Abstract
In Egypt, sunflower charcoal-rot caused by Macrophomina phaseolina and maize late-wilt caused by Magnaporthiopsis maydis are the most prevalent, and can lead to huge yield losses of both crops under epidemic conditions. In this study, the potential use of vermitea and wood vinegar for management of both diseases was investigated. Data revealed that, among the 17 bacterial strains obtained from vermitea, three strains named VCB-2, VCB-7 and VCB-11 were chosen for having the greatest in vitro inhibitory effect against M. phaseolina and M. maydis, with fungal inhibition values of 54.2; 61.7, 65.2; 74.0 and 57.1; 87.0% against both pathogens, respectively. These strains were identified as Bacillus amyloliquefaciens, Serratia marcescens and Bacillus velezensis, respectively. Wood vinegar significantly reduced the colony diameter of M. phaseolina and M. maydis in in vitro trials conducted on potato dextrose agar medium amended with the desired concentrations of 0.5, 1.0, 1.5, 2.0, and 2.5%. The efficiency increased with increasing wood vinegar concentration, and 2.0% was the most effective (100% suppression). Data from greenhouse experiments showed that the application of vermitea or wood vinegar tended to decrease the incidence (% dead plants) of sunflower charcoal-rot (by 61.1 and 66.7%) and maize late-wilt (by 70.6%). These treatments had positive impacts on the plant growth parameters, photosynthetic pigments and antioxidative enzymes of sunflower and maize plants. Data from field experiments showed that the application of vermitea or wood vinegar decreased the incidence of charcoal-rot (by 72.8 and 72.0%) and late-wilt (by 88.7 and 87.0%) as well as increased the production sunflower and maize plants.
Similar content being viewed by others
Introduction
Sunflower (Helianthus annuus L.) as the member of Asteraceae family is a significant global oil seed crop. It is a short-season crop (90–100 days) that can be grown twice a year, and there are significant efforts underway to expand the area under cultivation in order to increase local production of edible oil in Egypt. Unfortunately, several diseases, particularly charcoal rot disease, have a negative impact on sunflower production in Egypt1,2. Charcoal-rot is caused by the fungus Macrophomina phaseolina (Tassi) Goid (the pecnidial stage of Scletroium bataticola Taub.), which has a host range of more than 500 plant species3. Symptoms of charcoal-rot typically appear at the end of the growing season, after flowering3. Stunting, chlorosis and wilting are common symptoms2. It also causes lower yield and early senescence4. The development of microsclerotia at the base of the stems, which make them appear grey, is what is referred to as "charcoal-rot"5. The main source of inoculum is microsclerotia, which serve as survival structures and can germinate near roots during 2 days. However, germination can continue throughout the growing season. Root infection occurs very early in the season, but the factors influencing root colonization rate are not fully understood6. Macrophomina phaseolina infects and stays in plant tissue at the seedling stage. When conditions are favorable for disease development, which is typically when additional stress on the plant increases susceptibility to the pathogen, symptoms of charcoal-rot was appeared7.
On another way, maize (Zea mays L.) is considered the most significant cereal crops in Egypt. The most devastating or upsetting maize disease, late-wilt, which is caused by the soil-borne fungus Magnaporthiopsis maydis (also known as Cephalosporium maydis (Samra, Sabet, and Hingorani) and Harpophora maydis), results in significant yield and quality losses8,9. About 50 to 60 days after sowing, the plant begins to flower, at which point the first symptoms appear. The first signs are sudden wilting of nearby leaves, which advance upward over the following 2 weeks10. As the disease worsens, the leaves gradually lose their color and become dehydrated. Reddish-brown to yellowish streaks may be seen on the lower internode. The lower stem has dried out by that time, especially at the internodes, and looks hollow and shrunken. Later, M. maydis colonizes the entire stalk, suffocating the water supply and causing wilting by blocking the vascular tissue with hyphae and deposits that resemble gum. Secondary pathogen infection is frequently linked to late-wilt, which worsens the symptoms of the stem. Less ear growth occurs, and when it does, the kernels that do form are frequently damaged and immature and may harbor the pathogen. Disease severity is negatively correlated with grain supply and quality. These harmful processes may ultimately cause the plant’s death11.
Different disease management strategies have been put into practice to fight and eradicate sunflower charcoal-rot and maize late-wilt. Among them are cultural, physical, and biological techniques12,13,14,15. All of these techniques affect when used beforehand as a preventative measure16. As soon as a disease appeared, these techniques are no longer useful or effective. In that situation, chemical control is a good option for disease control. Chemical pesticides have been around for a while, and they offer quick, efficient, and affordable disease management17. The use of chemicals in agriculture has recently been shown to be less advantageous than previously believed. Both the person applying the chemical and the person using the treated material are at serious health risk. In addition to the target organism, pesticides also kill a number of beneficial organisms. Also, chemical pesticides continue to exist in toxic form in the soil, which pollutes the entire environment. Byproducts have taken a significant step in place of systemic materials due to humankind's growing awareness of its impact on ecosystems and the environment18.
Vermicompost (also known as organic fertilizer) is a byproduct of farmyard manure digested by the earthworm Eisenia foetida and the microorganisms that live within it. It has been widely used in organic agriculture not only for its beneficial effects on soil quality, but also for its remarkable ability to inhibit plant pathogens and promote plant growth19. Vermitea is a liquid vermicompost solution made by combining vermicast with water and fermenting it for a set period of time20. There are two kinds of vermitea: aerated and non-aerated vermitea. Nutrients and microorganisms are extracted during the steeping of vermicompost in water. The presence of microorganisms converts insoluble nutrients into soluble forms, which in turn promote a diverse range of organisms in vermitea during the brewing process. Both methods (aerated and non-aerated) of producing vermitea involve brewing matured vermicompost in water for a specific period of time and require filtration before application to plants21. Vermitea treatments have been shown to inhibit or kill a variety of fungi, parasitic nematodes, and other pests22,23.
A reddish-brown condensate liquid byproduct of the fresh wood gas combustion in an airless environment is called "wood vinegar," as one of biochar derivatives also known as "pyroligneous acid". Acetic acid, methanol, acetone, wood oils, and tars are all present in the liquid that is formed when the gas cools. It is widely used as a wood preservative, in health care products, and in agriculture. Liquid smoke, liquid wood, liquid vinegar, pyrolysis oil, pyrolysis liquid, wood liquid, wood acid, bio-oil, bio-crude oil, and wood distillate are synonyms for the wood vinegar24. Several substances, including hydroxy aldehydes, hydroxy ketones, sugars, carboxylic acid, and phenolic acid, are present in wood vinegar25. Recently, it has been used in agriculture (as a weed-control agent, anti-bacterial, and anti-fungal agent), the food industry, pharmaceuticals, and healthcare26,27. It is a cheap, all-natural product that doesn't harm living things in the environment28. According to the study of Matnork et al.29, it inhibits a number of fungal plant pathogens. However, no studies on the antifungal activity of byproducts (virmitea or wood vinegar) against sunflower charcoal-rot and maize late-wilt have been published. So, the goal of the current study was to estimate the effectiveness of vermitea and wood vinegar against M. phaseolina-caused charcoal-rot of sunflower and M. maydis-causes late-wilt of maize in vitro, in pots and field trials.
