Abstract
Lateral wall thickness is a known predictor for postoperative stability of trochanteric femoral fractures and occurrence of secondary lateral wall fractures. Currently, the AO/OTA classification relies on the absolute lateral wall thickness (aLWT) to distinguish between stable A1.3 and unstable A2.1 fractures that does not take interpersonal patient differences into account. Thus, a more individualized and accurate measure would be favorable. Therefore, we proposed and validated a new patient-specific measure—the relative lateral wall thickness (rLWT)—to consider individualized measures and hypothesized its higher sensitivity and specificity compared with aLWT. First, in 146 pelvic radiographs of patients without a trochanteric femoral fracture, the symmetry of both caput-collum-diaphyseal angle (CCD) and total trochanteric thickness (TTT) was assessed to determine whether the contralateral side can be used for rLWT determination. Then, data of 202 patients were re-evaluated to compare rLWT versus previously published aLWT. Bilateral symmetry was found for both CCD and TTT (p ≥ 0.827), implying that bone morphology and geometry of the contralateral intact side could be used to calculate rLWT. Validation revealed increased accuracy of the rLWT compared with the gold standard aLWT, with increased specificity by 3.5% (Number Needed to Treat = 64 patients) and sensitivity by 1% (Number Needed to Treat = 75 patients). The novel rLWT is a more accurate and individualized predictor of secondary lateral wall fractures compared with the standard aLWT. This study established the threshold of 50.5% rLWT as a reference value for predicting fracture stability in trochanteric femoral fractures.
Similar content being viewed by others
Introduction
Trochanteric femoral fractures (TFF) are common in the elderly population and represent a serious problem for both the patient and healthcare system1,2. The incidence is estimated at 6.26 million fractures per year by 2050 worldwide2,3. Despite advanced treatment options, the 2-year mortality rate after TFF is between 9 and 43%1,4,5. Postoperative complications are frequent and include implant failure, surgical site infection, deep vein thrombosis, and secondary lateral wall fracture (sLWF)1,4,5,6,7. The gold standard treatment of TFF is closed reduction and internal fixation (CRIF), where the correct implant choice and position after appropriate fracture reduction is paramount to minimize the risk of secondary complications1,5,6,7,8.
For TFF, lateral wall thickness (LWT) was shown to be a strong predictor of postoperative fracture stability6,7,8. A smaller LWT increases the risk of an intra-/postoperative sLWF6,8. Postoperative sLWF were reported in 20–30% of the cases treated with a dynamic hip screw (DHS; DePuy Synthes, Zuchwil, Switzerland)6,8,9. In case of sLWF, revision surgery is required in 22–45% of cases6,8.
In 2018, the AO/OTA classification has been revised regarding TFF stability based on new insights on the importance of the LWT10. The original classification assigned TFF into different categories based on fracture morphology contributing to fracture instability, such as comminution, subtrochanteric or femoral neck extension, and trochanter detachment10,11,12. The updated AO/OTA classification considers A2.1 fractures in the original classification as being either stable A1.3 or unstable A2.1 fractures, based on an absolute LWT (aLWT) threshold of 20.5 mm10,13. All A2 fractures are considered unstable, but the degree of their instability and therefore the required treatment remain controversial14. Consequently, the definition of an unstable A2 fracture pattern must be established so that treatment approaches can be differentiated12,14. The aLWT is evaluated in anteroposterior (AP) radiographs and defined as the distance between the fracture line and a lateral point located 3 cm distally of the innominate tubercle, measured at an angle of 135° with respect to the femoral shaft axis6,10. Although this is a straightforward method to determine aLWT, it does not consider interpersonal anatomical differences. A given aLWT could lead to a larger or smaller extent of instability depending on the size of the femur. Furthermore, the caput-collum-diaphyseal-angle (CCD) affects the load transmission between the femoral head and shaft and—assuming appropriate reduction—the patient-specific magnitude of this angle should therefore be considered in the analysis of fracture stability. Currently, a constant CCD of 135° is considered when assessing the aLWT6,10. Consideration of the patient-specific anatomy could lead to a more accurate fixation stability estimation15,16,17.
