004-0896ED (2020).
Funding
This research was supported by the Research Foundation of Nemonoxacin for Community Acquired Lower Respiratory Tract Infection (TJX024), the National Natural Science Foundation of China (82070049, 81400032) and the Natural Science Foundation of Hunan Province (2022JJ30060, 2019JJ50877, 09JJ3036).
Author information
Authors and Affiliations
Contributions
W.M and H.Z collected and analyzed the data and wrote the main manuscript text; Z.Z and R.X collected the data and revised the article; Y.C and Z.L designed the study and revised the article. All authors reviewed the manuscript and gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meng, W., Zeng, H., Zhao, Z. et al. Nemonoxacin achieved a better symptomatic improvement and a prolonged interval to next exacerbation than moxifloxacin for outpatients with acute exacerbations of chronic obstructive pulmonary disease. Sci Rep 13, 16954 (2023). https://doi.org/10.1038/s41598-023-44188-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44188-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.