Results
Laboratory trials
Antagonistic activity of vermitea bacteria
In vitro tests were performed on 17 bacterial strains obtained from vermitea and tested for their antagonistic activity against M. phaseolina and M. maydis (Table 1). Among them, three bacterial strains were found to have a significant antagonistic effect against the tested phytopathogens (Fig. 1). Macrophomina phaseolina growth inhibition percentages of 54.2, 65.2, and 57.1% were found in PDA plates treated with the three bacterial strains i.e., VCB2, VCB7, and VCB11, respectively. These strains had, also, the most antagonistic effect on M. maydis, inhibiting growth by 61.7, 74.0, and 87.0%, respectively. Of all, the strains VCB2, VCB7 and VCB11 had the greatest antagonistic effect on both pathogens, showing statistically significant differences respect to the other strains (P = 0.05) (Table 1). In contrast to M. phaseolina, M. maydis was more susceptible to all bacterial strains. The three strains VCB2, VCB7, and VCB11 were discovered to be the most noticeable and effective in inhibiting the growth of both pathogens in plate assays. Sequencing was performed on the PCR products from these strains (VCB2, VCB7, and VCB11), and the sequencing results were then compared for homology with the reported sequences listed in GenBank. These strains showed 99% homology with Bacillus amyloliquefaciens, Serratia marcescens, and B. velezensis, respectively, according to 16S rDNA gene sequence analysis. As a result, these strains were given the accession numbers OP546100, OP546101, and OP346607, respectively, when they added to the GenBank database (Fig. 2A–C).
Phylogeny analysis of vermtea bacteria and maize late-wilt pathogen Magnaporthiopsis maydis. (A–C) Phylogenetic tree based on 16S rDNA ITS sequence of B. amyloliquefaciens, Serratia marcescens and Bacillus velezensis (bold) and their closely related species and outgroup retrieved from the literature; (D) Phylogenetic tree based on 18S rDNA ITS sequence of M. maydis (bold) and its closely related species and outgroup retrieved from the literature.
Antifungal activity of wood vinegar
Table 2 displays the mycelium growth and percentage growth inhibition of wood vinegar against the charcoal-rot fungus M. phaseolina and late-wilt fungus M. maydis at concentrations ranging from 0.5 to 2.5% (v/v). In both fungi, wood vinegar performed better with increasing concentration and had antifungal activity in all concentrations (Fig. 3). Both pathogenic fungi colonies were able to grow on PDA mixed with wood vinegar at 0.5 and 1.0%. The means of the colony diameters of M. phaseolina and M. maydis were 7.7, 4.9, and 6.3, 2.8 cm, respectively. On the other hand, M. phaseolina, with a colony diameter of 3.8 cm, was able to grow on PDA mixed with wood vinegar at a 1.5%. Notably, hypha grew only upwards and not sideways. At 1.5, 2.0, and 2.5%, wood vinegar inhibited the late-wilt fungus, M. maydis, completely. However, at these concentrations, wood vinegar inhibited the charcoal-rot fungus, M. phaseolina by 57.8, 100 and 100%, respectively. PDA medium not mixed with wood vinegar showed no inhibition (Fig. 3).
Greenhouse trials
Effect on plant growth promotion
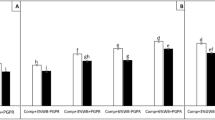
Vermitea and wood vinegar treatments significantly (P = 0.05) increased plant height, fresh weight and dry weight of the two crops (sunflower and maize) when compared to controls (Fig. 4). The plant height in the sunflower and maize negative controls was 88.0 and 105.1 cm, respectively, whereas the plant height in the sunflower and maize positive controls was reduced by 24.5 and 12.2% as a result of M. phaseolina and/or M. maydis inoculation. Treatments with vermitea or wood vinegar significantly increased plant height in comparison to the positive controls in both crops (Fig. 4). Vermitea or wood vinegar treatments against M. phaseolina and/or M. maydis stress significantly increased plant height in sunflower and maize, which increasing plant height by 36.5; 37.0, and 24.6; 24.4%, respectively. Sunflower and maize plant’s fresh weights were decreased by 32.9 and 13.3%, respectively, after M. phaseolina and/or M. maydis were introduced to the soil. When compared to the positive control, the vermitea and wood vinegar treatments significantly reduced fungal stress and increased fresh weight of the two crops (Fig. 4). The most significant impact on fresh weight against M. phaseolina and/or M. maydis stress was provided by these treatments, which had 179.9; 180.9, and 91.4; 91.0 g plant−1 in sunflower and maize, respectively (Fig. 4). The negative controls had dry weights of 10.5 and 6.4 g plant−1, while the positive controls had dry weights of 7.4 and 4.5 g plant−1 of 29.5 and 29.6% reduction, respectively. The vermitea and wood vinegar treatments significantly improved dry weight in sunflower and maize against the two pathogens stress, with 14.5; 15.2 and 8.7; 8.4 g plant−1, respectively (Fig. 4).
Effect of application with vermitea and wood vinegar on plant growth promotion of sunflower and maize plants, 35 days after sowing, grown under greenhouse conditions. (a) vermitea, (b) wood vinegar and (c) positive control. Values are mean of ten replications for each treatment as well as the positive and negative controls. Bars with the different letters within each variable indicate that the means ± standard errors are significantly different at P = 0.05, according to Duncan’s multiple range tests. Before sowing sunflower seeds and maize grains were soaked separately in each of vermitea (1:10, v:v) and wood vinegar (2.0%) for 2 h. After sowing, vermitea (1:10, v:v) and wood vinegar (2.0%) treatments at the rate of 200 mL pot−1 were applied four time intervals. The first application was 1 day before sowing. The three remains applications were applied weekly for 3 weeks starting 3 days after sunflower and maize seedlings emergence to the root zone and foliage of plants.
Effect on photosynthetic pigments and antioxidant enzymes
The effect of vermitea and wood vinegar on the photosynthetic pigments of sunflower and maize plants was studied, and the results were shown in Fig. 5. There were no significant differences in photosynthetic pigment enhancement between vermitea and wood vinegar treatments. Macrophomina phaseolina inoculation reduced chlorophyll a, chlorophyll b, and total carotenoids in the positive control by 26.7, 30.0, and 38.2%, respectively, when compared to the negative control of sunflower plants (Fig. 5). When compared to the positive controls, vermitea and wood vinegar increased chlorophyll a, chlorophyll b, and total carotenoids in sunflower plants by 54.0; 52.3, 58.8; 56.8, and 52.3; 51.2%, respectively. Treatments with vermitea and wood vinegar significantly increased the photosynthetic pigment content of maize plants grown in M. maydis contaminated soil. When compared to the positive control plants (5.3, 2.5, and 1.2 mg/g), these treatments had the highest chlorophyll a, chlorophyll b, and total carotenoids contents (12.7; 12.2, 7.5; 7.5, and 3.4; 3.2 mg/g) (Fig. 5). The effects of vermitea and wood vinegar on antioxidant enzymes were investigated, with the results shown in Fig. 6. The results reveal differences in enzyme activities between the control and plants grown in M. phaseolina or M. maydis contaminated soils treated with vermitea or wood vinegar. Plants treated with vermitea and wood vinegar had higher enzyme concentrations than plants grown in M. phaseolina or M. maydis contaminated soils. Sunflower plants grown in M. phaseolina-contaminated soil treated with vermitea or wood vinegar showed 12.2; 12.2, 5.3; 5.1, and 28.4; 27.2 U/g of CAT, POD, and SOD activity, respectively, compared to 12.7, 3.3, and 13.2 U/g in the positive control (Fig. 6). The CAT, POD, and SOD activity of maize plants grown in M. maydis contaminated soil treated with vermitea or wood vinegar was 14.4; 13.8, 11.2; 10.8, and 62.6; 61.5 U/g, respectively, compared to 8.0, 5.3, and 21.2 U/g in the positive control.
Effect of application with vermitea and wood vinegar on photosynthetic pigments of sunflower and maize plants, 35 days after sowing, grown under greenhouse conditions. Values are mean of ten replications for each treatment as well as the positive and negative controls. Bars with the different letters within each variable indicate that the means ± standard errors are significantly different at P = 0.05, according to Duncan’s multiple range tests. Chl a = chlorophyll a, Chl b = chlorophyll a, and TCAR = total carotenoids.