The mirrored contralateral femoral anatomy is commonly used for preoperative planning when the ipsilateral femur is fractured. Measurement accuracy relies on the assumption of bilateral femoral symmetry. Several studies supported the theory of proximal femoral bilateral symmetry in patients with normal morphology and confirmed the feasibility of contralateral preoperative planning18,19,20.
Therefore, this study aimed to investigate whether an adapted, patient-specific relative lateral wall thickness (rLWT) measuring protocol using the contralateral femur as a template could allow for higher sensitivity and specificity in sLWF prediction compared to the current standard aLWT.
Materials and methods
Study outline
Two sub-studies were performed to develop and validate the novel rLWT measure.
Sub-study 1 was designed to evaluate three important factors in patients without TFF (Dataset 1) before evaluating rLWT in sub-study 2. First, the bilateral symmetry of the parameters required for the rLWT measure was evaluated. Second, the importance of CCD for rLWT was predicted by determining the relationship between CCD and total trochanteric thickness (TTT), and third, the rLWT value corresponding to the previously published aLWT threshold of 20.5 mm was calculated to predict the rLWT threshold.
In sub-study 2, the prediction accuracy of rLWT was compared with the standard aLWT for assessment of TFF stability within a cohort of patients treated with DHS (previous published, Dataset 2), including cases with sLWF6.
Patients
Sub-study 1
Dataset 1 consisted of standardized AP pelvic radiographs of 146 adult patients acquired in standing or supine position (age 67.8 ± 17.0 years (mean value ± standard deviation (SD)), range 18–95 years, 69 women and 77 men). These radiographs were retrospectively collected from the database of the Lucerne Cantonal Hospital and anonymized prior to analyses. The local Ethical Committee (Swiss Association of Research Ethics Committees, Req-2021-01202) waived the need for obtained informed consent based on complete anonymization of the data in accordance with the Declaration of Helsinki. Exclusion criteria were signs of previous surgery, injury or disease around the greater trochanter, and a missing reference sphere.
Sub-study 2
Dataset 2 consisted of radiographs of AO/OTA 31-A1 and AO/OTA 31-A2 TFF cases treated with a DHS at the Department of Orthopedics, Taichung Veterans General Hospital, Taichung, Taiwan between January 2003 and May 2012. This cohort was identical to that of a previous study investigating aLWT6. The study was approved by the Institutional Review Board of Taichung Veterans General Hospital (number TCVGH-CE12183). Written informed consent was collected in accordance with the Declaration of Helsinki. Data between the study sites was shared in an anonymized manner. The rLWT measurements (described below) were performed retrospectively. Exclusion criteria were missing informed consent, non-traumatic fractures, no available intact femur radiograph (ipsi- and contra-lateral), previous fracture in the trochanteric region, osteosynthesis with a technique different from DHS, poor fracture reduction defined as either > 20° angulation on the lateral radiograph or > 4 mm displacement of any fragment, tip-apex distance (TAD) > 25 mm (measured according to the method by Baumgaertner et al.21), or a follow-up period shorter than six months21. In total, 202 patients were included, 101 females and 101 males (age 77.5 ± 10.36 years, range 33–94 years). All patients received DHS fracture fixation according to the manufacturer's instructions. Postoperative treatment consisted of first mobilization 24–72 h postoperatively with unrestricted weight-bearing under the supervision of a physiotherapist.
Measurements
Sub-study 1
In sub-study 1, non-fractured hip radiographs of Dataset 1 were used to evaluate the CCD and TTT differences between the left and right femur of the same patient and the rLWT value corresponding to the 20.5 mm aLWT threshold1. The radiographic images were calibrated with a 25 mm diameter reference sphere. Subsequently, CCD and TTT were measured on both sides by a single surgeon at two different time points to determine the intercorrelation coefficient (ICC), and the average values were used for analysis. TTT was defined as the distance between the lateral cortex of the femur and the intertrochanteric line measured along the caput-collum axis line used for CCD measurement.
