Abstract
Hyalomma dromedarii is an important tick species infesting livestock. This work evaluated the novel adulticidal, insect growth-regulating, and enzymatic efficacy of ethanol plant extracts of Aloe vera and Rheum rhabarbarum and their nanoemulsions against males and engorged females of the camel tick, H. dromedarii. The physicochemical properties of nanoemulsions were evaluated. The High-Performance Liquid Chromatography (HPLC) analyses indicated that the extracts contained polyphenols and flavonoids, which could enhance their acaricidal effect. Dynamic light scattering (DLS) of the nanoemulsions of A. vera and R. rhabarbarum were 196.7 and 291 nm, whereas their zeta potentials were − 29.1 and − 53.1 mV, respectively. Transmission electron microscope (TEM) indicated that nanoemulsions showed a regular spherical shape (less than 100 nm). Fifteen days post-treatment (PT) with 25%, the mortality% of A. vera and R. rhabarbarum were 88.5 and 96.2%, respectively. Five days PT, the median lethal concentration values of A. vera, R. rhabarbarum, and their nanoemulsions were 7.8, 7.1, 2.8, and 1.02%, respectively, and their toxicity indices were 91.02, 100, 36.4, and 100%, respectively. Their median lethal time values PT with 3.5% were 6.09, 5.09, 1.75, and 1.34 days, respectively. Nanoemulsions enhanced the efficacy of the crude extract 1–7 folds, 5 days PT, and accelerated their speed of killing ticks 2–4 times. The total protein and carbohydrates, Acetylcholinesterase, Alpha esterase, and Amylase were affected PT. The reproductive potential of engorged females was adversely impacted. In conclusion, the novel A. vera and R. rhabarbarum extracts were promising acaricides, and their nanoformulations enhanced their efficacies.
Similar content being viewed by others
Introduction
Ticks are serious ectoparasites transmitting severe infectious diseases1,2,3,4,5,6,7. Hyalomma dromedarii is a blood-feeding ectoparasite affecting livestock and causes severe economic losses because of retarded growth, weight loss, decreased milk and meat production, and transmission of serious diseases8. Various approaches for controlling ticks are dependent mainly on applying different conventional acaricides9. Despite their effectiveness, the development of resistance against many commercially available acaricides has been reported for H. dromedarii in Egypt10,11.
There is a persistent demand for searching for eco-friendly control strategies using Plant-based resources (botanicals), biological control agents, vaccination, photosensitizers, and safe acids12,13,14,15,16,17,18 to reduce the health and environmental hazards resulting from repeated applications of conventional pesticides19,20,21,22. Botanicals have been widely used, since ancient civilizations23, because of their high efficacy against pests and illness14,24,25,26,27,28,29,30,31,32, relative safety to non-target organisms27,33,34, rapid biodegradation, and prevention of development of resistance in pests because of their various active substances and mechanisms of actions19,20,21,35. Against pests of medical and veterinary importance, botanicals induce ovicidal36,37, larvicidal38,39,40,41,42,43, adulticidal36,44,45,46,47,48, repellent36,45,46, and insect growth regulating (IGR) effects38,49,50,51.
Secondary metabolites are chemical components that many plants produce such as flavonoids, phenols, carbohydrates, alkaloids, glycosides, saponins glycosides, amino acids, enzymes, tannins, essential oils, and pectins28,47,52,53,54. Botanicals contain numerous active ingredients acting as bio-pesticides, specifically as nematicides, protoscolicidal, fungicides, insect development regulators, and anti-feedants with toxicity to arthropod pests19,20,21,55,56,57.
Aloe, Aloe vera (L.) Burm.f. (Asphodelaceae) is a cactus-like plant used in numerous medicinal and cosmetic products58. It was used by the Ancient Egyptians to treat skin problems and scabies and used as pesticides and repellents30. It eases gastrointestinal disorders and dermal diseases. It has antiviral, antifungal, antibacterial, anti-inflammatory, antitumor, and immunomodulatory effects. A. vera contains diversified active components like essential and nonessential amino acids, chromones (isoaloeresin D and Isorabaichromone); carbohydrates (acetylated mannan and glucomannan); vitamins (B1, B2, α-tocopherol, and folic acid); enzymes (alkaline phosphatase, amylase,…etc); some other organic-based substances (arachidonic acid, linolic acid, triterpenoids, and lignin); and good command of organic-based active ingredients (syringic, sinapinic acid, myricetin, acid, and vanillic acid)53,58.
Rhubarb, Rheum rhabarbarum (L.) (Polygonaceae) is a medicinal plant grown worldwide (particularly in Asia) for its substantial edible petioles. Rhubarbs have long been, since the Middle Ages, used in Asia, Europe, and other places because of their antimicrobial, anti-inflammatory, antispasmodic, antioxidant, mucolytic, purgative, sedative, cardioprotective, and blood detoxifying efficacies. Moreover, R. rhabarbarum is a tough perennial herbaceous plant with close-knit and dense rhizomes. It has tuberous roots, numerous branches, triangular leaves, and long stems. It contains many biologically active compounds, the most important of which are stilbenes, flavonoids, and anthraquinones59. Rhapontigenin extracted from Rheum undulatum acts as an antioxidant protecting cells from damage caused by oxidative stress59 and the roots of R. emodi induce antioxidant and anticancer properties60.
Nanotechnology is recently used in fields related to humans and animals, especially pharmaceutical, food, and agricultural research developing nano-delivery systems to encapsulate, protect, and deliver lots of different compounds to achieve unique and picture-perfect outcomes. Colloidal dispersion is an important delivery system because of its compatibility, small size, good command of loading capacity, and stability because of its unique physiochemical properties and bioavailability. The diversity of encapsulated drugs comes from their nature. A microemulsion or nanoemulsion consists of two phases: aqueous phase, oil phase, and surfactant and/ or cosurfactant acting as an equalizer to assist the stability of nanoparticles. Such a structure makes nanoemulsion capable of accepting both hydrophilic and hydrophobic drug platforms. The aqueous phase consists of water beside some other phospholipids to enhance solubility and stability; whereas, the oil phase is varied according to the application, like oleic acid, ethyl oleate, mineral oils, vegetable oil, or triglycerides, which promotes a different type of microcrystalline called solid lipid nanoparticles (SLN) and their generations type II nanostructured lipid carrier (NLC) in size of 100 to 500nm if the triglyceride solid was used, but if liquid or semisolid was used at room temperature, it generates microemulsion with a relatively smaller size than those got from solid61,62,63. Sometimes oleic acid is used to obtain nanoemulsions based on natural extracts, trying to optimize the benefits of the two systems and continuing the co-author's work about nanomaterial research related to the insecticidal effect of natural resources like essential oils and plant extracts27,34.
Nanotechnology has been applied in numerous formulations to create many new products with faster effects and a wide range of applications in several fields such as pesticides27,33,34,41,42,64. This work aimed to evaluate the novel adulticidal, insect growth-regulating, and enzymatic efficacy of ethanol extracts of aloe and rhubarb and their nanoemulsions against males and engorged females of the camel tick, H. dromedarii.
Materials and methods
Plant source
Two plants, A.vera leaves and R. rhabarbarum stems were obtained from a local herbalist through a botanical specialist. Plant materials were collected according to institutional, national, and international guidelines and legislation. Plants were identified, and authenticated by Dr. Therese Labib, Botanical Specialist and Consultant of Plant Taxonomy, Department of Flora and Taxonomy, Ministry of Agriculture and Director of the Orman Botanical Garden, Giza, Egypt. Voucher specimens were deposited in the National Research Center’s Herbarium (CAIRC), Department of Phytochemistry and Plant Systematics, by Dr. Mona Mohamed Marzouk, Professor of Phytochemistry and Plant Chemosystematics, with respective voucher numbers for Rheum rhabarbarum L. (M180) and Aloe vera (L.) (M181).
Chemical and biochemical analysis
Chemical
Oleic acid 90%, polysorbate 20 (Tween 20), Sodium Glycocholate 97.5%, Sodium Cholate 99%, and Distilled water (de-ionized), all chemicals were purchased from Alfa Aesar, Karlsruhe, Germany, and used with no further purification. Chemicals used for biochemical analysis were the Bovine albumin standard, purchased from Stanbio Laboratory (Texas, USA); Commasie brilliant blue G-250, purchased from Sigma (Sigma Chemical Co.); P- nitroanisole (purity 97%), acquired from Ubichem Ltd. (Ham pshire); and nicotinamide ademine dinucleotide phosphate (reduced form, NADPH), got from BDH chemicals Ltd. (Poole, England). The rest of the chemicals were of high quality purchased from commercial local companies and used without further purification.
