Abstract
Geological carbon storage provides an efficient technology for the large-scale reduction of atmospheric carbon, and the drive for net-zero emissions may necessitate the future usage of oil reservoirs for CO2 projects (without oil production), hence, dynamic modeling of an oil reservoir for CO2 storage in the Bredasdorp basin, South Africa, was therefore conducted. Injection into the reservoir was for 20 years (2030–2050), and 100 years (2050–2150) to study the CO2–brine–oil interactions, with sensitivities carried out on reservoir boundary conditions. The closed boundary scenario experienced pressure buildup with a target injection rate of 0.5 Mt/year, and a cutback on injection rate progressively until 2050 to not exceed the fracture pressure of the reservoir. The CO2 plume migration was not rapid due to the reduced volume of CO2 injected and the confining pressure. The system was gravity dominated, and gravity stability was not attained at the end of the simulation as fluid interfaces were not yet flat. The open boundary reservoir did not experience a pressure buildup because all boundaries were open, the target injection rate was achieved, and it was a viscous-dominated system. In both cases, the dissolution of CO2 in oil and brine was active, and there was a growing increase of CO2 fraction dissolved in water and oil, a decline in gaseous mobile CO2 phase between 2050 and 2150, and active trapping mechanisms were structural trapping, dissolution in oil and water, and residual trapping. The study showed that boundary condition was very crucial to the success of the project, with direct impacts on injection rate and pressure. This pioneering study has opened a vista on the injection of CO2 into an oil reservoir, and CO2–brine–oil interactions, with sensitivities carried out on reservoir boundary conditions in a closed and an open hydrocarbon system in South Africa.
Similar content being viewed by others
Introduction
Global sea level and temperature rise (climate change) have been constantly perceived to be caused by the untoward concentration of greenhouse gases (GHGs) in the earth’s atmosphere, and carbon dioxide (CO2) the major constituent of GHGs1,2,3,4,5,6. Other energy sources especially renewables have received significant investments, but due to the ever-increasing demand for energy globally, fossil fuels will still be in the mix for the foreseeable future1,7,8. With man’s continued reliance on fossil fuels, and CO2 being a byproduct of fossil fuel combustion, drives and initiatives to mitigate the adverse effects of climate change must constantly be on the front burner. Goal 13 (climate action), one of the goals within the Sustainable Development Goals (SDGs) as adopted in 2015 by the United Nations (UN) speaks to the necessity of taking “urgent actions to combat climate change and its impact” and targets the integration of climate change strategies into national planning and policies9,10. Geological carbon storage is presently the most efficient technology for large-scale reduction of atmospheric carbon and stemming the upward tide of associated climate challenges, as the International Energy Agency asserts it can bring about around 19% reduction in emissions of CO2 worldwide by 205011. Yearly CO2 emissions on a global scale from cement manufacture and burning of fossil fuels were 36.58 Giga tonnes per annum (Gtpa) in 2018, with 10.06 Gt (28% of the world’s entirety) contributed by China, the USA emitted 5.42 Gt (15% of the world’s total sum), and India accounted for 2.65 Gt, representing 7% of total CO2 emission of the world12,13. With 28 carbon capture and storage (CCS) projects operational worldwide as of 2020 and sequestering 41 Mtpa14, this represents a meagre 0.12% of the CO2 emissions attributable to hydrocarbon and cement industries worldwide. About 37% of these are principally geological carbon storage projects, while 73% are for enhanced oil recovery (EOR) drives, with the USA, Brazil and, Australia being the nations that sequestered the most13. Some operational carbon storage projects around the world include the Gorgon Carbon Dioxide Project, Uthmaniyah CO2-EOR project15,16,17, CNPC Jilin Oil Field CO2-EOR18,19,20 and Alberta Carbon Trunk Line (ACTL) Sturgeon Refinery CO2 Stream project21,22,23. Some under construction presently include The Sinopec Qilu Petrochemical Carbon Store Project24,25, Guodian Taizhou Power Station Carbon Capture26,27, Air Liquide Refinery Rotterdam CCS28,29,30, The ZEROS Project31,32 and Norcem Brevik Cement Plant33,34 among others.