Sub-study 2
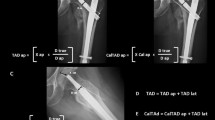
In sub-study 2, the new measurement protocol for evaluating rLWT threshold was established (Fig. 1). Two patient-specific dimensions parameters, determined in AP pelvic radiographs, were considered for measurements. CCD was chosen anticipating its influence on load transmission and TTT was considered to represent the femoral dimension. All measurements were performed on calibrated standard AP-view pelvic radiographs of Dataset 2. In seven cases, the contralateral side had a history of a hip fracture, disease, or total hip replacement. For these cases, CCD and TTT were measured on the ipsilateral side using a previous radiograph acquired before fracturing. No pelvic radiographs with an intact femur were present for three patients. For these cases, the contralateral TTT and CCD were measured on an abdominal radiograph. Subsequently, rLWT was calculated as rLWT = (LWT/TTT) × 100% where LWT was measured from the lateral cortex to the fracture line along the caput-collum axis line, considering the contralateral CCD. The distance from the tuberculum vastoadductorium (innominate tubercle) to the lateral cortex on the contralateral side was used for positioning the caput-collum axis line at the ipsilateral side (Fig. 1, green arrows). These measurements were performed by two independent surgeons to determine the ICC, and the average of the two measurements was used. The demographic data age, gender and fracture side of Dataset 2 demonstrated no significant differences between the non-sLWF (n = 166) and sLWF (n = 42) groups in the previous study6.
Illustration of the new measurement method for assessment of rLWT. (A) Calibration, (B) Defining CCD on the healthy contralateral side, (C) (red): Defining TTT as the distance from the lateral cortex to the intertrochanteric line along the caput-collum axis line, (D) Mirroring the CCD from the contralateral (B) to the fractured side and positioning the caput-collum axis line at the same distance to the innominate tubercle (green arrows), (E) (red): Measuring the LWT from the lateral cortex to the fracture line along the caput-collum axis line.
Statistical analysis
Normality distribution of the data was assumed based on the central limit theorem. Significant difference was set at 95% confidence level. IBM SPSS Statistics 26 (IBM Corp., Armonk, New York, USA) was used.
Sub-study 1
In sub-study 1, the differences between the left and right femurs were evaluated with regard to CCD and TTT using Paired-Samples t-test. Moreover, a linear regression analysis was performed to determine the CCD-TTT relationship, and the Pearson's correlation coefficient (r) was calculated. Additionally, the rLWT value corresponding to the previously published aLWT threshold of 20.5 mm was calculated to predict the rLWT threshold1.
Sub-study 2
In sub-study 2, the rLWT was compared between the sLWF and non-sLWF groups using Independent-Samples t-test. The Receiving Operating Characteristics (ROC) curve was calculated for rLWT and the area under the curve (AUC) was compared with the previously published aLWT results within the same cohort6. With regard to sLWF, the Number Needed to Treat was calculated as 1/absolute risk reduction for patients with aLWT < 20.5 mm compared to patients with rLWT < 50.5%, as well as for patients with aLWT > 20.5 mm compared to patients with rLWT > 50.5%.
Results
Sub-study 1: intra-patient symmetry (non-fractured Dataset 1)
The results from sub-study 1 (ICC, 0.953) are presented in Table 1. CCD and TTT did not differ significantly between the sides, p ≥ 0.827. Further stratification between men and women demonstrated no significant differences between left and right sides for either CCD or TTT, p ≥ 0.076. There was a moderate, positive correlation between these two variables, r = 0.438, N = 292; p < 0.001. The mean TTT increased by 0.4 mm for each degree increase of CCD. The aLWT threshold value of 20.5 mm corresponded to an rLWT value of of 52.5 ± 6.8%.