Synthesis of plant extracts
Synthesis of plant extracts before nanoformulations
Both A. vera leaves and R. rhabarbarum stems were separately washed with distilled water twice and attained to be dehydrated. After complete dryness for three days at 50 °C in a vacuum oven, the plants were ground to a fine powder and washed several times with distilled water. About 250 g of each plant was placed in a beaker containing about 600 ml of 10% ethanol (v/v). The beaker was transferred to a hotplate and the temperature was raised to 50 °C for 3 h with occasional mixing using a glass rod (every 10 min) with flipping the flakes up and down to achieve good extraction. Afterward, the beaker was attained to cool to room temperature and then cooled at a temperature of 5–10 °C for two hours. The cooled beaker was filtered several times using a cotton tissue and then filtered using a Whatman filter paper; the supernatant was re-concentrated using a vacuum rotary evaporator to 50 ml. The net solution was kept in a dark glass bottle and kept at a temperature of 5–10 °C. For easier concentration manipulation and definite solid-content weight quantification, a small volume of 15 ml (pre-weighted) was re-concentrated using freeze drier utile complete solvent evaporation and collection of solid contents.
Preparation of extract-nanoemulsions
Preparation of nanoemulsions of the plant extracts was done according to a previous portocol65 with little modifications, as follows: 2 gm of oleic acid, placed in a 50 ml beaker, was heated to 40 °C (solution I). On another 50-ml beaker, 2.5 ml solution of Tween 20 and 15 ml of concentrated extract were added to a well-stirred mixture consisting of 0.7 gm sodium glycolate, and 0.7 gm sodium taurocholate dissolved in 10 ml water, a portion-wise addition, and then the overall mixture heated to the same temperature 40 °C (solution 2). Solution I is then poured into solution II at the same temperature to get a solution of a clear nanoemulsion at 40 °C, which in turn is dispersed using an ultrasonic probe sonicator for 20 min at 500 W with the addition of 100 ml ice cold water. Mannitol was added to the dispersion as a cry protectant and then lyophilization was done to get a semi-solid substance.
Phytochemical analyses of plant extract
High-performance liquid chromatography (HPLC) analyses were done using an Agilent 1260 series. The separation process was carried out using the Eclipse C18 column (4.6 mm × 250 mm i.d., 5 μm). The mobile phase was comprised of water (A) and 0.05% trifluoroacetic acid in acetonitrile (B) at a flow rate of 0.9 ml/min. Such phase was programmed consecutively in a linear gradient as follows: 0 min (82% A); 0–5 min (80% A); 5–8 min (60% A); 8–12 min (60% A); 12–15 min (82% A); 15–16 min (82% A); and 16–20 (82%A). The multi-wavelength detector was monitored at 280 nm. The injection volume was 5 μl for each of the sample solutions. The column temperature was maintained at 40 °C.
Characterization of nanoemulsion
Droplet size and Zeta Potential
The hydrodynamic radius of the synthesized extract nanoemulsions and polydispersity index (PDI) were done by dynamic light scattering (DLS) at a fixed angle of 173° at room temperature. Zeta potential or the surface charge was measured by the frequency shift of scattered light at a scattering angle of 12°. Moreover, PDI, Radius, and Zeta potential were investigated by a Zetasizer nano Zs analyzer (Malvern instruments) at the Egyptian Petroleum Research Institute (EPRI), Cairo, Egypt. About 5–10 mg of each powder was dispersed in 10 mL of distilled water at a temperature of 25 °C.
NLC surface morphology by transmission electron microscope (TEM)
The morphology and internal structure visualization of obtained nanoemulsions were investigated using field transmission electron microscopy (HR-TEM, JSM-7100F) at EPRI. Images were recorded with JEOL JEM-2100-115 a high-resolution transmission electron microscope with an accelerating voltage of 200 kV. Nearly 1 µL of NLCs was diluted with double distilled water (1:200) and placed on a 200 mesh carbon-coated grid and attained for two min. and the excess liquid was disposed of by filter paper. One to two drops of 2% (w/w) phosphotungstic acid (PTA) were added to the grid for 10 s to achieve negative staining, the excess PTA was removed via adsorption on a filter paper.
Tick
Adult males and engorged females of H. dromedarii were collected from places around infested camels in Toukh (35 km north Cairo: 30° 21′ 11.6″ N and 31° 11′ 31.5″ E), Qalyubia Governorate, Egypt. Ticks were transferred to the laboratory in plastic cups covered by a piece of cotton net gauze. Morphological identification was performed66.
N.B. This study involved the treatment of ticks and did not involve live vertebrates. All experiments were accomplished in agreement with the relevant guidelines and regulations of the Ethical Committee of the Faculty of Veterinary Medicine, Benha University, Egypt (BUFVTM 02–10-22).
Adult immersion tests
Adulticidal effect
An in-vitro adult immersion test (AIT) was used to evaluate the toxicity of plant extracts against H. dromedarii in line with a previously described protocol67. Five and six concentrations were diluted in distilled water for each plant extract and its nanoemulsions, respectively. Ten active males were immersed for 60 s in a 100 ml solution at each concentration.
Three replicates were used for each concentration (30 ticks/ concentration) and the control group was treated with distilled water. After immersion, ticks were added to a Petri dish with filter paper (Whatman no. 1) and kept at 27 ± 2 °C and 80 ± 5% relative humidity. Tick mortalities were checked up to 15 days post-treatment (PT) and recorded as dead when no reaction was shown after stimulation with a fine brush.
Insect growth regulating effect
Tests were carried out to determine the efficacy of plant extracts before and after nanoformulations against engorged females of H. dromedarii, according to a previously described protocol18 with a slight modification. Five concentrations were freshly prepared in distilled water. Thirty ticks were individually weighted and treated as mentioned in the previously mentioned AIT protocol for each concentration. Each immersed tick was kept uprightly in a labeled vertical test tube covered with a cotton plug at 27 ± 2 °C and 80 ± 5% relative humidity. The weight of the egg mass was measured and the number of hatched eggs was counted.
Biochemical and enzyme assay analyses
Apparatus
Ticks were treated with the LC50 of each tested material and homogenized for biochemical analysis in a chilled glass Teflon tissue homogenizer (ST–2 Mechanic-Preczyina, Poland). After homogenization, supernatants were kept in a deep freezer at -20 °C till used for biochemical assays. A double-beam ultraviolet/ visible spectrophotometer (Spectronic 1201, Milton Roy Co., USA) was used to determine the optical density of the colored substances or metabolic compounds.
Preparation of ticks for analyses
Treated ticks were homogenized in distilled water (50 mg /1 ml); homogenates were centrifuged at 8000 r.p.m. for 15 min at 2 °C in a refrigerated centrifuge. After that, the deposits were discarded and the supernatant (enzyme extract) was stored at < 0 °C for less than a week until used. All experiments contained three replicates (tick homogenates) and the results of biochemical determinations were pooled in triplicates68.
Total proteins69 and total carbohydrates were assessed in an acid extract of the sample by the phenol-sulphuric acid reaction70, extracted and prepared for the assays71. Acetylcholinesterase (AchE) activity was measured using acetylcholine bromide (AchBr) as a substrate72; Alpha esterases (α-esterases) were determined using α-naphthyl acetate as a substrat73. Determination of amylase activity was also revealed68.
Data analyses
The data were analyzed through SPSS V23 (IBM, USA) to perform the one-way analysis of variance (ANOVA) (Post Hoc/Tukey's HSD (honestly significant difference) to compare the significant difference within and between groups and the Probit analyses to calculate the lethal concentration (LC) and time (LT) values. All significant levels were set at P<0.05.
The mortality data were corrected74 according to the following equation:
\({\text{Corrected Mortality}}\% \, = \, \left( {{\text{MT}}\% - {\text{ MC}}\% } \right)/ \, \left( {{1}00 - {\text{MC}}\% } \right){\text{ X 1}}00\)
MT: mortality of the treated group; MC: mortality of the control group.
The relative toxicities46 and the toxicity indices were determined75 for a comparison of the tested extracts, where the most toxic plant extract had given 100 units on the toxicity index scale.
Ethical approval
The protocol of this work was approved by the Ethical Committee related to the Faculty of Veterinary Medicine at Benha University, Egypt (BUFVTM 02-10-22).
Results and discussion
Hyalomma ticks have economic importance76. Previous studies indicated that H. dromedarii had acquired resistance against the commercially and widely used acaricides, Deltamethrin (Butox®)10 and Phoxim® (50%, an analogous dimethyl ester, C12H15N2O3PS) in Egypt11, in Qalyubia Governorate, Egypt, the same locality of the present study. Consequently, searching for eco-friendly acaricides is a pressing need12,16,67. Besides their pesticidal effects, botanicals have fungicidal, bactericidal, and antioxidant properties; therefore, they are used in medicine and cosmetics15,24,32,49. There are few studies on the efficacy of herbal extracts against H. dromedarii10,78,79,80,81 and very rare studies tested their effect on its reproductive potential50. This study evaluated the innovative acaricidal efficacy of ethanolic extracts of A. vera and R. rhabarbarum and their nanoemulsions against males and engorged females of H. dromedarii to break its life cycle.
Phytochemical analyses
HPLC of the ethanol extracts
To identify the components presented in the ethanol extracts of A. vera and R. rhabarbarum extracts in this study, HPLC analysis was carried out with 19 standard polyphenols (Table 1). Active ingredient polyphenols were revealed for A. vera (Table 2) and R. rhabarbarum (Table 3) and the HPLC analysis indicated that both ethanol extracts were enriched with polyphenolic and flavonoid active ingredients that may give a good interpretation of their acaricidal and IGR effects. Alike findings were reported28,35,81. The most active ingredients identified by GC-MS analysis in A. vera gel extract were Terpene and Sesquiterpene hydrocarbons82. Moreover, a combination of active ingredients in the extract could synergistically increase the biological activity of the extract19,20,51.