Within the South African context, studies have shown that South Africa has the potential of storing ~ 150 Gt of CO2, with the offshore basins showing greater prospects35,36,37,38,39,40, but it is unlikely that commercially available CCS will be in place in the country before 2030 due to the present challenges of doubts in policies around climate action, delayed pilot/test underground carbon injection projects, low-priced carbon rates, dearth of commercial or profitable case studies, and the acceptability of the public to carbon storage projects in regions hosting potential storage sites41,42,43,44,45,46,47. South Africa fulfils its energy needs principally from fossil fuels, with 77% of the nation’s total electricity generation from coal-fired power plants and its power sector the 9th biggest emitter among power sectors in the world (~ 218 Mt of CO2) (Fig. 1), though these are low emissions in comparison to developed nations, cumulative emissions from the next nine African nations are below it48. Preliminary studies have been carried out to identify and estimate the capacities of potential geological CO2 reservoirs in South Africa39, with particular focus on the Zululand basin49, Algoa36,50, and the Bredasdorp basin51,52,53, with a pilot CO2 injection test scheduled for 202354,55. These continued inquests into underground carbon storage are a pointer that it will play a big part in the decarbonization of South Africa.
Core point sources of carbon dioxide emissions in South Africa are in the Free State, Gauteng, and Mpumalanga provinces hosting the coal mines and most of the coal-fired power plants. Emissions from oil refineries, gas-to-liquid, and coal-to-liquid firms are also covered here. Adapted after48.
In the bid to attain climate neutrality, Denmark, currently the largest oil producer in the European Union (EU), is set to completely phase out the production of hydrocarbon, new oil and gas extraction permits have been cancelled and when the calendar reads 2050, the hydrocarbon valves will be shut off for good. Presently, subsidies are being provided to stimulate large-scale CCS projects56,57. This means more searchlights will be beamed on oil reservoirs (without oil recovery) to make them viable CO2 sinks. Oil reservoirs present many advantages for CO2 storage, such as the presence of below-surface and on-the-ground installations and equipment that can be tailored to CO2 injection and storage (usually with some modifications), the presence of quality seal and establishment of caprock integrity which have held oil in place through geologic time, and availability of geological, hydrogeological, geophysical and engineering data for characterization of the reservoir and other petroleum system elements, among others5,58,59,60,61,62,63,64.
Therefore, this research aims to consider an oil reservoir in the offshore Bredasdorp basin, South Africa, for CO2 storage, with no enhanced oil recovery (EOR). The knowledge gained from the study will also be useful for reservoirs with pockets of unrecoverable oil due to economic, technical, commercial, or logistics reasons, and bypassed oil/unswept zones.
Methodology
With the aid of the equation-of-state CMG-GEM compositional simulator (2022.30v), dynamic simulation was conducted for CO2 injectivity and storage in the oil reservoir, with a focus on CO2 plume migration, active trapping mechanisms, and supercritical CO2–oil–brine interactions. The reservoir used for this study is a clastic type with shales, siltstones, and sandstone units, and it has a permeability range of 3–560 mD and average effective porosity of 14%51. For CO2 solubility in the aqueous phase, Henry’s law was employed65,66,67,68,69, with the formation and fluid values, as well as the composition of the oil used for the study presented in Tables 1, 2.
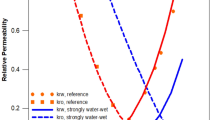
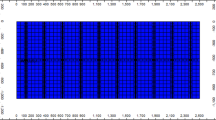
Two rock types were delineated in the reservoir based on permeability zones70, namely high (rock type 1) and low (rock type 2) permeability zones, with permeability cut-off set at 150 mD as the reservoir had prevailing permeability values of 100–560 mD51 (Fig. 2). Given three-phase flow in the reservoir (oil, CO2, and water) and at connate water saturation, for both zones, there were liquid–gas relative permeability values71, and water–oil relative permeability values (generated from Corey correlation)72,73,74,75,76 (Fig. 3).
Dynamic simulations were carried out to simulate the injection of 0.5 Mt/year of CO2 (762,000 m3/day) in the oil reservoir for 20 years (2030–2050) with one injection well, and a further simulation period of 100 years (2050–2150) to study the CO2–oil–brine interactions. Two scenarios were considered based on the boundary conditions of the reservoir, namely (i) open system (all boundaries open); and (ii) closed system (all boundaries closed).