Effect of application with the vermitea and wood vinegar on antioxidant enzymes of sunflower and maize plants, 35 days after sowing, grown under greenhouse conditions. Values are mean of ten replications for each treatment as well as the positive and negative controls. Bars with the different letters within each variable indicate that the means ± standard errors are significantly different at P = 0.05, according to Duncan’s multiple range tests. CAT = catalase, POX = peroxidase and SOD = superoxide dismutase.
Effect on charcoal- rot and late -wilt incidence
There was no disease found in either the sunflower or maize negative controls. Positive controls, on the other hand, had disease incidence of 90.0 and 85.0% for sunflower charcoal-rot and maize late-wilt, respectively (Fig. 7). Treatments with vermitea or wood vinegar reduced the occurrence of both diseases significantly. In sunflower, vermitea or wood vinegar treatments resulted in disease incidence of 35.0 and 30.0%, respectively. With a 25.0% incidence of late-wilt in maize, vermitea or wood vinegar treatments were found to be effective against M. maydis stress (Fig. 7). The wood vinegar and vermitea did not differed significantly.
Effect of application with vermitea and wood vinegar on sunflower charcoal-rot and maize-late wilt under greenhouse and field conditions. In greenhouse, values are mean of ten replications for each treatment as well as the positive and negative controls. In field, values are mean of four replications for each treatment as well as the controls. Bars with the different letters within each variable indicate that the means ± standard errors are significantly different at P = 0.05, according to Duncan’s multiple range tests. Percentages data of disease incidence were transformed into arcsine square-root transformation for analyses of variance, however untransformed data are presented.
Field trials
Effect on charcoal-rot and late-wilt incidence
Throughout the crop season, diseased plants with sunflower charcoal-rot and maize late-wilt symptoms were observed in the field experiment. When compared to controls, treatment with vermitea or wood vinegar significantly reduced disease development in both crops (Fig. 7). In contrast, the progression of charcoal-rot and late-wilt diseases was unaffected, and severe disease symptoms appeared 60–80 days after sowing in control plants that had not been treated with vermitea or wood vinegar. As a result, the control set had a high mean disease incidence value of 26.8% for sunflower charcoal-rot and 42.3% for maize late-wilt, respectively (Fig. 7). When compared to the control, all treatments in the case of sunflower significantly decreased the incidence of charcoal-rot. The percentage reduction in charcoal-rot in the vermitea and wood vinegar treatments was 72.8 and 72.0%, respectively. In comparison to control plots of maize, vermitea and wood treatments showed a significant level of disease suppression, with 88.7 and 87.0%, respectively. There was no significant difference in overall disease control efficacy between vermitea and wood vinegar.
Effect on plant yield
In general, all treatments significantly increased sunflower and maize yield over the non-treated control (Fig. 8). In sunflower, the seeds yield resulted from the vermitea and wood vinegar treatments was 4.95 and 4.89 kg plot−1, respectively, compared to 3.15 kg plot−1 in the control. In maize, vermitea (37.8 kg plot−1) and wood vinegar (37.3 kg plot−1) treatments produced the highest ears yield, which was significantly higher than the non-treated control (17.5 kg plot−1) treatment.
Effect of application with vermitea and wood vinegar on sunflower and maize yield under field conditions. Values are mean of four replications for each treatment as well as the controls. Bars with the different letters within each variable indicate that the means ± standard errors are significantly different at P = 0.05, according to Duncan’s multiple range tests.
Discussion
In the current study, a total of seventeen bacteria strains from vermitea were tested for their antifungal activity to M. phaseolina and M. maydis, the pathogens responsible for sunflower charcoal-rot and maize late-wilt, respectively. Surprisingly, these isolates showed promising biological control potential against both pathogens. Three bacterial strains were chosen for molecular characterization due to their high antifungal activity against M. phaseolina and M. maydis. They were identified as Bacillus amyloliquefaciens OP546100, Serratia marcescens OP546101, and Bacillus velezensis OP346607. Many researchers have discovered that B. amyloliquefaciens, S. marcescens and B. velezensis are effective in plant disease prevention and control30,31,32. Beneficial microbes are in abundance in vermitea, which has a wide variety of bacteria that help with the biocontrol of diseases caused by different soil borne pathogens33. For instance, Fusarium oxysporum f. sp. ciceri, which causes Fusarium wilt of chickpeas, was able to be inhibited by 33 actinomycetes strains that were isolated from 25 different herbal vermicomposts34. Pathma and Sakthivel35 discovered 96 bacterial strains from vermicompost that had antagonistic effect against phytopathogenic fungi. Liu et al.36 reported that a total of 28 bacterial strains from vermicompost exhibited antagonistic activity against Fusarium oxysporum f. sp. cucumerinum (FOC) in cucumber. Soltan et al.37 found that the ten bacterial isolates obtained from vermicompost had an in vitro antagonistic effect against Fusarium solani, Fusarium spp., M. phasolenia and Rhizoctonia solani.
The wood vinegar as biochar derivatives used in this study performed best at 1.5 and 2.0%, which completely inhibiting the both pathogens. Wood vinegar has the ability to inhibit pathogenic fungi due to its chemical properties, including acidity and other chemical components. It has been demonstrated that the components of wood vinegar like acetic acid, formaldehyde, and methanol kill fungi, bacteria, and virus particles38,39. In the present study, the highest antifungal activity of wood vinegar was found to be associated with total phenol, total organic acids and total alcohols (Table 3). In comparison to the charcoal-rot fungus M. phaseolina, the antifungal activity of wood vinegar was significantly higher against late-wilt fungus M. maydis. Even at the highest dosage (1.5%) of wood vinegar, M. phaseolina only slightly expanded (57.8% inhibition). Chalermsan and Peerapan40 found that some pathogenic fungi, such as R. solani, Sclerotium oryzae, Helminthosporium maydis, Pythium sp., Colletotrichum gloeosporioides, and Choanephora cucurbitarum, were inhibited by wood vinegar solutions on PDA medium. Matnork et al.29 discovered that wood vinegar at 1.0% completely inhibited the mycelial growth and sclerotial germination of S. rolfsii.
In this study, the application of vermitea or wood vinegar reduced the incidence of sunflower charcoal-rot and maize late-wilt diseases in both greenhouse and field trials. This study suggests that indigenous vermitea bacteria and wood vinegar inhibitor components may be involved in this suppression. From vermitea, we isolated 17 bacterial strains. B. amyloliquefaciens, S. marcescens, and B. velezensis were identified and having high antagonistic activity against the causal pathogens. These bacterial strains exert biocontrol effects through a variety of mechanisms, including direct antibiosis and competition through the secretion of a variety of secondary metabolites in the rhizosphere, as well as stimulation of plant-induced systemic resistance41. The results are in line with a number of recent studies. Sabaté et al.42 discovered that in the presence of M. phaseolina, B. amyloliquefaciens B14 synthesized various lipopeptides such as surfactin, iturin, fengycin, kurstatin, and polymyxin, implying that they are the primary cause of the antagonistic effect observed. Chen et al.43 reported that B. velezensis ZW-10 liquid culture and cell-free culture filtrate had significant inhibitory effects on rice blast. In Korea, Kim et al.44 reported that B. velezensis has biocontrol activity against apple bitter rot. Also, B amyloliquefaciens AW3 causes mycelial cell malformation, swelling, and distortion in the fungus Fusarium oxysporum45. According to the obtained data, the fungicidal activity of wood vinegar against the occurrence of charcoal-rot and late-wilt might be caused by a combination of its acidic nature and the presence of components like phenolic, polyphenolic compounds, and organic acids. Wood vinegar has the capacity to inhibit the two pathogenic fungi because of its chemical components such as acetic acid, formaldehyde and methanol, as previously reported40. Using GC–MS analysis, Velmurugan et al.46 identified seven major compounds in neutralized wood vinegar and eleven major compounds in acidic wood vinegar. Moreover, Mahmud et al.47 reported that the presence of phenols and their major derivatives, as indicated by GC–MS and FTIR analysis, is what gives pyroligneous acid its antifungal properties.