This study revealed that A. vera extract was rich in many active ingredients, such as catechin, gallic acid, naringenin ellagic acid, and ferulic acid (567.51, 441.29, 346.56, 273.18, and 210.02 µg/g, respectively) and good amounts of methyl gallate, chlorogenic acid, coumaric acid, syringic acid, daidzein, hesperetin, querectin, apigenin, pyro catechol, rutin, and cinnamic acid (93.59, 90.73, 78.17, 58.09, 58.00, 47.23, 36.88, 19.53, 15.69, 13.64 and 3.11 µg/g, respectively). In contrast, kaempferol, vanillin, and coffeic acid had no participation from A. vera extract (Table 2).
In contrast to our findings, another study implied that A. vera contains acemannan, valoin a and b, homonataloin, Aloe emodin, etc. in its latex. Furthermore, aloe leaf extract contains 99% water and 75 active compounds including vitamins, minerals, amino acids, and enzymes (peroxidase, lipase, cellulase, catalase, carboxypeptidase, bradykinase, amylase, and alkaline phosphatase)83.
Similar to the contents of A. vera in the present study, R. rhabarbarum contained catechin, querectin, daidzein, ferulic acid, gallic acid, and naringenin (6476.79, 2316.96, 2163.61, 1573.21, 1350.86, and 1113.26 µg/g, respectively); whereas, coumaric acid, cinnamic acid, apigenin, chlorogenic acid, hesperetin, coffeic acid, methyl gallate, and pyro catechol have good abundance (561.51, 320.55, 290.25, 231.19, 180.59, 59.14, 17.28, and 3.93 µg/g, respectively). On the other hand, rutin, vanillin, kaempferol, ellagic, and syringic acids had no abundance in R. rhabarbarum (Table 3).
Phytochemicals in Rhubarbs in another study included stilbenes, anthraquinones, and flavonoids59. The phytochemical characterization of the extracts of Aloe arborescens enabled the identification of the presence of condensed and water-soluble tannins, besides anthraquinones, including aloeresin, aloenin, aloin A and B, homonataloin, and 4′-O-glucosylisoaloeresin. Water-soluble tannins were the main components of the extracts with acaricidal activity84. The presence of the phenolic contents may play an important role in the elimination of ticks and mites through their action on the GABA receptor and the octopamine receptor85.
Against H. dromedarii in Egypt, Saussurea costus extract contains mainly sesquiterpene, fatty acid esters, phenols, and acyclic hydrocarbons which might explain its pesticidal effect86. A similar study showed that myrrh is slightly more effective than ginger against H. dromedarii and this may be due to its high contents of flavonoids and phenols10. The total phenolic and flavonoid contents of nine aqueous plant extracts explain their acaricidal efficacy; Ricinus communis possessed the highest total phenolic contents (95.50 ± 0.17 mg/g), while Quercus cortex contained the highest flavonoid contents (70.78 ± 0.17 mg QE/g)77.
Physicochemical properties of nanoemulsion- plant extracts
Dynamic light scattering (DLS) and Zeta potential (z.p)
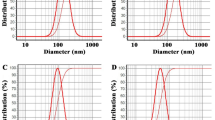
According to the "Brownian motion", when a small suspended particle moves through a liquid solution, it will undergo randomized motion because of the collisions by the molecules themself and; consequently, particles. Furthermore, DLS is a very reliable and powerful technique to study the diffusion attitude of the small to relatively large particles in a solution depending on the particle’s size (hydrodynamic radii) and shape variations. After size and shape data collection, the data were analyzed internally by the device to obtain the degree of the heterogeneity, or what so-called poly dispersity index (PDI), of the solution based on size variation, which may occur as a result of agglomeration or aggregation. The International Organization for Standardization (ISOs) declared that if the values of PDI were less than 0.05, the solution under investigation is more likely to be monodispersed or homogenous, while values > 0.7 indicated that the solution is commonly of broad size or polydisperse particle distribution27,87. In this study, DLS of the prepared A. vera extract nanoemulsion pretended particle size of 196.7 nm with pdi of 0.300, confirmed that the synthesized A. vera extract nanoemulsion had a good particle size with polydisperse particle distribution. Like A. vera extract nanoemulsion, R. rhabarbarum nanoemulsions showed a slightly larger average size of 291nm with little reduction in the pdi value of 0.239, but it was still fit to be described by" polydispersed” particle distribution (Table 4).
The stability of the colloidal system related to the dispersion of solid materials in the liquid was monitored with zeta potential. It is caused by the net electrical charges in a specific region in a slipping plane depending on the location of such plane. Zeta potential magnitude indicates the degree of electrostatic repulsion forces between the same adjacent charged particles in the dispersion. For small particles like molecules, a higher zeta potential will promote stability, i.e., dispersion will resist aggregation, and vice versa when the zeta potential is small enough to make the attractive forces exceed the repulsion forces, i.e., particles become closer to each other and the dispersion will be flocculated34. Consequently, colloids with high values of zeta potential, even negative or positive, are electrically stabilized while colloids with low zeta potential values have a large tendency to flocculate or coagulate. It is interesting that the results of this study showed values of zeta potential of R. rhabarbarum extract nanoemulsion of −53.1 mV. The larger negatively charged numerical value reflects the higher repulsion forces exerted on the system due to the similar negative charges. Increasing the number and concentrations of polyphenols and flavonoids in the extract promoted extra negative charges because of a wide spread of hydroxyl groups. From this point of view, A. vera nanoemulsion extract contained less concentration of polyphenolic active ingredients that may affect the net charge or zeta potential to be −29.1 mV. Comparatively, the zeta potential of the nanoemulsion of R. rhabarbarum is much greater than that of A. vera indicating that R. rhabarbarum nanoemulsin had excellent stability. In the meantime, both nanoemulsions have good stability attitudes88.
Transmission electron microscope (TEM) findings of plant extract-loaded nanoemulsions
The transmission electron microscope is one of the most important analyses to confirm the internal structure of nanoemulsions prepared in this study (Figs. 1 and 2). Nanoemulsions of R. rhabarbarum and A. vera extracts showed regular and spherical shapes in various sizes (less than 100nm); whereas that of R. rhabarbarum presented a slightly higher size near 200 to 600nm confirming the polydispersity depicted by PDI findings. The revealed size in this study came along with data obtained by zeta sizer; the results of DLS measure the average size, not definite particle size like that of TEM27.
Acaricidal activity of the plant extracts against H. dromedarii
The mortality data of the present study showed a time and concentration-dependent relationship; similar studies were recorded12,67. Three days post-treatment (PT) with 25% of A. vera and R. rhabarbarum, the mortality percentage (MO%) reached 50 and 53.6%, respectively. Five days PT, MO% reached 61.5 and 65.4%, respectively. Meanwhile, 15 days PT, MO% reached 88.5% and 96.2%, respectively (Table 5). Three days PT, the LC50 and LC95 values of A. vera were 9.8 and 25.6%; whereas those of R. rhabarbarum were 8.9 and 24.8%, respectively (Table 6). Such values five days PT were 7.8 and 22.9% for A. vera and 7.1 and 21.4% for R. rhabarbarum, respectively (Table 7). The toxicity index values were 90.8% and 100%, at three days PT, and reached 91.02 and 100%, respectively, at five days PT (Tables 6 and 7). Meanwhile, LT50 values PT with 25% and 3.5% of A. vera were 1.86 and 6.09 days; while those of R. rhabarbarum were 1.65 and 5.09 days, respectively (Table 8).
Parallel to our findings, the hydroethanolic extract of the leaves of Aloe rupestris induced an acricidal effect (66.6%) against the cattle tick, Rhipicephalus turanicus (Acari: Ixodidae) at a concentration of 20% (200 mg/mL)89. In addition, aqueous extracts (5%) of A. vera, garlic, Allium sativum, and ginger, Zingiber, officinale, against the brown dog tick, Rhipicephalus sanguineus, showed acaricidal effect (100%) against females, males, and larvae and showed cuticular damage along with breaching and loss of homogeneity of both epicuticle and endocuticle90. The findings of A. vera in this study came along with some other studies as it has a great potential for development as a botanical acaricide against the carmine spider mite, Tetranychus cinnabarinus (LC50 values of the acetone extract were 0.614 and 0.099 mg/ml, 48 h and 72 h PT, respectively)91 and the females of the two-spotted spider mite, Tetranychus urticae (LC50 values of petroleum ether, methanol, ethanol, and acetone extracts reached 1.279, 0.953, 0.667, and 0.446%, respectively)92.