Results and discussion
Closed boundary system
Injection and pressure
The maximum allowable pressure (well bottom-hole pressure, BHP) in the reservoir was set at 30,000 kPa, equivalent to 90% of reservoir lithostatic pressure77,78. With the target gas rate set at 0.5 Mt/year (762,000 m3/day) and the inability of pressure to dissipate in the system due to closed reservoir boundaries, the plot of BHP against time (Fig. 4) showed a steadily rising gas rate until it reached the maximum rate allowable (653,649 m3/day) to not exceed the maximum pressure, then the injection/gas rate was cut back to ensure caprock was not damaged. The average reservoir pressure rose from 25,500 kPa at the onset of injection in 2030, peaked at 30,000 kPa in 2050 and this pressure was maintained from 2050 to 2150. BHP and Well block pressure dropped to 29,741 kPa in 2050 to 2150, as the pressure due to injection of CO2 into the reservoir had ceased.
In any underground carbon storage project, a target amount of CO2 is proposed for injection into the ground, usually accompanied by pressure buildup in the system. The attainment of this desired injection rate is dependent on the injectivity of the reservoir, as an injection rate that is too high can cause the downhole pressure to exceed the fracture pressure, inducing reservoir and seal fracturing, or reactivation of existing sealing faults, all leading to environmental concerns64. A typical project that has had to deal with this scenario is the CO2 injection into the Tubåen Formation at Snøhvit, where a considerable rate of pressure at the early onset of the project led to cutting down of injection rates, and subsequent abandonment of injection into the formation when the pressure increase considerably approached the fracture pressure79,80,81.
CO2 plume migration and active flow regime
CO2 plume did not migrate rapidly away from the wellbore due to confining pressure, and reduction in the volume of CO2 injected into the reservoir with the inability to attain the intended constant injection rate of 0.5 Mt/year (Fig. 5a,c). Supercritical CO2 accumulated at the top of the reservoir due to buoyancy in 2150, rather than moving rapidly laterally (Fig. 5b,d)82. The system was gravity dominated, as counter-current movement ensued in the vertical route owing to the gravity segregation of CO2, water, and oil, and the CO2 invasion into the reservoir being stable due to gravity stability and gravity exceeding viscous forces83, it is also pertinent to note that low injection rate is accompanied with less lateral spreading of gas84. Though the gravity segregation was reduced because of relative permeability85,86,87,88, with increasing residence time, the system will be fully gravity stable evidenced by fluid interfaces being flat, as interfaces are usually destabilized by viscous forces83. There was structural trapping under the caprock, and the occurrence of non-reservoir rocks also provided local compartmentalization in the reservoir, aiding the reduction of the rapid migration of the plume.
CO2 dissolution in oil and brine
Displacement of oil by injected CO2 is active in the reservoir, though only 71% of the oil can be displaced (Fig. 6a), the heavier hydrocarbon components (29%) are not dissolved into the gas phase. Oil mass density (Fig. 6b) ranged from 650 kg/m3 in the CO2 uninvaded zones to 699 kg/m3 in the zones invaded by CO2, with water mass density (Fig. 6c) also increasing to 1031 kg/m3 in blocks that CO2 had invaded. This indicates active dissolution of CO2 in the reservoir fluids, as formation fluids density increases progressively as they become enriched with CO289,90.
Active trapping mechanism
Active CO2 trapping mechanisms were structural trapping under the caprock (supercritical mobile), dissolution and residual trapping (Fig. 7a,c). At the end of injection in 2050, 74.9 billion moles (3.3 Mt) of CO2 had been successfully injected into the reservoir, 63.7% was in the gaseous mobile phase, 27.7% had dissolved in oil, 4% was trapped residually and 4.5% had dissolved in brine (Fig. 7b,c). In 2150, 4.8% had dissolved in water, 35.9% dissolved in oil, 3.6% was trapped residually and 55.5% was in the gaseous mobile phase.
There was a growing increase in CO2 fraction dissolved in oil and water and a decline in the residually trapped and gaseous mobile CO2 phase. With further residence time, which is a core element of the CO2 storage process91,92,93, this trend should continue.
Open boundary system
Injection and pressure
With all boundaries open, there was pressure dissipation in the system. In Fig. 8, the average reservoir pressure was constant at 25,395 kPa from 2030 to 2150, maximum well bottom-hole pressure recorded was 31,000 kPa in 2050, which was below the lithostatic pressure of the formation (33,440 kPa). The pressure dropped to 25,290 kPa after cessation of injection, which was maintained till 2150. Well block pressure rose from 26,000 kPa in 2030 to 26,297 kPa in 2050 and dropped to 25,290 kPa from 2050 to 2150. Pressure dissipation in the system because of open boundaries also ensured the proposed gas injection rate of 762,000 m3/day (0.5 Mt/year) was fully attained, with no direct risks of caprock and reservoir fracture, or storage capacity destruction of the rock94.