It has been reported that the induction of antioxidant enzymes is associated with plant tolerance to fungal disease stress. Thus, in M. phaseolina or M. maydis infested soil, the antioxidant activity of vermitea and wood vinegar treated sunflower and maize was investigated. The activity of CAT, POD, and SOD enzymes were differed from the negative control, positive control, and plants grown in pathogen-contaminated soils treated with vermitea or wood vinegar. Plants treated with vermitea or wood vinegar had higher concentrations of enzyme activities than both the negative and positive controls. According to Mahmud et al.47, wood vinegar may have a second mode of action in disease control in addition to direct antifungal activity, which could be the induction of resistance in plants to particular diseases. Furthermore, Rathika et al.48 found that the vermi-wash treatment increased the antioxidant enzyme activity in Sorghum bicolor plants (140, 125, and 152 U/mg of CAT, SOD, and POD, respectively). Plant polyphenols act as reducing agents, scavengers, metal chelators, and oxygen radical quenchers in the cell to provide antioxidant activity. Antioxidant enzymes catalyze the reaction that involves the breakdown of H2O2, in which one molecule of H2O2 acts as a substrate donor and another molecule of H2O2 acts as an oxidant or electron acceptor. This molecule may be in charge of producing stress defense signals because an increase in catalase causes an increase in H2O2. In addition, H2O2 and other free radicals are toxic to a wide range of microbial pathogens49. When a plant interacts with a pathogen, the oxidative potential of H2O2 helps to produce lignin through the peroxidase-mediated crosslinking of structural proteins rich in proline and phytoalexin biosynthesis as well as the polyphenoloxidase-mediated conversion of O-dihydroxyphenols to harmful O-quinones50.
In the current study, the application of vermitea and wood vinegar improved the growth and photosynthetic pigments of sunflower and maize plants grown in pots trials. In addition, these treatments increased the yield of both crops in field trials. This is because vermitea and wood vinegar had beneficial properties. Ruiz et al.51 discovered that inoculating vermicompost tea with plant growth promoting bacteria resulted in significant increases in tomato fruit yield and quality. Mineral nutrients in plant-available forms, a hormone-like effect on plant growth, and stimulation of plant mineral nutrition are all potential beneficial mechanisms of vermitea and wood vinegar on plants47,52. Increased leaf photosynthetic pigment content (chlorophyll and carotenoid) of sunflower and maize as a result of vermitea treatment can be used to indicate improved plant physiological state53,54. This effect was also observed in the wood vinegar treatment in the current study as well as in other experiments55,56, indicating that vermitea and wood vinegar components activate photosynthesis-related processes. When used as a foliar fertilizer, wood vinegar increases cucumber, lettuce, and cole yields55,56. Tikoria et al.57 reported that when tomato seedlings were treated with 100% vermicompost extract, root and shoot length, root and shoot fresh weights increased by 28, 36.53, 68.20 and 43%, respectively.
Conclusion
The most harmful pathogens that severely reduce sunflower and maize plant yields are M. phaseolina and M. maydis, respectively. The effectiveness of vermitea and wood vinegar in preventing these fungi-related diseases was assessed in this study. Out of 17 bacterial strains obtained from vermitea, three demonstrated the strongest inhibitory effect against M. phaseolina and M. maydis. Obtained data also stated that wood vinegar, as biochar derivative, prevented the representative pathogens from growing in in vitro tests. The greenhouse and field results showed that vermitea and wood vinegar were effective in preventing sunflower charcoal-rot and maize late-wilt, while also significantly increased plant growth, antioxidant enzymes, photosynthetic pigments and yield for both crops when compared to the untreated control. This discovery raises the possibility that vermitea and wood vinegar may work in two different ways to combat charcoal-rot of sunflower and late-wilt of maize. Direct antifungal activity and the development of plant resistance to the responsible pathogens are two examples of these. To increase the use of vermitea and wood vinegar in agricultural production, more thorough studies are required.
Materials and methods
Soil, vermicompost and wood vinegar
The greenhouse experiment took place in clay soil located at El-Gharbia governorate, Egypt. The vermicompost used in this study was provided by the Environment and Bio-Agricultural Department, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt. It consisted of horse dung and mushroom compost 1:1 (v:v) processed in outdoor beds by earthworms Eisenia foetida. The study's wood vinegar was made from Psidium guajava wood and obtained from the Egyptian Company for Modern Agriculture in Cairo, Egypt. The best pyrolysis conditions were attained by heating at a rate of 1.4 °C per minute to a final temperature of 550 °C. Wood vinegar was infused through Whatman filter paper No. 1 and sterilized using a 0.22 μm filters syringe before being tested for antifungal activity. The soil, vermicompost, and wood vinegar were chemically analyzed using standard procedures at the Soils, Water & Environment Research Institute, Agriculture Research Centre, Giza, Egypt. Table 3 shows the chemical properties of soil, vermicompost and wood vinegar used in this study.
Sunflower charcoal-rot pathogen
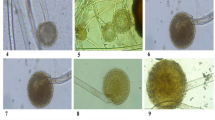
In the second half of August and later in 2021, diseased samples exhibiting symptoms of charcoal-rot of sunflower (cv. Sakha 53) were collected from some infected field localities in El-Gharbia Governorate, Egypt. Infected sunflower plants died early and took on a silvery gray appearance (Fig. 9A). In the lower portion of their stem, black microsclerotia were found (Fig. 9B). The infected stem and root samples were washed by running tap water and then cut into small (1 cm) pieces. By dipping the pieces in 0.5% sodium hypochlorite for 45 s, followed by 3 min of washing with sterile distilled water, the pieces were surface sterilized. After drying on sterilized filter paper, the fragments were kept on 90 mm Petri-plates of sterilized potato dextrose agar medium (PDA, Difco, Detroit, MI, USA) that has been modified by adding chloramphenicol as antibacterial agent then incubated at 25 ± 2 °C for 7 days. The fungus was identified based on host plant symptoms, colony color, and the morphology of the microsclerotia and pycnidia after the colonies were purified using single hyphal tip techniques. Sunflower seeds were planted in 35-cm diameter pots (2 seeds/pot) with sterilized soil that had been infused with the previously obtained strain of M. phaseolina under greenhouse conditions. For a period of 12 weeks, sunflower plants were watched for indications of charcoal-rot. Ninety days after sowing, 95.0% of the M. phaseolina-inoculated pots had disease incidence (dead plants as a result of charcoal-rot). Sclerotia in the plant base, a symptom of charcoal-rot, are present on all infected plants (Fig. 9E). The negative control (non-infested pots) did not induce disease (Fig. 9C and D). In order to confirm Koch’s hypotheses, M. phaseolina was once again isolated from infected tissues of cultivated sunflower plants. The infected stem inoculated on PDA produced grey hyphae that eventually turned black and formed microsclerotia (Fig. 9F).
Typical natural symptoms of charcoal-rot on sunflower plant behind healthy one (A). The black microsclerotia are visible on root and the lower part of stem of diseased plant (B). Symptoms of charcoal rot disease on foliage and root parts of sunflower plants artificially infected with M. phaseolina isolate, 35 days after sowing (C) (left: infected plants and right: healthy plants). Charcoal-rot disease signs on infected stem and roots (left: infected plants and right: healthy plants) (D). Symptoms and signs of charcoal-rot disease on foliage and root parts of sunflower plants artificially infected with M. phaseolina isolate, 68 days after sowing (E) Pure culture of M. phaseolina isolate grew on the PDA medium (F).