Sub-study 2: comparison of rLWT and aLWT (Dataset 2)
The results from sub-study 2 (ICC, 0.960) are presented in Table 2. Fourty-two (20.8%) of the 202 patients had sLWF. Patients without sLWF had a significantly larger rLWT (61.8 ± 15.0%) compared to the rLWT (42.7 ± 10.5%) of patients with sLWF, (p < 0.001, 95% confidence interval of the difference (CI) 14.4–24.0). After grouping the patients based on the AO/OTA fracture type, a significantly larger rLWT was seen in patients without sLWF compared to patients with sLWF in both groups with AO/OTA 31-A1 and AO/OTA 31-A2 fractures, p ≤ 0.002. Furthermore, patients with AO/OTA 31-A1 fractures had a significantly greater rLWT compared with AO/OTA 31-A2 fractures (p < 0.001, 95% CI 11.4–19.3).
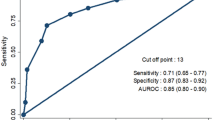
The ROC analysis revealed an rLWT AUC of 0.861 (95% CI 0.80–0.92, p < 0.001), being higher compared with the aLWT AUC of 0.823 (95% CI 0.76–0.90, p < 0.001) (Fig. 2). The optimal rLWT threshold defined by maximizing the Youden's index (specificity = 83.7% and sensitivity = 81.3%) was 50.5%, which is 2% less than the one predicted in sub-study 1. The specificity and sensitivity were improved for rLWT in comparison to aLWT by 3.5% and 1%, respectively. The Number Needed to Treat for specificity and sensitivity—64 and 75 patients, respectively—was calculated based on the data presented in Table 3 (e.g., treating 64 patients will prevent 1 patient from being categorized as stable while being unstable—subsequently having an sLWF, and treating 75 patients will prevent one patient from being categorized as unstable while being stable).
Discussion
TFF stability was reported to be partly predictable by aLWT with a 20.5 mm threshold, which has been incorporated in the preoperative treatment decision6,7,8,10. However, an absolute threshold ignores anatomical differences, which can result in an inaccurate evaluation of stability and subsequently in a higher rate of sLWF. This study established an individualized measure of rLWT for assessment of TFF stability. The feasibility of measuring rLWT using the contralateral femur was demonstrated. Furthermore, a threshold of rLWT = 50.5% was demonstrated to be a more accurate sLWF predictor after DHS implantation compared with the standard aLWT.
Anatomical variation in the proximal femur is known to have an influence on fracture risk. With increased femoral dimensions, such as femoral neck length and thickness, a rising risk of TFF was reported16,17. Contrarily, the risk in primary TFF fractures is not affected by CCD16,17. However, as shown in part one of this study, CCD correlates with TTT. Therefore, CDD indirectly influences rLWT and accordingly the stability prediction for osteosynthesis. Also, the direct influence of postoperative CCD on stability has previously been demonstrated5. The standard aLWT measure is evaluated at a fixed 135° angle with an absolute threshold of 20.5 mm, thus not considering anatomical variation6,10. The novel rLWT incorporates this variability by measuring the ratio between LWT and TTT under consideration of an individual CCD, resulting in a patient-specific value and a more accurate measure. Moreover, the 135° angle used in the definition of aLWT was based on the corresponding angle of DHS. TFF might require an implant with a different angle to gain a more anatomical fracture reduction. This raises the question whether or not aLWT is suitable in cases where an implant with a different angle is used. rLWT incorporates the angle variation and is therefore independent of the implant angle, which makes rLWT more suitable in such cases.