CO2 plume migration and active flow regime
There was gradual and active lateral migration of injected CO2 away from the injection well (Fig. 9a–d), as there was no confining pressure in the reservoir. The system was viscous-dominated as increased injection rates were accompanied by increasing viscous forces95, though gravity was also active, as shown by up-dip movement (Fig. 9c,d) and vertical segregation of the CO2 plume from the bottom of the reservoir due to buoyancy, with supercritical CO2 accumulating and moving directly below the seal but did not get to the reservoir flanks (Fig. 9b,d).
CO2 dissolution in oil and brine
Oil displacement by injected CO2 was active in the reservoir, though only 70.5% of the oil could be displaced (Fig. 10a), the heavier hydrocarbon components (29.5%) are not dissolved into the gas phase. Oil mass density (Fig. 10b) ranged from < 640 kg/m3 in the CO2 uninvaded zones to 695 kg/m3 in the zones invaded by CO2, with water mass density (Fig. 10c) also increasing from 1021 kg/m3 in uninvaded zones to 1030 kg/m3 in blocks that CO2 had invaded. This is indicative of the active dissolution of CO2 in the reservoir fluids, as formation fluids density increases progressively as they become CO2 enriched89,90.
Active trapping mechanism
Active CO2 trapping mechanisms were structural trapping under the caprock (supercritical mobile), dissolution in oil and brine, and residual trapping (Fig. 11a,c). At the end of injection in 2050, 236 billion moles (10.4 Mt) of CO2 had been successfully injected into the reservoir, 67.9% was in the gaseous mobile phase, 22.8% had dissolved in oil, 4.3% was trapped residually and 4.3% had dissolved in brine (Fig. 11b,c). In 2150, 4.7% had dissolved in water, 29.2% dissolved in oil, 4.2% was trapped residually and 61.7% was in the gaseous mobile phase.
There was a gradual decline in the gaseous mobile CO2 phase and an increase in the CO2 fraction dissolved in oil and water. This leaning should continue upon further residence time, and a marked increase in CO2 dissolution in water occasioned by an influx of brine into the reservoir.
Conclusion
In this study, an oil reservoir in the offshore lying Bredasdorp basin, South Africa, has been considered for CO2 storage, without enhanced oil recovery (EOR). The closed boundary scenario experienced a pressure buildup with a target injection rate of 0.5 Mt/year, and therefore a cutback on injection rate progressively until 2050 to ensure the reservoir and overlying seal were not damaged. Migration of the CO2 plume was not rapid, due to the reduced volume of CO2 that was injected and confining state of the reservoir, the system was gravity dominated but did not attain gravity stability at the end of the simulation. There was a growing increase of CO2 fraction dissolved in water and oil and a decline in the gaseous mobile CO2 phase between 2050 and 2150. In 2150, 4.8% had dissolved in water, 35.9% dissolved in oil, 3.6% was trapped residually and 55.5% was in the gaseous mobile phase. The open boundary state experienced no pressure buildup in the reservoir and the target injection rate of 0.5 Mt/year was achieved, and 10.4 Mt of CO2 had been successfully injected into the reservoir. CO2 plume migrated up-dip without getting to the reservoir flanks, it was a viscous-dominated system attended with gravity movement and segregation. With an increase in the density of formation fluids, the dissolution of CO2 in brine and oil was active, active trapping mechanisms were structural trapping, dissolution in oil and water and residual trapping. There was a decline in the gaseous mobile CO2 phase and an increase in CO2 fraction dissolved in oil and water between 2050 and 2150.
With further residence time, fractions of CO2 dissolved in the oil and brine phases would increase, as well as residually trapped fractions, with the CO2 gaseous mobile phase experiencing a continuous decline. Therefore, this study showed that boundary condition was key to the success of the project, as it impacts injection rate and pressure.
Data availability
The data that support the findings of this study are available from the Petroleum Agency of South Africa (PASA) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Petroleum Agency of South Africa (PASA).