Maize late-wilt pathogen
A virulent isolate of Magnaporthiopsis maydis was used. Isolation, morphology identification and pathogenicity test of this isolate was confirmed in previous study8. In this study, the molecular identification of this isolate was verified. Whole genomic DNA was extracted using the i-genomic BYF DNA extraction Mini Kit as directed by the manufacturer (iNtRON Biotechnology Inc., South Korea). PCR amplification and sequencing of transcribed spacers (ITS), using the forward primer ITS1 (5′ TCCGTAGGTGAACCTGCGG-3′) and the reverse primer ITS4 (5′ TCCTCCGCT TATTGATATGC-3′), were used to identify the pathogen molecularly58. A polymerase chain reaction (PCR) was used to amplify a particular region of the rDNA primer (2 min at 94 °C, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, elongation at 72 °C for 60 s, and final extension at 72 °C for 10 min). The amplified products were separated on 1% agarose gels in 1X TBE (Tris–borate-EDTA) buffer for approximately 2 h at a constant voltage of 100 V. The nucleotide sequence similarity of the PCR products was examined using BLAST (http://www.ncbi.nlm.nih.gov) analysis against the National Center for Biotechnology Information database after the PCR products were sent to GATC Company's sequencing laboratories in Germany for analysis. The phylogenetic trees showed that this isolate shared many characteristics with the isolate of the M. maydis type that was deposited in the NCBI Center and uploaded to GenBank under the accession number ON564309 (Fig. 2D).
Laboratory experiments
Isolation and antagonistic activity of vermitea bacteria
In this study, the vermitea was prepared from vermicompost (cured for 3 months). For vermitea extraction procedures, 1 L of vermicompost was mixed with 10 L of deionized water (25–30 °C). The vessel was a typical polymer container with an air pump and interior tubings that enabled thorough mixing and simultaneous aeration of the liquid inside, but no thermostating. The extraction time was 24 h unless otherwise stated. To boost microbial growth, 1 g L−1 of dry humic acid and 0.5 g L−1 of citric acid were added before extraction. Just before application, the vermitea was filtered through a nylon membrane. According to Mu et al.22 and Darwesh et al.59, total culturable bacteria from vermitea sample were counted by dilution plating onto Nutrient Agar (NA) medium and purified on Luria–Bertani (LB) agar medium. The purified bacterial strains were examined for antagonistic activity against M. phaseolina and M. maydis using the dual culture method60. The bacterial strains were streaked on one side of a Petri dish (1 cm from the edge) containing PDA medium, and a 5 mm mycelial disc from a 7-day old pathogenic fungal culture was placed on the opposite side. The plates were incubated for 7 days at 27 ± 2 °C. Only dishes inoculated with the pathogen were used as control. After an incubation period, the linear growth of the pathogen was assessed. Each treatment was replicated four times over the course of the experiment. According to the following formula the percentage of growth inhibition was calculated as follow: Growth inhibition % = (Linear growth of the pathogen colony in the control- linear growth of the pathogen colony in the treatment/ Linear growth of the pathogen colony in the control) × 100.
Molecular identification of vermitea bacteria
The strains showing the greatest inhibition in in vitro tests were chosen. The morphology and biochemical tests on the bacterial strains were carried out utilizing Bergey's Manual of Systematic Bacteriology 61. For molecular identification, lysozyme (20 mg/mL) and proteinase K (1 mg/mL) were used to extract genomic DNA 33. Total genomic DNA was purified using isopropanol buffer. The 16S rRNA genes were amplified using extracted DNA and 2 primers, F: (5'- AGAGTTTGATCCTGGCTCAG -3') and R: (5'- TACGGTTACCTTGTTACGACTT -3'). Initial denaturation at 95°C for 5 min was followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s to complete extension in the PCR amplification. The QIAquick Gel Extraction Kit (QIAGEN, USA) was used to purify the PCR product before it was run on an agarose gel for sequencing. The BLAST program was used to identify bacterial strains (National Centre for Biotechnology Information). The Jukes Cantor Model was used to align the sequences. The neighbor joining (NJ) algorithm was used for the phylogenetic reconstruction, along with bootstrap values, and the results were submitted to Gene Bank.
Antifungal activity of wood vinegar
The fungal inhibition bioassay of wood vinegar against M. phaseolina and M. maydis was performed according to previous manuscript 39. Macrophomina phaseolina, a charcoal-rot fungus, and M. maydis, a late-wilt fungus, were grown on PDA medium in dishes for 7 days at 25 ± 2 °C and used as inoculants. PDA medium was autoclaved at 121 °C for 15 min. It was then amended with 0.5, 1.0, 1.5, 2.0, and 2.5% (v/v) wood vinegar before being poured into Petri-dishes. The Petri-dishes were then cooled before being centrally inoculated with a single plug (5 mm in diameter) from the pre-cultured PDA plate of each fungus. As controls, PDA dishes containing zero percent wood vinegar were used. Each fungus was replicated four times, once for the control and once for each concentration. Dishes with the treated and untreated ingredient were incubated at 27 ± 2 °C for 7 days. The percentages of mycelial growth inhibition were assessed as mentioned before.
Greenhouse experiments
Pot experiments were carried out in an open greenhouse during the summer of 2022 at the Plant Pathology Department of the National Research Centre in Giza, Egypt. The experiment's minimum and maximum temperatures were 20 to 30 °C and 32 to 40 °C, respectively. We bought maize grains cv. Landraces and sunflower seeds cv. Sakha 53 from the Egyptian Agricultural Research Center, Egypt.
Preparation of pathogens inocula
Inoculants were grown on potato dextrose agar (PDA) and potato dextrose agar yeast extract (PDAY) dishes for 7 days at 25 ± 2 °C for M. phaseolina, a charcoal-rot fungus, and M. maydis, a late-wilt fungus. A 1000 mL Erlenmeyer flask containing 200 g of wet, sterile sorghum grain was inoculated with 10 colony agar disks (5 mm in diameter each), taken from the edges of a 7-day-old culture, to create inocula for each fungus. Cultures were incubated in the incubator for 4 weeks in a dark at 25 ± 2 °C. The inoculum was then collected and homogenized.
Preparation of pots
The experiment was conducted in 35 cm diameter pottery pots filled with 8 kg sterilized clay soil that had been pre-mixed with 4% inocula of any of M. phaseolina and M. maydis. Negative control treatment pots were made of non-infested soil. The soil and pots were sterilized before filling with a formalin solution made up of 2.5 L of concentrated solution (40% formaldehyde) to 50 L of water. The soil was then covered with a plastic sheet for 7 days to keep the gas in place. The soil was not planted until the formaldehyde odor had subsided. After filling, the pots were watered, and a week was given for the inoculum to establish. Sunflower seeds and maize grains were sown in experimental units (pots) on May 20, 2022, at a rate of 5 seeds or grains per pot. Twelve days later, plants of each crop were thinned to two plants per pot. Each pot for each crop received 15 g of phosphate rock (P2O5) and 8.0 g of ammonium nitrate prior to sowing. Ammonium nitrate fertilizer was applied at rates of 8.0 g pot−1 at 20, 30, and 40 days after sowing, and plants were irrigated when it was necessary.