Very recent and similar studies revealed the efficacy of other botanicals as acaricides. Complete mortalities of H. dromedarii and Rhipicephalus (Boophilus) annulatus were recorded PT with 25 mg/mL of Araucaria heterophylla and Commiphora molmol extracts for seven days. The LC50 values PT of H. dromedarii with the methanol and hexane extracts were 1.13 and 1.04 mg/mL and 1.47 and 1.38 mg/mL, respectively; whereas such values against R. annulatus were 1.09 and 1.41 as well as 1.55 and 1.08 mg/mL, respectively28. The influence of the ethanol extracts (25%) of costus, Vitex castus, and, Z. officinale, against H. dromedarii demonstrated that MO% 15 days PT reached 80.8 and 84.7%, respectively; their LC50 values, five days PT, were 10.50 and 9.60%, respectively; and their toxicity indices reached 91.43 and 100.00%, respectively. On the other hand, their LT50 values PT with 25% were 2.6 and 2.5 days, respectively78. Moreover, the adulticidal effect of olive, Olea europaea L, oil against H. dormidarii males reached 83.33%, 15 days PT with 25%, and its LT50 and LT95 were 5.161 and 17.072 days, respectively, whereas its LC50 and LC95 values were 12.715 and 46.386%, respectively, 12 days PT50.
Analogs to our findings, another species of R. palmatum extract and five of its isolated anthraquinones possessed insecticidal activity against the brown planthopper, Nilarparvata lugens, and the northern armyworm, Mythimna separate. A high amount of R. palmatum compounds (82.87 mg/g) were detected in its acetone extract inducing stronger insecticidal effect than those of the aqueous and ethanol extracts (yields = 8.84, 37.17, and 36.92%, respectively). Amongst the isolated compounds in such study, emodin displayed very high and moderate insecticidal activity, as its LC50 values were 84.30 and 548.74 μg/mL, respectively). A similar pattern was recorded for toosendanin (LC50= 89.34 and 418.24 μg/mL, respectively93. In contrast to our superior acaricidal effect, fractions of R. palmatum root extract and anthraquinone (aloe-emodin, emodin, chrysophanol, and physcion) showed no acaricidal activities against the mite, T. urticae94. Different outcomes might be related to using different pest species and extracts. Moreover, R. palmatum has fungicidal93,94 and herbicidal activitie94.
Similar adulticidal efficacies were reported after treating the engorged females of H. dromedarii, collected from the same locality of the present study and treated with the same in vitro immersion bioassays, and a 100% lethal effect was recorded at 8 h PT with 2% rose Bengal (a photosensitizer) and 24 h PT with 2.5%, ivermectin (a macrocyclic lactone). Their LT50 values were 0.92 and 2.63 h PT with 2% rose bengal and 2.5% ivermectin, respectively, whereas their LC50 values were 0.08 and 0.35%, respectively, and their LC95 values reached 1.45 and 30.07%, respectively18. An analogous study specified that a complete adulticidal effect was recorded PT of the engorged females of H. dromedarii with 4% safranin and tetramethrin, for 8 and 48 h, respectively. LC50 values eight and 24 h PT were 0.08 and 0.03% as well as 0.78 and 0.20%, respectively. In addition, LT50 of safranin and tetramethrin were 0.80 and 2.17 h, respectively, PT with 4%67. Against H. dromedarii, Methylene blue was the most effective material 3 days PT (LC50=127 ppm) followed by safranin, field stain, rhodamine 6G, phthalocyanine, echinochrome, ribofavin, and chlorophyllin (LC50= 209, 251, 271, 303, 324, 332, and 362 ppm, respectively). Their LT50, values reached 45, 87, 96, 72, 129, 115, 131, and 137 h, respectively, PT with 240 ppm11.
A comparable study revealed the acaricidal activity of Citrus limetta seed oil against the cattle tick, Rhipicephalus microplus (LC50 = 2.87 and 3.96% PT of larvae and adults, respectively) and 100% MO were reached PT with 12.5%95. Furthermore, the crude extract along with water and petroleum ether fractions of Areca (A.) catechu seeds were effective against cypermethrin-resistant R. (R.) microplus53. In addition, Saussurea costus as methanol and hexane extracts of effectively controlled cattle and camel ectoparasites; MO% of H. dromedarii, seven days, PT with 12.5 and 25 mg/ml was 100 and 90% (LC50 = 1.37 and 2.33 mg/ml, respectively). In the meantime, such values against R. (B) annulatus were 100 and 93.33% coupled with 1.23 and 1.95 mg/ml, respectively86. A comparable acaricidal effect of an aqueous neem extract against Sarcoptes scabiei var. cuniculi in vitro and experimentally-infested rabbits was recorded14.
Insect growth regulating effects of plant extracts against H. dromedarii
After treatment with A. vera and R. rhabarbarum in this study, the reproductive potential of engorged females was adversely affected when compared to that of the control group. After treatment with the highest concentration, 25%, egg production had ceased and engorged female weight reached 54.00 and 51.83 g, respectively (Table 9). Analogs result was recorded as the ethanol extract of A. vera effectively reduced egg production/ female of T. urticae, followed by acetone, methanol, and petroleum ether extracts by 96.0, 94.0, 85.0, and 83.0%, respectively (LC50 = 0.950, 1.406, 2.115, and 3.312%, respectively). Moreover, ethanol extract was the most effective repellent against T. urticae females92.
A similar finding revealed a strong effect on the reproductive parameters represented by a marked decrease in the number of laid eggs PT of fresh and dry Aloe arborescens Mill. extracts (solvents pure ethanol, ethanol-dichloromethane binary mixture, and ethanol-dichloromethane-acetone ternary mixture, contained water-soluble tannins) against engorged females of Rhipicephalus (Boophilus) microplus84. A related finding revealed that Citrus limetta seed oil significantly (p < 0.001) lowered the oviposition rate, egg hatching, and reproduction efficiency of treated ticks of R. microplus94. Furthermore, Azadirachtin, a tetranortriterpene extracted from neem (LC50 = 0.47 ppm) adversely affected the fecundity and the development of the follicular epithelial cells of ovaries of female N. lugens, and the weight of the treated females was significantly reduced, 23, 40, and 64% PT with 0.1, 0.25, and 0.5 ppm, respectively96.
Few studies evaluated IGRs against H. dromedarii collected from the same locality of this study, olive oil induced IGR effect and adversely impacted the reproduction of the engorged females of H. dromedarii PT with 25% and adversely affected the number of hatched eggs (2.83 ± 2.31), hatchability (32.7%), as well as weights of females (52.50 ± 2.88 g) and egg masses (0.27 ± 0.27 g)50. Moreover, the number of survived females, ovipositing females, eggs per female, ticks laid hatched eggs, and hatched eggs were reduced PT with 0.01% of rose bengal (48.98%, 93.33%, 1854.53 ± 45, 97.5%, and 93.64%, respectively) and 0.02% of ivermectin (26.53%, 86.67%, 7661.27 ± 377, 87.80%, and 89.40%, respectively), respectively18. A comparable results were also recorded, PT with a low concentration (0.03%) of both safranin (75.0%, 89.13%, 3116 ± 70.26, 95.24%, and 81.00%, respectively) and tetramethrin (33.3%, 100.00%, 0.00, 100.00, and 100.00%, respectively67.
Acaricidal activity of nanoemulsions against H. dromedarii
This study indicated the efficacy of nanoemulsions of A. vera and R. rhabarbarum against H. dromedarii PT with 15%; MO% reached 83.3 and 76.7%, respectively, three days PT; 86.6 and 90%, respectively, five days PT; and 100% for both extracts, 12 and nine days PT, respectively (Table 10). The LC50 and LC95 values were 4.2 and 17.67% PT for three days with nanoemulsions of A. vera and reached 3.5 and 17.4% PT with nanoemulsions of R. rhabarbarum, respectively (Table 11). Five days PT, the LC50 and LC95 values were 2.8 and 15.8% as well as 1.02 and 32.72%, respectively (Table 12). Regarding the LC50 values, the prepared nanoformulations enhanced the efficacy of the ethanol extracts of A. vera and R. rhabarbarum 2.3 and 2.5 times, three days PT (Table 11), and 2.8 and 7 times, five days PT (Table 12). R. rhabarbarum induced a superior effect and its toxicity index reached 100% (Tables 11 and 12). The LT50 values of A. vera and R. rhabarbarum were 0.898 and 0.839 days, respectively, PT with 15% and 1.75 and 1.34 days, respectively, PT with 3.5%. Nanoemulsions accelerated the speed of killing of ticks 2-4 times faster than the ethanol extracts (Table 13).
The finding of this study came along with a previous study that used different concentrations of methanol leaf extract, green synthesized silver (AgNPs), and chitosan nanoparticles (CsNPs) using A. vera and Nerium oleander against Musca domestica and indicated that the nanoparticles were more potent than the methanol extract97. Furthermore, a corresponding study indicated that the larvicidal effect of silver nanoparticles AgNPs and CsNPs encapsulated A. vera gel extract against M. domestica was documented and their relative efficacies were almost 40.65 and 148.51 times more effective than the A. vera crude extrac82.