Stability is crucial in fracture management. Fracture morphology, defining fracture stability, therefore determines the treatment. Fracture classification systems help orthopaedic trauma surgeons to predict fracture stability based on fracture morphology10. Tawari et al. summarized the following unstable fracture configurations in TFF: fractures with posteromedial comminution, reverse oblique fractures, fractures with subtrochanteric extension, avulsed greater trochanter and lateral wall fractures12. The lateral wall importance was discussed by Gotfried et al. in understanding the fracture collapse after DHS implantation7. After validation in multiple studies, lateral wall thickness was generally accepted as a stability factor for TFF6, 8,9,10,12,14. The lateral wall acts as a buttress during fracture compression allowed by the dynamic characteristics of the DHS and other dynamic implants creating stability. In case of sLWF, extramedullary implants cannot replace the loss in stability, while intramedullary implants medialize the load transmission and the proximal end of the nail is at the level of the greater trochanter, thus providing additional stability5,10,14,22. Moreover, lateral wall weakening during surgery is associated with the drillhole of the implant and may result in sLWF, converting TFF into an unstable AO/OTA 31-A3 or subtrochanteric fracture, risking excessive telescoping and collapse5,7,9. Subsequently, the collapse contributes to postoperative morbidity, disability and the need for reoperation7,10,14.
This study investigated whether an adapted, patient-specific rLWT measure could allow for higher sensitivity and specificity in sLWF prediction compared to the current standard aLWT. The results revealed increased sensitivity and specificity (by 1.0% and 3.5%, respectively) for rLWT (Fig. 2). The predicted rLWT threshold was 52.5% based on the 20.5 mm aLWT threshold. However, the rLWT threshold, determined by maximizing sensitivity and specificity, was 50.5%, suggesting that the 20.5 mm aLWT threshold is less accurate. Moreover, rLWT was found to be relevant for both AO/OTA 31-A1 and AO/OTA 31-A2 fractures, demonstrating better differentiation compared to aLWT that reached significance only for AO/OTA 31-A2 fractures6. Both of these improvements render rLWT a promising candidate for sLWF prediction.
sLWF is associated with an incidence of 20–30% and has a major impact on the patient and the health system requiring revision surgery in 22–45% of cases6,8,9. Moreover, sLWF without the need or wish for a reoperation presumably contributes to morbidity, disability, longer rehabilitation and/or mortality. Preventing sLWF is expected to lead to better patient outcome and reduced healthcare costs.
This study has several limitations. First, the proposed measuring protocol relies on the presence of a healthy, non-deformed contralateral femur or a previous pelvic radiograph. aLWT can be a good alternative in cases where this data is not available. Second, TTT is a newly proposed measure that has not yet been validated in other studies. Third, the measuring protocol described in this study relies on a two-dimensional radiograph, which is challenging for measurement of a three-dimensional fracture line in an externally rotated femur. However, the same applies to the already established aLWT measure. Evaluating rLWT in a three-dimensional aspect from a computed tomography (CT) scan should be more accurate but would come at a higher cost and radiation exposure23,24. Today's standard in diagnosing TFF does not imply a CT scan and that is why the latter cannot be used for rLWT definition25,26. Third, the evaluation has been performed retrospectively. Further prospective clinical studies are needed to confirm its proposed accuracy.
The findings of this study suggest that rLWT should be favored over the aLWT as it achieves improved accuracy in predicting sLWF. The high incidence of TFF will continue to increase with the aging population2,3. Although rLWT has not yet been used in clinical practice, this study demonstrates increased accuracy compared with aLWT based on the same dataset. The obtained increase in specificity of sLWF prediction by 3.5% via rLWT corresponds to a Number Needed to Treat of 64 patients for preventing one more sLWF case, which has an impact on TFF treatment. Therefore, the slightly decreased reproducibility and increased complexity of the rLWT versus aLWT measurement should not be limiting factors. rLWT with the 50.5% stability threshold could be used for implant selection; however, future studies are needed to investigate this aspect. Moreover, implant selection depends on multiple factors and should be evaluated by the surgeon per individual case.
Conclusion
The novel rLWT is a more accurate and individualized predictor of sLWF after DHS fixation compared withto the standard aLWT. The 50.5% rLWT threshold could be used as an indicator for implant selection, with more sLWF and re-operations expected in extramedullary DHS treatment when the rLWT is lower than 50.5%. The current findings indicate that DHS should not be used with a rLWT below 50.5% but future studies will be needed to investigate the aspect of implant selection.
Data availability
Data are available from the authors upon reasonable request by contacting the corresponding author (Kenneth P. van Knegsel).