Treatments and experimental design
Before planting, sunflower seeds and maize grains were soaked separately for 2 h in vermitea (1:10, v:v) or wood vinegar (2.0%). Four times after sowing, vermitea (1:10, v:v) or wood vinegar (2.0%) treatments were applied at a rate of 200 mL pot−1. The first application was occurred out 1 day prior to sowing. The three residue applications were applied weekly for 3 weeks, beginning 3 days after the emergence of sunflower and maize seedlings to the root zone and foliage of plants. Half of the vermitea and/or wood vinegar were applied to the root zone and the other half to the foliage; however, once the foliage was saturated, the solutions applied to the foliage were moved to the root zone. In the greenhouse experiments, a randomized complete block design (RCBD) with 10 replications was used. Vermitea application, wood vinegar application, negative control (non-treated seeds or grains sown in uninfested and untreated soil), and positive control were the treatments used for each crop. For every crop, there are two separate experiments. The first experiment examined the effects of vermitea and wood vinegar on plant physiological traits and growth promotion 35 days after planting. In order to determine the impact of vermitea and wood vinegar on the incidence of charcoal-rot and late-wilt, a second experiment was conducted 90 days after planting.
Experiment I: effect on physiological traits and growth promotion
To investigate the effect of vermitea and wood vinegar applications on physiological traits (photosynthetic pigments and antioxidant enzyme activities) and growth promotion of sunflower and maize, the following four treatments were set up, each with 10 replications for each crop:
Sunflower | Maize | |
|---|---|---|
T1, | Negative control | Negative control |
T2, | Positive control (M. phaseolina) | Positive control (M. maydis) |
T3, | Vermitea application | Vermitea application |
T4, | Wood vinegar application | Wood vinegar application |
The sunflower and maize plants were pull-off 35 days after they were planted by mulching them out of their pots. Plant height (cm plant−1) and fresh weight (g plant−1) as well as dry weight (g plant−1) were measured for each crop. Before mulching, sunflower and maize leaf samples were separated for the analysis of photosynthetic pigments and antioxidant enzyme activity. According to Porra62, photosynthetic pigments [Chlorophyll: (Chl a, Chl b, and total carotenoids (TCAR)] were measured in fresh sunflower and maize leaves and the results were expressed as mg/g−1 fresh weight. The extraction and determination of antioxidant enzymes were carried out63, and the activity was expressed as unit/g fresh weight/min. At 240 nm, catalase activity (EC 1.11.1.6) was detected. By monitoring the rise in absorbance at 470 nm brought on by guaiacol oxidation, the activity of peroxidase (EC 1.11.1.7) (POX) was ascertained. At 560 nm, superoxide dismutase (EC 1.15.1.1) activity (SOD) was measured using the photochemical technique.
Experiment II: effect on charcoal-rot and late-wilt incidence
To determine the impact of vermitea and wood vinegar applications on the incidence of sunflower charcoal-rot and maize late-wilt diseases, the previous treatments were set up with ten replications for each. At the end of the experiment (90 days after planting), the percentage of infected plants during the growing season was calculated as follows: Charcoal-rot and/or late-wilt infection (%) = (number of dead plants due to the disease during the growing season/total number of plants) × 100.
Field experiments
The goal of the experiment was to ascertain how vermitea and wood vinegar affected both the incidence of diseases like sunflower charcoal-rot and maize late-wilt as well as the yield of both crops. The trial took place in the Egyptian Governorate of El-Gharbia from May to August 2022. The experiment's natural soil type was clay. The contiguous maize-growing region that made up this field site stood out because it was naturally infested with a high M. maydis inoculum and suffered from severe late-wilt disease8. Before seeding, soil samples were taken, and the Mihail and Alcorn64 method was used to check for the presence of M. phaseolina isolates. A fungus population was discovered in the field. The experiment was set up in a randomized block design for each crop, with three treatments and four replications (plots). Sunflower seeds cv. Sakha 53 and maize grains cv. Landraces were sown with hand drill in holes (two seeds/hole) and a plot size of 4 lines each equaling 0.75 m width × 5.0 m length was set up. Each crop's seedlings were thinned 7 days after emergence to maintain a plant-to-plant distance of 20 cm and a row-to-row distance of 75 cm. As per traditional sunflower and maize cultivation technology, recommended agronomic practices such as hoeing, weeding, inorganic fertilizer (N:P:K) doses, and insect management were carried out as and when required. The following were the applications of vermitea and wood vinegar: Before planting, sunflower seeds and maize grains were soaked separately for 2 h in vermitea (1:10, v:v) or wood vinegar (2.0%). Four times after sowing, vermitea (1:10, v:v) and wood vinegar (2.0%) treatments were applied at a rate of 100 mL hole−1. The first application occurred immediately following sowing and before irrigation. The three residue applications were applied weekly for 3 weeks, beginning 3 days after the emergence of sunflower and maize seedlings to the root zone and foliage of plants. Half of the vermitea and/or wood vinegar were applied to the root zone and the other half to the foliage; however, once the foliage was saturated, the solutions applied to the foliage were moved to the root zone. The following treatments were used on each crop:
Sunflower | Maize | |
|---|---|---|
T1, | Un-treated control | Un-treated control |
T2, | Vermitea application | Vermitea application |
T3, | Wood vinegar application | Wood vinegar application |
Throughout the crop season, the plants were checked for symptoms of sunflower charcoal-rot and maize late-wilt, and the number of infected plants (dead plants) in each plot was counted and expressed as a percentage of disease incidences. Sunflower plant heads from each plot were manually harvested 90 days after planting, dried, and seed yield was weighed (kg). The quantitative maize yield was determined by weighing the harvested plant ears from each plot 110 days after planting, drying them, and the quantitative maize yield was calculated as the weight (kilograms) of harvested ears per plot. The yield of each crop is given in kg/plot.
Statement on guidelines
Experimental procedures and field studies on plants comply with relevant institutional, national, and international guidelines and legislation.
Statistical analysis
The CoStat6303Win.exe software program was used to conduct a one-way analysis of variance (ANOVA) to examine differences among treatments. Mean differences were compared using Duncan's multiple range tests, and a P value of 0.05 was regarded as significant. The percentage data were transformed using arcsine square root prior to data analysis. The untransformed means are included in the results.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Aboshosha, S. S. et al. Characterization of Macrophomina phaseolina isolates affecting sunflower growth in El-Behera governorate. Egypt. Int. J. Agri. Biol. 9, 807–815 (2007).
Taha, M. M. et al. Potential resistance of certain sunflower cultivars and inbred lines against charcoal rot disease caused by Macrophomina phaseolina (Tassi) Goid. J. Phytopathol. Pest Manag. 3, 55–66 (2018).
Ijaz, S., Sadaqat, H. A. & Khan, M. N. A review of the impact of charcoal rot (Macrophomina phaseolina) on sunflower. J. Agric. Sci. 151, 222–227. https://doi.org/10.1017/S0021859612000512 (2012).
Jordaan, E., van der Waals, J. E. & McLaren, N. W. Effect of irrigation on charcoal rot severity, yield loss and colonization of soybean and sunflower. Crop Prot. 122, 63–69. https://doi.org/10.1016/j.cropro.2019.04.026 (2019).
Kaur, S. et al. Emerging phytopathogen Macrophomina phaseolina: biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 38, 136–151. https://doi.org/10.3109/1040841X.2011.640977 (2012).
Chowdhury, S., Basu, A., Chaudhuri, T. R. & Kundu, S. In vitro characterization of the behavior of Macrophomina phaseolina (Tassi) Goid. at the rhizosphere and during early infection of roots of resistant and susceptible varieties of sesame. Eur. J. Plant Pathol. 138, 361–375. https://doi.org/10.1007/s10658013-0335-z (2014).
Gupta, G. K., Sharma, S. K. & Ramteke, R. Biology, epidemiology and management of the pathogenic fungus Macrophomina phaseolina (Tassi) Goid. with special reference to charcoal rot of soybeans (Glycine max (L) Merril). J. Phytopathol. 160, 167–180. https://doi.org/10.1111/j.1439-0434.2012.01884.x (2012).