A similar study revealed the acaricidal effect of the aqueous extracts of C. molmol and Z. officinale against H. dromedarii in Egypt as their MO% reached 96 and 84.01%, respectively, 15 days PT with 17%, whereas complete MO was reached seven and nine days PT with 12% of their corresponding AgNPs extracts, synthesized physically via laser ablation. Their LC50 values reached 10.37, 12.81, 2.38, and 4.12%, respectively, three days PT and their LT50 values were 5.6, 6.73, 2.25, and 3.56 days, respectively, PT with 4%. Such extracts reduced R. (Boophilus) microplus three days PT of naturally infested cattle by 54.45, 45.73, 100, and 100%, respectively, whereas such ticks acquired resistance against Deltamethrin (Butox®)10. In addition, AgNPs of R. rhabarbarum have antibacterial activity towards Gram-positive (+ve) strains of Staphylococcus aureus and Gram-negative (−ve) strains of Escherichia coli98.
Insect growth regulating effects of nanoemulsions against H. dromedarii
This study illustrated the significant (p < 0.05) adverse effect of nanoemulsions of A. vera and R. rhabarbarum as IGRs on the reproduction of the engorged females. After treatment with their lowest concentrations (0.75%), the hatchability%, the number of hatched eggs, and the weights of engorged females and egg masses were 61.9%, 116.66, 51.33, and 2.86 g as well as 51.9%, 138.33, 51.0, and 3.93 gm, respectively. Whereas PT with the highest concentration, 25%, egg production was suppressed and engorged female weights were 48.50 and 51.50g, respectively. Nanoemulsions of both extracts adversely affected the reproductive potential of treated engorged females than those of the crude ethanol extracts (Table 14). A similar study proved the IGR effect of AgNPs and CsNPs encapsulated A. vera gel extract after treatment of larvae of M. domestica as they prolonged larval duration and reduced the pupation and adult emergence rates82.
Biochemical characterization
Enzymes are usually used as reliable indicators for evaluating the impact of the applied toxic materials against insects99,100; the mechanisms of insecticide resistance mostly include enzymes involved in the detoxification of carbamates, organophosphates, pyrethroids, and growth regulators such as non-specific esterase, Glutathione-S-transferase (GSTs), and P450-mediated monooxygenase (MFOs)100. Generally, biochemical analyses of the present work revealed that the total protein, carbohydrates, AChE, Alpha esterase, and Amylase were affected after treatments; their values PT with nanoformulations were significantly (p < 0.05) more affected than those of the control group, except for the total protein level. The level of Alpha esterase protein was significantly (p < 0.05) increased in the case of Rheum extract (Table 15).
Analog results revealed that Citrus limetta seed oil disturbed the defensive and target enzymes of Rhipicephalus microplus via reducing the levels of SOD, GST, MAO, and AChE and increasing NOS level in ticks when compared with the control group95. Furthermore, azadirachtin significantly inhibited the activity of AChE when compared with control of N. lugens96. Essential oils can diminish esterase, glutathione S-transferases (GSTs) activities, and the total carbohydrate, lipid, and protein contents in Tribolium castaneum101,102.
Aloe and Rheum extracts in this study significantly decreased the total protein as compared to the control group. Similarly, reduction in total protein content (representing defensive and target enzymes) is a common occurrence in insects after treatment with toxic compounds, but increased after treatment of a field strain of the mosquito, Culex pipiens, with some IGRs like lufenuron and novaluron100. Such variation might be due to using different species and compounds.
In this study, there was an increased level of AChE after treatment with A. vera ethanol extract but decreased PT with its nanoemulsion and R. rhabarbarum extracts. Similarly, essential oils as well as carvacrol have an AChE inhibitory effect indicating that the position of the hydroxyl group in the structure plays an important role85. AChE is the main physiological target of many synthetic acaricides such as organophosphate (OP) and carbamate leading to overstimulation of the neurons and rapid twitching of the muscles, convulsions, and death. Treatment with A. vera extract significantly increased AChE level in this work. A parallel study indicated that imidacloprid significantly increased AChE, Glutathione-S-transferases, GSTs, and activities per protein content of the water flea Daphnia magna (Daphniidae: Anomopoda) 21 days PT103. A similar recent study showed elevated levels of AChE, α and β esterases, and GSH after treatment of Cx. pipiens with IGRs like lufenuron100.
In contrast, AChE level was decreased PT with Rheum nanoemulsion in this study. Alike, AchE activity was significantly inhibited in larvae of R. annulatus PT with fennel oil and its main constituents, trans-anethole and fenchone104; and larvae of Rhipicephalus microplus PT with the n-hexane extract of Calea serrate105.. Moreover, emodin, extracted from Rheum palmatum, exhibited significant AChE and GST inhibitory activities when applied against Nilarparvata lugens and Mythimna separate (IC50 = of 11.36 and 4.18 μg/mL, respectively)93.
It is worth mentioning that silver and graphene oxide nanoparticles affected insect antioxidant and detoxifying enzymes, inducing oxidative stress and cellular death106. The pesticidal action of the synthesized green nanoparticles could be induced because of their penetration into insect exoskeleton, ability to bind to the sulfur element with proteins or DNA phosphorylation, and rapid denaturation of saturation106,107.
Plant extracts contain secondary compounds derived from plants that perform useful functions against insects by acting as repellents, antifeedants, and toxins and have provided alternative resources for insect control. They are also characterized by their biodegradation and minimal harmful effects on non-target organisms19,55,56. In general, botanicals could be recognized as safe19,20,21. R. rhabarbarum is a medicinal edible plant consumed worldwide59. A. vera has anticoccidial, antibacterial, and immunomodulatory effects; therefore, it enhances the intestinal health and performance of birds when used as a safe feed additive108. The aqueous and hydroethanolic extracts of Tabernaemontana elegans; Calpurnia aurea, Schkuhria pinnata, and Aloe rupestris (leaves, stems, whole plant, and leaves, respectively) not only effectively controlled R. turanicus, but also were safe or very safe on human Vero kidney and liver HepG2 cells89. Temulawak (Curcuma xanthorrhiza Roxb) nanoemulsion is safe and improves chickens’ productivity and performance and could prevent the risks of antibiotic residues and resistance109. No symptoms of skin irritation or abnormal health observation were observed among operators as well as birds44 and buffaloes36, post spraying and pour-on applications, respectively, of essential oils; rabbits PT with aqueous neem extract14; and cattle after spot-on application with aqueous and silver nanoformulations of C. molmol and Z. officinale10.
Conclusions
This investigation proved that the novel ethanol extracts of A. vera and R. rhabarbarum and their nanoemulsion induced effective acaricidal and growth-regulating effects against H. dromedarii. Nanoformulations of A. vera and R. rhabarbarum enhanced the efficacy of the ethanol extracts 1.5- 2.5 times three days PT, and 1-7 times five days PT and accelerated the speed of killing ticks 2-4 times faster than the ethanol extracts. They also adversely affected the reproductive potential of engorged females. Consequently, they could prevent tick bites and their associated diseases as eco-friendly acaricides. It is indicated from this investigation that botanicals could be used for the progress of benign and eco-friendly acaricides against H. dromedarii. Further studies could be directed towards in vivo and ecotoxicological studies of A. vera and R. rhabarbarum.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali, S. et al. Species distribution and seasonal dynamics of equine tick infestation in two subtropical climate niches in Punjab, Pakistan. Pak. Vet. J. 40, 25–30 (2020).
Farooq, R. et al. Molecular characterization and phylogenetic analysis of Babesia species isolated from domestic cattle. Pak. Vet. J. 40, 224–228 (2020).
Hegab, A., Fahmy, M., Omar, H., Abuowarda, M. & Gattas, S. Investigation of tickborne pathogens within naturally infected brown dog tick (ixodidae: Rhipicephalus Sanguineus) in Egypt by light and electron microscopy. Int. J. Vet. Sci. 9, 476–482 (2020).
Peter, S. Zoonotic Anaplasma and Ehrlichia infections and their potential reservoirs: A review. Int. J. Vet. Sci. 9, 1–9 (2020).
Ceylan, O., Uslu, A., Ozturk, O. & Sevinc, F. Serological investigation of some vector-borne parasitic and rickettsial agents in dogs in the western part of Turkey. Pak. Vet. J. 41, 386–392. https://doi.org/10.29261/pakvetj/2021.036 (2021).
Hussain, S., Saqib, M., Ashfaq, K. & Sindhu, Z. U. D. First molecular evidence of coxiella burnetii in ticks collected from dromedary camels in Punjab, Pakistan. Pak. Vet. J https://doi.org/10.29261/pakvetj/2021.073 (2021).
Rahman, A. et al. A review of tick and tick control strategies in Pakistan. Pak. J. Med. Health Sci. 16, 652–655. https://doi.org/10.53350/pjmhs22161652 (2022).
Perveen, N., Muzaffar, S. B., Vijayan, R. & Al-Deeb, M. A. Microbial communities associated with the camel tick, Hyalomma dromedarii: 16S rRNA gene-based analysis. Sci. Rep. 10, 1–11 (2020).
George, J. E. Present and future technologies for tick control. Ann. N. Y. Acad. Sci. 916, 583–588. https://doi.org/10.1111/j.1749-6632.2000.tb05340.x (2000).