References
Knobe, M. & Siebert, C. H. Hip fractures in the elderly: Osteosynthesis versus joint replacement. Orthopade. 43(4), 314–324. https://doi.org/10.1007/s00132-014-2265-7 (2014).
Hagino, T. et al. Comparison of the prognosis among different age groups in elderly patients with hip fracture. Indian J. Orthop. 42(1), 29–32. https://doi.org/10.4103/0019-5413.38577 (2008).
Dhanwal, D. K., Dennison, E. M., Harvey, N. C. & Cooper, C. Epidemiology of hip fracture: Worldwide geographic variation. Indian J. Orthop. 45(1), 15–22. https://doi.org/10.4103/0019-5413.73656 (2011).
van Knegsel, K. P. et al. Trochanteric femur fractures: Application of skeletal traction during surgery does not alter soft-tissue microcirculation. Medicina 57, 9. https://doi.org/10.3390/medicina57090884 (2021).
Knobe, M., Drescher, W., Heussen, N., Sellei, R. M. & Pape, H. C. Is helical blade nailing superior to locked minimally invasive plating in unstable pertrochanteric fractures?. Clin. Orthop. Relat. Res. 470(8), 2302–2312. https://doi.org/10.1007/s11999-012-2268-9 (2012).
Hsu, C. E., Shih, C. M., Wang, C. C. & Huang, K. C. Lateral femoral wall thickness: A reliable predictor of post-operative lateral wall fracture in intertrochanteric fractures. Bone Joint J. 95(8), 1134–1138. https://doi.org/10.1302/0301-620x.95b8.31495 (2013).
Gotfried, Y. The lateral trochanteric wall: A key element in the reconstruction of unstable pertrochanteric hip fractures. Clin. Orthop. Relat. Res. 425, 82–86 (2004).
Palm, H., Jacobsen, S., Sonne-Holm, S. & Gebuhr, P. Integrity of the lateral femoral wall in intertrochanteric hip fractures: An important predictor of a reoperation. J. Bone Joint Surg. Am. 89(3), 470–475. https://doi.org/10.2106/jbjs.F.00679 (2007).
Langford, J., Pillai, G., Ugliailoro, A. D. & Yang, E. Perioperative lateral trochanteric wall fractures: Sliding hip screw versus percutaneous compression plate for intertrochanteric hip fractures. J. Orthop. Trauma. 25(4), 191–195. https://doi.org/10.1097/BOT.0b013e3181ecfcba (2011).
Meinberg, E. G., Agel, J., Roberts, C. S., Karam, M. D. & Kellam, J. F. Fracture and dislocation classification compendium—2018. J. Orthop. Trauma. 32, S1–S10 (2018).
Marsh, J. L. et al. Fracture and dislocation classification compendium-2007: Orthopaedic trauma association classification, database and outcomes committee. J. Orthop. Trauma. 21(10 Suppl), S1-133. https://doi.org/10.1097/00005131-200711101-00001 (2007).
Tawari, A. A., Kempegowda, H., Suk, M. & Horwitz, D. S. What makes an intertrochanteric fracture unstable in 2015? Does the lateral wall play a role in the decision matrix?. J. Orthop. Trauma. 29(Suppl 4), S4-9. https://doi.org/10.1097/bot.0000000000000284 (2015).
Klaber, I. et al. The new AO classification system for intertrochanteric fractures allows better agreement than the original AO classification: An inter- and intra-observer agreement evaluation. Injury. 52(1), 102–105. https://doi.org/10.1016/j.injury.2020.07.020 (2021).
Knobe, M., Gradl, G., Ladenburger, A., Tarkin, I. S. & Pape, H. C. Unstable intertrochanteric femur fractures: Is there a consensus on definition and treatment in Germany?. Clin. Orthop. Relat. Res. 471(9), 2831–2840. https://doi.org/10.1007/s11999-013-2834-9 (2013).