Elshahawy, I. E. & Abd El-Wahed, M. S. Suppression of Cephalosporium maydis by the resistance inducer beta-sitosterol. Eur. J. Plant Pathol. 163, 673–693. https://doi.org/10.1007/s10658-022-02506-w (2022).
Elshahawy, I. E. & Khattab, A. A. Endophyte Chaetomium globosum improves the growth of maize plants and induces their resistance to late wilt disease. J. Plant Dis. Prot. 129, 1125–1144. https://doi.org/10.1007/s41348-022-00626-3 (2022).
Degani, O. A review: Late wilt of maize-the pathogen, the disease, current status, and future perspective. J. Fungi 7, 989. https://doi.org/10.3390/jof7110989 (2021).
Sabet, K. A., Zaher, A. M., Samra, A. S. & Mansour, I. M. Pathogenic behavior of Cephalosporium maydis and C. acremonium. Ann. Appl. Biol. 66, 257–263. https://doi.org/10.1111/j.1744-7348.1970.tb06432.x (1970).
Elshahawy, I. E. & El-Sayed, A. E. Maximizing the efficacy of Trichoderma to control Cephalosporium maydis, causing maize late wilt disease, using freshwater microalgae extracts. Egypt. J. Biol. Pest Control 28, 48. https://doi.org/10.1186/s41938-018-0052-1 (2018).
Hussien, Z. N., Ibrahim, M. M., Ahmed, M. I. M. & Khalil, A. A. Thermotherapy of sunflower seeds controlling charcoal-rot caused by Macrophomina phaseolina. Egypt. J. Phytopathol. 46, 179–194 (2018).
Khan, I. H. & Javaid, A. Biocontrol Aspergillus species together with plant biomass alter histochemical characteristics in diseased mungbean plants. Microsc. Res. Techn. 85(8), 2953–2964. https://doi.org/10.1002/jemt.24145 (2022).
Uroos, M. et al. Green synthesis of coumarin derivatives using bronsted acidic pyridinium based ionic liquid [MBSPy][HSO4] to control an opportunistic human and a devastating plant pathogenic fungus Macrophemina phaseolina. RSC Adv. 12, 23963–23972. https://doi.org/10.1039/d2ra03774b (2022).
Kata, J. Physical and cultural methods for the management of soil borne pathogens. Crop Prot. 19, 725–731. https://doi.org/10.1016/S0261-2194(00)00096-X (2000).
Degani, O., Weinberg, T. & Graph, S. Chemical control of maize late wilt in the field. Phytoparasitica 42, 559–570. https://doi.org/10.1007/s12600-014-0394-5 (2014).
Pylak, M., Oszust, K. & Fra, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 18, 597–616. https://doi.org/10.1007/s11157-019-09500-5 (2019).
Joshi, R., Singh, J. & Vig, A. P. Vermicompost as an effective organic fertilizer and biocontrol agent: Effect on growth, yield and quality of plants. Rev. Environ. Sci. Biotechnol. 14, 137–159. https://doi.org/10.1007/s11157-014-9347-1 (2015).
Edwards, C. A. et al. Suppression of green peach aphid (Myzus persicae) (Sulz.), citrus mealybug (Planococcus citri) (Risso), and two spotted spider mite (Tetranychus urticae) (Koch) attacks on tomatoes and cucumbers by aqueous extracts from vermicomposts. Crop Prot. 29, 80–93. https://doi.org/10.1016/j.cropro.2009.08.011 (2010).
Yatoo, A. M., Ali, M. N., Baba, Z. A. & Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron. Sustain. Dev. 41, 7. https://doi.org/10.1007/s13593-020-00657-w (2021).
Mu, J. et al. Biocontrol potential of vermicompost through antifungal volatiles produced by indigenous bacteria. Biol. Control 112, 49–54. https://doi.org/10.1016/j.biocontrol.2017.05.013 (2017).
Gudeta, K. et al. Vermicompost and its derivatives against phytopathogenic fungi in the soil: A review. Horticulturae 8, 311. https://doi.org/10.3390/horticulturae8040311 (2022).
Zulkarami, B., Ashrafuzzaman, M., Husni, M. O. & Razi Ismail, M. Effect of pyroligneous acid on growth, yield and quality improvement of rock melon in soilless culture. Aust. J. Crop Sci. 5, 1508–1514 (2011).
Guillen, M. D. & Manzanos, M. J. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem. 79, 283–292. https://doi.org/10.1016/S0308-8146(02)00141-3 (2002).
Liu, X., Wang, J., Feng, X. & Yu, J. Wood vinegar resulting from the pyrolysis of apple tree branches for annual blue grass control. Ind. Crops Prod. 174, 114193. https://doi.org/10.1016/j.indcrop.2021.114193 (2021).
Yildizli, G., Coral, G. & Ayaz, F. Anti-bacterial, anti-fungal, and anti-inflammatory activities of wood vinegar: A potential remedy for major plant diseases and inflammatory reactions. Biomass. Convers. Biorefin. https://doi.org/10.1007/s13399-022-02482-5 (2022).
Koç, İ et al. The effects of wood vinegar on some soil microorganisms. Appl. Ecol. Environ. Res. 17, 2437–2447. https://doi.org/10.15666/aeer/170224372447 (2019).
Matnork, S., Thitla, T., Imaiam, N. & Nalumpang, S. In vitro evaluation of fungicides and wood vinegar to control Sclerotium rolfsii causing potato sclerotium rot disease. Asian J. Plant Pathol. 15, 14–21. https://doi.org/10.3923/ajppaj.2021.14.22 (2021).
Dawei, L., Dong, L., Li, L., Wang, Y. & Zhang, Y. Screening and identification of antagonistic bacteria from vermicompost against Fusarium oxysporum f. sp. cucumerinum. Acta Agric. Scand. - B Soil Plant Sci. 71, 266–272. https://doi.org/10.1080/09064710.2021.1893807 (2021).
Darwesh, O. M. & Elshahawy, I. E. Silver nanoparticles inactivate sclerotial formation in controlling white rot disease in onion and garlic caused by the soil borne fungus Stromatinia cepivora. Eur. J. Plant. Pathol. 160, 917–934. https://doi.org/10.1007/s10658-021-02296-7 (2021).
Dawwam, G. E. & Sehim, A. Promissing biological agents represented in Bacillus velezensis 33RB and Aspergillus niger 46 SF endophytic isolates for controlling Populus tomentosa wilt and anthracnose diseases. Egypt. J. Biol. Pest Control 32, 144. https://doi.org/10.1186/s41938-022-00644-1 (2022).
Darwesh, O. M., El-Hawary, A. S., El Kelany, U. S. & El-Sherbiny, G. M. Nematicidal activity of thermostable alkaline protease produced by Saccharomonospora viridis strain Hw G550. Biotechnol. Rep. 24, e00386. https://doi.org/10.1016/j.btre.2019.e00386 (2019).
Gopalakrishnan, S. et al. Evaluation of Actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 30, 1070–1078. https://doi.org/10.1016/j.cropro.2011.03.006 (2011).
Pathma, J. & Sakthivel, N. Molecular and functional characterization of bacteria isolated from straw and goat manure based vermicompost. Appl. Soil Ecol. 70, 33–47. https://doi.org/10.1016/j.apsoil.2013.03.011 (2013).
Liu, D., Liu, D., Liu, L., Wang, Y. & Zhang, Y. Screening and identification of antagonistic bacteria from vermicompost against Fusarium oxysporum f. sp. cucumerinum. Acta Agric. Scand. B Soil Plant Sci. 71, 266–272. https://doi.org/10.1080/09064710.2021.1893807 (2021).