Nabil, M. et al. Acaricidal efficacy of silver nanoformulations of Commiphora molmol and Zingiber officinale against the camel Tick, Hyalomma dromedarii (Ixodida: Ixodidae). Inorg. Chem. Commun. 147, 110229. https://doi.org/10.1016/j.inoche.2022.110229 (2023).
Mohammed, S. H. et al. Correction: Acaricide resistance and novel photosensitizing approach as alternative acaricides against the camel tick, Hyalomma dromedarii. Photochem. Photobiol. Sci. 22, 693. https://doi.org/10.1007/s43630-022-00329-6 (2023).
Khater, H. F. & Ramadan, M. Y. The acaricidal effect of peracetic acid against Boophilus annulatus and Argas persicus. Acta Sci. Vet. 35, 29–40 (2007).
Khater, H. F., Seddiek, S. A., El-Shorbagy, M. M. & Ali, A. M. Erratum to: The acaricidal efficacy of peracetic acid and deltamethrin against the fowl tick, Argas persicus, infesting laying hens. Parasitol. Res. 112, 3669–3678. https://doi.org/10.1007/s00436-013-3563-4 (2013).
Seddiek, S. A., Khater, H. F., El-Shorbagy, M. M. & Ali, A. M. The acaricidal efficacy of aqueous neem extract and ivermectin against Sarcoptes scabiei var. cuniculi in experimentally infested rabbits. Parasitology Research 112, 2319–2330. https://doi.org/10.1007/s00436-013-3395-2 (2013).
Pavela, R. & Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant. Sci. 21, 1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005 (2016).
Benelli, G., Pavela, R., Canale, A. & Mehlhorn, H. Tick repellents and acaricides of botanical origin: A green roadmap to control tick-borne diseases?. Parasitol. Res. 115, 2545–2560. https://doi.org/10.1007/s00436-016-5095-1 (2016).
Muhammad, G., Naureen, A., Firyal, S. & Saqib, M. Tick control strategies in dairy production medicine. Pak. Vet. J. 28, 43–50 (2008).
Khater, H. & Hendawy, N. Photoxicity of rose bengal against the camel tick, Hyalomma dromedarii. Int. J. Vet. Sci. 3, 78–86 (2014).
Khater, H. F. Prospects of botanical biopesticides in insect pest management. Pharmacologia 3, 641–656 (2012).
Khater, H. F. Ecosmart biorational insecticides: Alternative insect control strategies. in Advances in integrated pest management (eds: Farzana Preveen) 17–60 (InTechopen, 2012).
Khater, H. F. Bioactivity of essential oils as green biopesticides: recent global scenario. in Recent progress in medicinal plants Vol. 37 (eds: Govil JN and Bhattacharya S) 151–218 (Studium Press LLC, 2013).
Zahoor, M. A. et al. Teratogenic effects of thiamethoxam (a neonicotinoid) on development of chick embryo. Pak. Vet. J. 42, 179–184. https://doi.org/10.29261/pakvetj/2022.033 (2022).
Khater, H. F. Herbal and horticultural Remedies: Gardening for the Elderly and Physically and Mentally Disabled. 257 (Authorhouse UK Ltd, 2020).
Khater, H. F. et al. Avian coccidiosis: Recent advances in alternative control strategies and vaccine development. Agrobiol. Records 1, 11–25 (2020).
Seddiek, S. A., El-Shorbagy, M. M., Khater, H. F. & Ali, A. M. The antitrichomonal efficacy of garlic and metronidazole against Trichomonas gallinae infecting domestic pigeons. Parasitol. Res. 113, 1319–1329. https://doi.org/10.1007/s00436-014-3771-6 (2014).
Abbas, R. Z. et al. Anthelmintic effects and toxicity analysis of herbal dewormer against the infection of Haemonchus contortus and Fasciola hepatica in goat. Pak. Vet. J. 40, 455–460 (2020).
Radwan, I. T., Baz, M. M., Khater, H. & Selim, A. M. Nanostructured lipid crriers (nlc) for biologically active green tea and fennel natural oils delivery: Larvicidal and adulticidal activities against. Culex pipiens Mol. 27, 1939. https://doi.org/10.3390/molecules27061939 (2022).
Baz, M. M. et al. Novel pesticidal efficacy of Araucaria heterophylla and Commiphora molmol extracts against camel and cattle blood-sucking ectoparasites. Plants 11, 1682. https://doi.org/10.3390/plants11131682 (2022).
Vaz, N. P., De Oliveira, D. R., Abouelella, G. A. & Khater, H. The black seed, Nigella sativa (Ranunculaceae), for prevention and treatment of hypertension. in Metabolic Disorders: Hypertension" of the Series "Recent Progress in Medicinal Plants Vol. 48 (eds: JN Govil and Bhardwaj N) 221–244 (Studium Press LLC, 2018).
Khater, H. F. Introductory chapter: Back to the future-solutions for parasitic problems as old as the pyramids. in Natural remedies in the fight against parasites (eds: Govindarajan M Khater HF, Benelli G) 4–19 (IntechOpen, 2017).
Imran, A. & Alsayeqh, A. Anticoccidial efficacy of citrus sinensis essential oil in broiler chicken. Pak. Vet. J. 42(461), 466. https://doi.org/10.29261/pakvetj/2022.082 (2022).
Altaf, S. et al. Antioxidant rich medicinal plants as a potential candidate to treat gastric ulcer. Boletin Latinoamericano Y Del Caribe De Plantas Medicinales Y Aromáticas 22, 560–580. https://doi.org/10.37360/blacpma.23.22.5.41 (2023).
Murugan, K. et al. Predation by Asian bullfrog tadpoles, Hoplobatrachus tigerinus, against the dengue vector, Aedes aegypti, in an aquatic environment treated with mosquitocidal nanoparticles. Parasitol. Res. 114, 3601–3610. https://doi.org/10.1007/s00436-015-4582-0 (2015).
Radwan, I. T., Baz, M. M., Khater, H., Alkhaibari, A. M. & Selim, A. M. Mg-LDH Nanoclays Intercalated fennel and green tea active ingredient: Field and laboratory evaluation of insecticidal activities against Culex pipiens and their non-target organisms. Molecules 27, 2424. https://doi.org/10.3390/molecules27082424 (2022).
Khater, H. F. Bioactivity of essential oils as green biopesticides: Recent global scenario. Recent Progr. Med. Plants 37, 151–218 (2013).
Khater, H. F., Ramadan, M. Y. & El-Madawy, R. S. Lousicidal, ovicidal and repellent efficacy of some essential oils against lice and flies infesting water buffaloes in Egypt. Vet. Parasitol. 164, 257–266. https://doi.org/10.1016/j.vetpar.2009.06.011 (2009).
Khater, H. F. et al. Ovicidal aroma shields for prevention of blow fly strikes caused by Lucilia sericata (Meigen), Diptera: Calliphoridae. Vector-Borne Zoonot. Dis. 22, 459–464. https://doi.org/10.1089/vbz.2021.0107 (2022).
Khater, H. F. Bioactivities of some essential oils against the camel nasal botfly, Cephalopina titillator. Parasitol. Res. 113, 593–605. https://doi.org/10.1007/s00436-013-3688-5 (2014).
Khater, H. F., Hanafy, A., Abdel-Mageed, A. D., Ramadan, M. Y. & El-Madawy, R. S. Control of the myiasis-producing fly, Lucilia sericata, with Egyptian essential oils. Int. J. Dermatol. 50, 187–194. https://doi.org/10.1111/j.1365-4632.2010.04656.x (2011).
Alkenani, N. A. et al. Molecular identification and bio-control of mosquitoes using black seeds extract in Jeddah. Pak. Vet. J. 41, 359–364 (2021).
Govindarajan, M., Khater, H. F., Panneerselvam, C. & Benelli, G. One-pot fabrication of silver nanocrystals using Nicandra physalodes: A novel route for mosquito vector control with moderate toxicity on non-target water bugs. Res. Vet. Sci. 107, 95–101 (2016).
Govindarajan, M. et al. Single-step biosynthesis and characterization of silver nanoparticles using Zornia diphylla leaves: A potent eco-friendly tool against malaria and arbovirus vectors. J. Photochem. Photobiol. B: Biol. 161, 482–489 (2016).
Alkenani, N. A. et al. Molecular identification and bio-control of mosquitoes using black seeds extract in Jeddah. Pak. Vet. J. https://doi.org/10.29261/pakvetj/2021.025 (2021).
Khater, H. F., El-Shorbagy, M. M. & Seddiek, S. A. Lousicidal efficacy of camphor oil, d-phenothrin, and deltamethrin against the slender pigeon louse, Columbicola columbae. Int. J. Vet. Sci. Med. 2, 7–13 (2014).
Khater, H. F. & Geden, C. J. Efficacy and repellency of some essential oils and their blends against larval and adult house flies, Musca domestica L. (Diptera: Muscidae). J. Vector Ecol. 44, 256–263 (2019).
Khater, H. F. & Geden, C. J. Potential of essential oils to prevent fly strike and their effects on the longevity of adult Lucilia sericata. J. Vector Ecol. 43, 261–270. https://doi.org/10.1111/jvec.12310 (2018).