Gilligan, I., Chandraphak, S. & Mahakkanukrauh, P. Femoral neck-shaft angle in humans: Variation relating to climate, clothing, lifestyle, sex, age and side. J. Anat. 223(2), 133–151. https://doi.org/10.1111/joa.12073 (2013).
Dinçel, V. E., Sengelen, M., Sepici, V., Cavuşoğlu, T. & Sepici, B. The association of proximal femur geometry with hip fracture risk. Clin. Anat. 21(6), 575–580. https://doi.org/10.1002/ca.20680 (2008).
Faulkner, K. G. et al. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. J. Bone Miner. Res. 8(10), 1211–1217. https://doi.org/10.1002/jbmr.5650081008 (1993).
Zhao, R., Cai, H., Tian, H. & Zhang, K. Morphological consistency of bilateral hip joints in adults based on the X-ray and CT data. Surg. Radiol. Anat. 43(7), 1107–1115. https://doi.org/10.1007/s00276-020-02676-4 (2021).
Boese, C. K. et al. The femoral neck-shaft angle on plain radiographs: A systematic review. Skeletal. Radiol. 45(1), 19–28. https://doi.org/10.1007/s00256-015-2236-z (2016).
Young, E. Y., Gebhart, J., Cooperman, D. & Ahn, N. U. Are the left and right proximal femurs symmetric?. Clin. Orthop. Relat. Res. 471(5), 1593–1601. https://doi.org/10.1007/s11999-012-2704-x (2013).
Baumgaertner, M. R., Curtin, S. L., Lindskog, D. M. & Keggi, J. M. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J. Bone Joint. Surg. Am. 77(7), 1058–1064. https://doi.org/10.2106/00004623-199507000-00012 (1995).
Hardy, D. C. et al. Use of an intramedullary hip-screw compared with a compression hip-screw with a plate for intertrochanteric femoral fractures. A prospective, randomized study of one hundred patients. J. Bone Joint Surg. Am. 80(5), 618–630. https://doi.org/10.2106/00004623-199805000-00002 (1998).
Hecht, G. et al. CT scans better assess lateral wall morphology of “stable appearing” intertrochanteric (IT) femur fractures and predict early failure of sliding hip screw (SHS) fixation. OTA Int. 4(3), e140. https://doi.org/10.1097/oi9.0000000000000140 (2021).
Sharma, G. et al. Which AO/OTA 31–A2 pertrochanteric fractures can be treated with a dynamic hip screw without developing a lateral wall fracture? A CT-based study. Int. Orthop. 40(5), 1009–1017. https://doi.org/10.1007/s00264-015-2835-2 (2016).
Wendt, K. et al. Recommendations on hip fractures. Eur. J. Trauma Emerg. Surg. 42(4), 425–431. https://doi.org/10.1007/s00068-016-0684-3 (2016).
Jordan, R., Dickenson, E., Westacott, D., Baraza, N. & Srinivasan, K. A vast increase in the use of CT scans for investigating occult hip fractures. Eur. J. Radiol. 82(8), e356–e359. https://doi.org/10.1016/j.ejrad.2013.02.033 (2013).
Acknowledgements
The authors are not compensated and there are no other institutional subsidies, corporate affiliations, or funding sources supporting this work unless clearly documented and disclosed. This investigation was performed with the assistance of the AO Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization, K.P.v.K., M.K.; methodology, K.P.v.K., E.B., T.P., P.V., M.K.; validation, K.P.v.K., P.V., C.-E.H., K.-C.H.; formal analysis, K.P.v.K., K.-C.H.; data curation, K.P.v.K., C.-E.H., K.-C.H.; writing—original draft preparation, K.P.v.K.; writing—review and editing, K.P.v.K., E.B., T.P., K.-C.H., Be.G., P.V.; supervision, C.-E.H., Bo.G., M.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Knegsel, K.P., Hsu, CE., Huang, KC. et al. Relative lateral wall thickness is an improved predictor for postoperative lateral wall fracture after trochanteric femoral fracture osteosynthesis. Sci Rep 13, 17750 (2023). https://doi.org/10.1038/s41598-023-43929-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43929-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.