Soltan, H. A. H., Dakhly, O. F., Mahmoud, M. A. & Fikry, Y. F. M. Microbiological and genetical identification of some vermicompost beneficial associated bacteria. SVU-IJAS 4, 21–36. https://doi.org/10.21608/svuijas.2021.106875.1154 (2022).
Oramahi, H. A. & Yoshimura, T. Antifungal and antitermitic activities of wood vinegar from Vitex pubescens Vahl. J. Wood Sci. 59, 344–350. https://doi.org/10.1007/s10086-013-1340-8 (2013).
Theapparat, Y., Chandumpai, A., Leelasuphakul, W. & Laemsak, N. Pyroligneous acids from carbonization of wood and bamboo: their components and antifungal activity. J. Trop. For. Sci. 27, 517–526 (2015).
Chalermsan, Y. & Peerapan, S. Wood vinegar: by-product from rural charcoal kiln and its role in plant protection. As. J. Food Ag-Ind. 2, S89–S195 (2009).
Darwesh, O. M., Mahmoud, R. H., Abdo, S. M. & Marrez, D. A. Isolation of Haematococcus lacustris as source of novel anti-multi-antibiotic resistant microbes agents; Fractionation and identification of bioactive compounds. Biotechnol. Rep. 35, e00753. https://doi.org/10.1016/j.btre.2022.e00753 (2022).
Sabaté, D. C., Brandan, C. P., Petroselli, G., Erra-Balsells, R. & Audisio, M. C. Decrease in the incidence of charcoal root rot in common bean (Phaseolus vulgaris L.) by Bacillus amyloliquefaciens B14, a strain with PGPR properties. Biol. Control 113, 1–8. https://doi.org/10.1016/j.biocontrol.2017.06.008 (2017).
Chen, Z. et al. Isolation and evaluation of Bacillus velezensis ZW-10 as a potential biological control agent against Magnaporthe oryzae. Biotechnol. Biotechnol. Equip. 34, 714–724. https://doi.org/10.1080/13102818.2020.1803766 (2020).
Kim, Y. S. et al. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 11, 626. https://doi.org/10.1038/s41598-020-80231-2 (2021).
Zhang, P., Hao, H., Wang, L., Liu, Z. & Ma, L. Endophytes Bacillus amyloliquefaciens AW3 (CGMCC1.16683) improves the growth of Populus davidiana × Populus bolleana (PdPap) and induces its resistance to wilt disease by Fusarium oxysporum Fox68 (CFCC86068). Eur. J. Plant Pathol. 162, 1–17. https://doi.org/10.1007/s10658-021-02381-x (2022).
Velmurugan, N., Han, S. S. & Lee, Y. S. Antifungal activity of neutralized wood vinegar with water extracts of Pinus densiflora and Quercus serrata saw dusts. Int. J. Environ. Res. 3, 167–176. https://doi.org/10.22059/ijer.2009.45 (2009).
Mahmud, K. N. et al. Evaluation on efficiency of pyroligneous acid from palm kernel shell as antifungal and solid pineapple biomass as antibacterial and plant growth promoter. Sains Malays. 45, 1423–1434 (2016).
Rathika, R. et al. Effect of citric acid and vermi-wash on growth and metal accumulation of Sorghum bicolor cultivated in lead and nickel contaminated soil. Chemosphere 243, 125327. https://doi.org/10.1016/j.chemosphere.2019.125327 (2020).
Wu, G. et al. Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell 9, 1357–1368. https://doi.org/10.1105/tpc.7.9.1357 (1995).
Mayer, A. M. & Harel, E. Polyphenol oxidases in plants. Phytochemistry 18, 193–215. https://doi.org/10.1016/0031-9422(79)80057-6 (1997).
Ruiz, J. L. & Salas Sanjuan, M. D. C. The use of plant growth promoting bacteria for biofertigation; effects on concentrations of nutrients in inoculated aqueous vermicompost extract and on the yield and quality of tomatoes. Biol. Agric. Hortic. 38, 145–161. https://doi.org/10.1080/01448765.2021.2010596 (2022).
Zhang, H. et al. Analysis of phytohormones in vermicompost using an ovel combinative sample preparation strategy of ultrasound-assisted extraction and solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. Talanta 139, 189–197. https://doi.org/10.1016/j.talanta.2015.02.052 (2015).
Ievinsh, G. Vermicompost treatment differentially affects seed germination, seedling growth and physiological status of vegetable crop species. Plant Growth Regul. 65, 169–181. https://doi.org/10.1007/s10725-011-9586-x (2011).
Voko, M. P. et al. Vermicompost leachate, seaweed extract and smoke-water alleviate drought stress in cowpea by influencing phytochemicals, compatible solutes and photosynthetic pigments. Plant Growth Regul. 97, 327–342. https://doi.org/10.1007/s10725-022-00815-y (2022).
Mu, J., Yu, Z. M., Wu, W. Q. & Wu, Q. L. Preliminary study of application effect of bamboo vinegar on vegetable growth. For. Stud. China 8, 43–47. https://doi.org/10.1007/s11632-006-0023-6 (2006).
Jothityangkoon, D., Koolachart, R., Wanapat, S., Wongkaew, S. & Jogloy, S. Using wood vinegar in enhancing peanut yield and in controlling the contamination of aflatoxin producing fungus. Inter. Crop Sci. 4, 253–253 (2008).
Tikoria, R., Kaur, A. & Ohri, P. Potential of vermicompost extract in enhancing the biomass and bioactive components along with mitigation of Meloidogyne incognita-induced stress in tomato. Environ. Sci. Pollut. Res. 29, 56023–56036. https://doi.org/10.1007/s11356-022-19757-z (2022).
Darwesh, O. M., Li, H. & Matter, I. A. Nano-bioremediation of textile industry wastewater using immobilized CuO-NPs myco-synthesized by a novel Cu-resistant Fusarium oxysporum OSF18. Environ. Sci. Pollut. Res. 30, 16694–16706. https://doi.org/10.1007/s11356-022-23360-7 (2023).
Darwesh, O. M., Eida, M. F. & Matter, I. A. Isolation, screening and optimization of L-asparaginase producing bacterial strains inhabiting agricultural soils. Biosci. Res. 15, 2802–2812 (2018).
Elshahawy, I. E., Saied, N. M., Abd-El-Kareem, F. & Morsy, A. A. Field application of selected bacterial strains and their combinations for controlling onion and garlic white rot disease caused by Stromatinia cepivora. J. Plant Pathol. 100, 493–503. https://doi.org/10.1007/s42161-018-0113-z (2018).
Holt, J. G. et al. Bergey’s Manual of Determinative Bacteriology 786–788 (Williams and Wilikins, 1994).
Porra, R. J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73, 149–156. https://doi.org/10.1023/A:1020470224740 (2002).
Rios-Gonzalez, K., Erdei, L. & Lips, S. H. The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Sci. 162, 923–930. https://doi.org/10.1016/S0168-9452(02)00040-7 (2002).
Mihail, J. D. & Alcorn, S. M. Quantitative recovery of Macrophomina phaseolina sclerotia from soil. Plant Dis. 66, 662–663 (1982).
Acknowledgements
This work was supported financially by National Research Centre, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
O.M.D. and I.E.E. contributed in the conception and design of the study, the acquisition of data, and the analysis and interpretation of data. Also, they drafted the article and critically revised its important intellectual content. All authors read and approved the version submitted of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Darwesh, O.M., Elshahawy, I.E. Management of sunflower charcoal-rot and maize late-wilt diseases using the aqueous extract of vermicompost (vermitea) and environmental-safe biochar derivative (wood vinegar). Sci Rep 13, 17387 (2023). https://doi.org/10.1038/s41598-023-43974-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43974-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.