Baz, M. M., Selim, A., Radwan, I. T., Alkhaibari, A. M. & Khater, H. F. Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. Sci. Rep. 12, 4406. https://doi.org/10.1038/s41598-022-08223-y (2022).
Abdel-Meguid, A. D., Ramadan, M. Y., Khater, H. F. & Radwan, I. T. Louicidal efficacy of essential oils against the dog louse, Trichodectes canis (Mallophaga: Trichodectidae). Egypt. Acad. J. Biol. Sci. E. Med. Entomol. Parasitol. 14, 1–16. https://doi.org/10.21608/EAJBSE.2022.218673 (2022).
Khater, H. F. Biocontrol of Some Insects (Benha University, 2003).
Abosalem, H. S., Ramadan, M. Y., Selim, A. M. & Khater, H. F. Novel acaricidal and insect growth regulating activity of olive oil against Hyalomma dromedarii (Acari: Ixodida). Benha J. Appl. Sci. 7, 91–96. https://doi.org/10.21608/bjas.2022.253618 (2022).
Khater, H. F., Ramadan, M. Y. & Mageid, A. D. A. In vitro control of the camel nasal botfly, Cephalopina titillator, with doramectin, lavender, camphor, and onion oils. Parasitol. Res. 112, 2503–2510. https://doi.org/10.1007/s00436-013-3415-2 (2013).
Sirikantaramas, S., Yamazaki, M. & Saito, K. Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochem. Rev. 7, 467–477. https://doi.org/10.1007/s11101-007-9080-2 (2008).
Naseer, M. S., Iqbal, Z. & Aslam, B. In vitro efficacy of areca catechu against cypermethrin resistant Rhipicephalus microplus and its phytochemical analysis. Pak. Vet. J. 42, 414–418 (2022).
Baz, M. M., Hegazy, M. M., Khater, H. F. & El-Sayed, Y. A. Comparative evaluation of five oil-resin plant extracts against the mosquito larvae, Culex pipiens Say (Diptera: Culicidae). Pak. Vet. J. 41, 191–196. https://doi.org/10.29261/pakvetj/2021.010 (2021).
Ahmed, N. et al. Botanical insecticides are a non-toxic alternative to conventional pesticides in the control of insects and pests. in Global Decline of Insects (eds: Hamadttu El-Shafie) 1–19 (IntechOpen 2021).
Iqbal, T. et al. Botanical Insecticides and their Potential as Anti-Insect/Pests: Are they Successful against Insects and Pests? in Global Decline of Insects (eds: Hamadttu El-Shafie) 123–149 (IntechOpen, 2021).
Shnawa, B. H., Jalil, P. J., Aspoukeh, P., Mohammed, D. A. & Biro, D. M. Protoscolicidal and biocompatibility properties of biologically fabricated zinc oxide nanoparticles using Ziziphus spina-christi Leaves. Pak. Vet. J. 42, 517–525. https://doi.org/10.29261/pakvetj/2022.058 (2022).
Zagórska-Dziok, M. et al. Evaluation of clinical effectiveness of Aloe vera—A review. J. Pre-Clin. Clin. Res. https://doi.org/10.26444/jpccr/74577 (2017).
Kolodziejczyk-Czepas, J. & Liudvytska, O. Rheum rhaponticum and Rheum rhabarbarum: A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 20, 589–607. https://doi.org/10.1007/s11101-020-09715-3 (2021).
Rajkumar, V., Guha, G. & Ashok Kumar, R. Antioxidant and anti-cancer potentials of Rheum emodi rhizome extracts. Evid. -Based Complement. Alternat. Med. 2011, 697986. https://doi.org/10.1093/ecam/neq048 (2011).
Pandey, V., Gajbhiye, K. R. & Soni, V. Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv. 22, 199–205. https://doi.org/10.3109/10717544.2013.877100 (2015).
Singh, J., Garg, T., Rath, G. & Goyal, A. K. Advances in nanotechnology-based carrier systems for targeted delivery of bioactive drug molecules with special emphasis on immunotherapy in drug resistant tuberculosis—A critical review. Drug Deliv. 23, 1676–1698. https://doi.org/10.3109/10717544.2015.1074765 (2016).
Patra, J. K. et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 16, 71. https://doi.org/10.1186/s12951-018-0392-8 (2018).
Roni, M. et al. Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf. 121, 31–38 (2015).
Moradi, S. & Barati, A. Essential oils nanoemulsions: Preparation, characterization and study of antibacterial activity against Escherichia coli. Int. J. Nanosci. Nanotechnol. 15, 199–210 (2019).
Apanaskevich, D. A., Schuster, A. L. & Horak, I. G. The genus Hyalomma: VII. Redescription of all parasitic stages of H.(Euhyalomma) dromedarii and H.(E.) schulzei (Acari: Ixodidae). J Med Entomol 45, 817–831 (2008).
Khater, H., Hendawy, N., Govindarajan, M., Murugan, K. & Benelli, G. Photosensitizers in the fight against ticks: Safranin as a novel photodynamic fluorescent acaricide to control the camel tick Hyalomma dromedarii (Ixodidae). Parasitology Research 115, 3747–3758. https://doi.org/10.1007/s00436-016-5136-9 (2016).
Amin , T. R. Biochemical and physiological studies of some insect growth regulators on the cotton leafworm , Spodoptera littoralis (Boisd.) Ph.D. thesis thesis, Cairo University., (1998).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Dubios, M., Gilles, K., JK, H., PA, R. & F, S. Colorimetric method for determination of sugars and related substances. Anal Chem 28, 350–356 (1956)
Crompton, M. & Birt, L. Changes in the amounts of carbohydrates, phosphagen, and related compounds during the metamorphosis of the blowfly, Lucilia cuprina. J. Insect Physiol. 13, 1575–1592. https://doi.org/10.1016/0022-1910(67)90180-1 (1967).
Simpson, D., Bull, D. & Lindquist, D. A semimicrotechnique for the estimation of cholinesterase activity in boll weevils. Ann. Entomol. Soc. Am. 57, 367–371. https://doi.org/10.1093/aesa/57.3.367 (1964).
Van Asperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 8, 401–416 (1962).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 18, 265–267 (1925).
Zidan, Z. & Abdel-Megeed, M. New approaches in pesticides and insect control. Sun, J, 78–85 (1988).
Jonsson, N., Davis, R. & De Witt, M. An estimate of the economic effects of cattle tick (Boophilus microplus) infestation on Queensland dairy farms. Aust Vet J 79, 826–831. https://doi.org/10.1111/j.1751-0813.2001.tb10929.x (2001).
Abd Elgawad, S., Baz, M., Taie, H., Mustafa, S. & Khater, H. Novel acaricidal efficacy of nine Egyptian plants against the camel tick, Hyalomma dromedarii (Ixodida: Ixodidae). Persian J. Acarol. 12, 121–136. https://doi.org/10.22073/pja.v12i1.76977 (2023).
Abdel-Ghany, H., Allam, S. A., Khater, H., Selim, A. & Abdel-Shafy, S. Effects of commercial oils on the camel tick Hyalomma dromedarii (Acari: Ixodidae) and their enzyme activities. Persian J. Acarol. 12, 137–149 (2023).
Eltaly, R. et al. Novel acaricidal activity of Vitex castus and Zingiber officinale extracts against the camel tick, Hyalomma dromedarii. Int. J. Vet. Sci. 12, 255–259. https://doi.org/10.47278/journal.ijvs/2022.184 (2023).
Mohammed, S. H. et al. Acaricide resistance and novel photosensitizing approach as alternative acaricides against the camel tick, Hyalomma dromedarii. Photochem. Photobiol. Sci. 22, 87–101. https://doi.org/10.1007/s43630-022-00301-4 (2023).
Baz, M. M., Hegazy, M. M., Khater, H. F. & El-Sayed, Y. A. Comparative evaluation of five oil-resin plant extracts against the mosquito larvae, Culex pipiens Say (Diptera: Culicidae). Pak. Vet. J 41, 191–196. https://doi.org/10.29261/pakvetj (2021).
El-Monairy, O. M., El-Sayed, A. A., Emara, M. M. & Abdel-Meguid, A. D. Larvicidal activity of green synthesized silver nanoparticles and chitosan nanoparticles encapsulated Aloe vera gel extract against Musca domestica (Diptera: Muscidae). Curr. Mater. Sci.: Former.: Recent Patents Mater. Sci. 15, 102–114. https://doi.org/10.2174/2666145414666210602151312 (2022).
Adlakha, K., Koul, B. & Kumar, A. Value-added products of Aloe species: Panacea to several maladies. S. Afr. J. Bot. https://doi.org/10.1016/j.sajb.2020.12.025 (2021).
De Matos, A. C., Ribeiro, C. M., Scarminio, I. S., Afonso, S. & Vidotto, O. Phytochemical analysis and acaricidal activity of Aloe arborescens Mill. extracts against Rhipicephalus (Boophilus) microplus. Semina Ciências Agrárias 38, 3113–3121. https://doi.org/10.5433/1679-0359.2017v38n5p3113 (2017).
Camilo, C. J. et al. Acaricidal activity of essential oils: A review. Trends Phytochem. Res. 1, 183–198 (2017).
Hegazy, M. M. et al. The efficacy of Saussurea costus extracts against hematophagous arthropods of camel and cattle. Pak. Vet. J. https://doi.org/10.29261/pakvetj/2022.064 (2022).
Mudalige, T. et al. Chapter 11 - Characterization of Nanomaterials: Tools and Challenges. in Nanomaterials for Food Applications (eds: Amparo López Rubio, Maria José Fabra Rovira, Marta martínez Sanz, & Laura Gómez Gómez-Mascaraque) 313–353 (Elsevier, 2019).
Kumar, A. & Dixit, C. K. 3 - Methods for characterization of nanoparticles. in Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids (eds: Surendra Nimesh, Ramesh Chandra, & Nidhi Gupta) 43–58 (Woodhead Publishing, 2017).
Fouche, G. et al. Acaricidal activity of the aqueous and hydroethanolic extracts of 15 South African plants against Rhipicephalus turanicus and their toxicity on human liver and kidney cells. Onderstepoort. J. Vet. Res. 86, e1–e7. https://doi.org/10.4102/ojvr.v86i1.1665 (2019).
Jeyathilakan, N., Sundar, S., Sangaran, A. & Latha, B. In vitro acaricidal properties of aqueous extracts of Allium sativum, Zingiber officinale and Aloe vera on brown dog tick, Rhipicephalus sanguineus. J. Vet. Parasitol. 33, 41–46. https://doi.org/10.5958/0974-0813.2019.00008.1 (2019).
Wei, J., Ding, W., Zhao, Y.-G. & Vanichpakorn, P. Acaricidal activity of Aloe vera L. leaf extracts against Tetranychus cinnabarinus (Boisduval)(Acarina: Tetranychidae). J. Asia-Pacif. Entomol. 14, 353–356. https://doi.org/10.1016/j.aspen.2011.04.006 (2011).
Basunia, R. et al. Acaricidal and repellent effects of Aloe vera L. leaf extracts against Tetranychus urticae Koch (Acari:Tetranychidae). J. Med. Plants 9, 10–15. https://doi.org/10.22271/plants.2021.v9.i6a.1345 (2021).
Shang, X.-F. et al. Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crops Prod. 137, 508–520. https://doi.org/10.1016/j.indcrop.2019.05.055 (2019).
Jang, S. J. & Kuk, Y. I. Effects of different fractions of Rheum palmatum root extract and anthraquinone compounds on fungicidal, insecticidal, and herbicidal activities. J. Plant Dis. Protect. 125, 451–460. https://doi.org/10.1007/s41348-018-0179-z (2018).
Jain, P., Satapathy, T. & Pandey, R. K. Acaricidal activity and biochemical analysis of Citrus limetta seed oil for controlling Ixodid Tick Rhipicephalus microplus infesting cattle. System. Appl. Acarol. 26(1350–1360), 1311. https://doi.org/10.11158/saa.26.7.13 (2021).
Senthil Nathan, S. et al. Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotoxicol. Environ. Saf. 70, 244–250. https://doi.org/10.1016/j.ecoenv.2007.07.005 (2008).
Ramadan, R. H., Abdel-Meguid, A. & Emara, M. Effects of synthesized silver and chitosan nanoparticles using Nerium oleander and Aloe vera on antioxidant enzymes in Musca domestica. Catrina Int. J. Environ. Sci. 21, 9–14 (2020).
Reddy, P. R., Ganesh, S. D., Saha, N., Zandraa, O. & Sáha, P. Ecofriendly synthesis of silver nanoparticles from garden rhubarb (Rheum rhabarbarum). J. Nanotechnol. 1–9, 2016. https://doi.org/10.1155/2016/4964752 (2016).
Lushchak, V. I., Matviishyn, T. M., Husak, V. V., Storey, J. M. & Storey, K. B. Pesticide toxicity: A mechanistic approach. EXCLI J. 17, 1101. https://doi.org/10.17179/excli2018-1710 (2018).
El-Sitiny, M. F. A., Gad, M. E., Khater, H. F. & Mahmoud, M. G. Susceptibility of Culex pipiens L. (Diptera, Culicidae) in Sharkia Governorate, Egypt to Chitin Synthesis Inhibitors and their Biochemical Characterizations. Benha J. Appl. Sci. (2023).
Ebadollahi, A., Khosravi, R., Sendi, J. J., Mahboubi, M. & Kosari, A. A. Chemical composition of essential oil from Zhumeria majdae Rech. F. & Wendelbo and its bioactivities against Tribolium castaneum Herbst (Tenebrionidae) larvae. J. Essent. Oil Bear. Plants 17, 824–831. https://doi.org/10.1080/0972060X.2014.935038 (2014).
Ebadollahi, A., Khosravi, R., Sendi, J. J., Honarmand, P. & Amini, R. M. Toxicity and physiological effects of essential oil from Agastache foeniculum (Pursh) Kuntze against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) larvae. Annu. Res. Rev. Biol. 3, 649–658 (2013).
Jemec, A., Tišler, T., Erjavec, B. & Pintar, A. Antioxidant responses and whole-organism changes in Daphnia magna acutely and chronically exposed to endocrine disruptor bisphenol A. Ecotoxicol Environ Saf 86, 213–218. https://doi.org/10.1016/j.ecoenv.2012.09.016 (2012).
Aboelhadid, S., Kamel, A., Arafa, W. & Shokier, K. Effect of Allium sativum and Allium cepa oils on different stages of Boophilus annulatus. Parasitol. Res. 112, 1883–1890. https://doi.org/10.1371/journal.pone.0260172 (2013).
Ribeiro, V. L. S. et al. Effect of Calea serrata Less n-hexane extract on acetylcholinesterase of larvae ticks and brain Wistar rats. Vet. Parasitol. 189, 322–326. https://doi.org/10.1016/j.vetpar.2012.04.033 (2012).
Benelli, G., Caselli, A. & Canale, A. Nanoparticles for mosquito control: Challenges and constraints. J. King Saud Univ. Sci. 29, 424–435. https://doi.org/10.1016/j.jksus.2016.08.006 (2017).
Sultana, N. et al. Bio-nanoparticle assembly: A potent on-site biolarvicidal agent against mosquito vectors. RSC Adv. 10, 9356–9368 (2020).
Khan, R. U. et al. Aloe vera: A sustainable green alternative to exclude antibiotics in modern poultry production. Antibiotics 12, 44. https://doi.org/10.3390/antibiotics12010044 (2022).
Orinetha, J., Salsabil, J. K., Putri, S. M. & Pratama, A. M. Temulawak (Curcuma xanthorrhiza Roxb.) nanoemulsion can be substituted as natural growth promoter in broiler chickens. Pak. Vet. J. 42, 409–413. https://doi.org/10.29261/pakvetj/2022.022 (2022).
Acknowledgements
The co-authors would like to thank LEAP-Agri and STIFA for partially supporting this work. Grant numbers: 220-MeTVAC and 13520-220. The co-authors appreciated the help of Ms. Therese Labib, Botanical Specialist and consultant at Orman Botanical Garden, Giza, Egypt, for plant identification and Dr. Azza A. Moustafa, Moustafa, Professor at the Research Institute of Medical Entomology, Egypt for her help and support.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was partially supported by the funding agencies, LEAP-Agri (A Long-term EU-Africa Research and Innovation Partnership on Food and Innovation on Food and Nutrition Security, and Sustainable Agriculture), project No: 220-MeTVAC, as well as Science, Technology & Innovation Funding Authority, Egypt, Project ID: 13520-220. Project title: "Ecosmart Alternative Control Strategies against Theileria annulata and its Tick Vectors".
Author information
Authors and Affiliations
Contributions
Conceptualization, I.R., H.K., R.E., and M.B.; methodology, H.K, M.B.; software, R.E., and A.S.; validation, H.K., M.B., R.E., and H.T.; formal analysis, R.E., and H.K.; investigation, A.S., R.E., M.Y.; resources, R.E., E.M., and K.K.; data curation, K.K., I.R., R.E., and M.B.; writing—original draft preparation, R.E., H.K., E.M., M.Y.; writing—review and editing, R.E., E.M., H.T., M.B. and H.K.; visualization, H.K., I.R, H.T., R.E., and M.B.; supervision, H.K.; project administration, H.K.; funding acquisition, H.K. All authors have read and agreed to the published version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Radwan, I.T., Eltaly, R.I., Baz, M.M. et al. Novel acaricidal and growth-regulating activity of Aloe vera and Rheum rhabarbarum extracts and their oil/water nanoemulsions against the camel tick, Hyalomma dromedarii. Sci Rep 13, 16802 (2023). https://doi.org/10.1038/s41598-023-43776-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43776-6
This article is cited by
-
Synthesis of eco-friendly layered double hydroxide and nanoemulsion for jasmine and peppermint oils and their larvicidal activities against Culex pipiens Linnaeus
Scientific Reports (2024)
-
Acaricidal Efficacy of Thirty-Five Egyptian Plants Against the Camel Tick, Hyalomma Dromedarii
Acta Parasitologica (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.