References
Farajzadeh, R., Eftekhari, A. A., Dafnomilis, G., Lake, L. W. & Bruining, J. On the sustainability of CO2 storage through CO2 enhanced oil recovery. Appl. Energy 261, 114467–114745 (2012).
Tkemaladze, G. S. & Makhashvili, K. A. Climate changes and photosynthesis. Ann. Agrar. Sci. 14, 119–126 (2016).
IPCC. IPCC report Global warming of 1.5°C. Glob. Warm. 1.5°C. An IPCC Spec. Rep. impacts Glob. Warm. 1.5°C above pre-industrial levels Relat. Glob. Greenh. gas Emiss. pathways, Context Strength. Glob. response to Threat Clim. Change 2, 17–20 (2018).
Ginbo, T., Di Corato, L. & Hoffmann, R. Investing in climate change adaptation and mitigation: A methodological review of real-options studies. Ambio https://doi.org/10.1007/s13280-020-01342-8 (2020).
Fagorite, V. I., Onyekuru, S. O., Opara, A. I. & Oguzie, E. E. The major techniques, advantages, and pitfalls of various methods used in geological carbon sequestration. Int. J. Environ. Sci. Technol. 20, 4585–4614 (2022).
Zhao, K. et al. Recent advances and future perspectives in carbon capture, transportation, utilization, and storage (CCTUS) technologies: A comprehensive review. Fuel 351, 128913 (2023).
Petroleum, B. BP Energy Outlook 2035 (BP Stats, 2014).
Mohr, S. H., Wang, J., Ellem, G., Ward, J. & Giurco, D. Projection of world fossil fuels by country. Fuel https://doi.org/10.1016/j.fuel.2014.10.030 (2014).
UN. Department of Economic and Social Affairs (United Nations, 2014).
United Nations: Department of Economic and Social Affairs. #Envision2030 Goal 13: Climate Action | United Nations Enable. United Nations https://www.un.org/development/desa/disabilities/envision2030-goal13.html (2020).
Cao, C. et al. A review of CO2 storage in view of safety and cost-effectiveness. Energies 13, 600 (2020).
Ritchie, H., Roser, M. & Rosado, P. CO2 and greenhouse gas emissions. Our world data (2020).
Li, H., Lau, H. C., Wei, X. & Liu, S. CO2 storage potential in major oil and gas reservoirs in the northern South China Sea. Int. J. Greenh. Gas Control 108, 103328 (2021).
Global CCS Institute. Global Status of CCS (2020).
Saini, D. Simultaneous CO2-EOR and storage projects. In Springer Briefs in Petroleum Geoscience and Engineering 11–19 (Springer Nature, 2017).
Weyand, K., Haider, B. A. & Zhang, L. A young professional’s guide to lucky no. 7: A tale of oil in the Saudi Arabian Desert. W. Ahead 11, 35–36 (2015).
Liu, H., Consoli, C. & Zapantis, A. Overview of carbon capture and storage (CCS) facilities globally. SSRN Electron. J. https://doi.org/10.2139/ssrn.3366353 (2020).
Hill, L. B., Li, X. C. & Wei, N. CO2-EOR in China: A comparative review. Int. J. Greenh. Gas Control 103, 103173. https://doi.org/10.1016/j.ijggc.2020.103173 (2020).
Song, X., Wang, F., Ma, D., Gao, M. & Zhang, Y. Progress and prospect of carbon dioxide capture, utilization and storage in CNPC oilfields. Pet. Explor. Dev. 50, 229–244 (2023).
Yuan, S. et al. Progress and prospects of carbon dioxide capture, EOR-utilization and storage industrialization. Pet. Explor. Dev. 49, 955–962 (2022).
Heal, K. & Kemp, T. North west sturgeon refinery project overview—Carbon capture through innovative commercial structuring in the Canadian oil sands. In Energy Procedia Vol. 37 7046–7055 (Elsevier, 2013).
Novak Mavar, K., Gaurina-Međimurec, N. & Hrnčević, L. Significance of enhanced oil recovery in carbon dioxide emission reduction. Sustainability (Switzerland) 13, 1–27. https://doi.org/10.3390/su13041800 (2021).
Zhang, K., Lau, H. C. & Chen, Z. Regional carbon capture and storage opportunities in Alberta, Canada. Fuel 322, 124224 (2022).
Guo, Y. et al. The influence of narrative versus statistical evidence on public perception towards CCS in China: Survey results from local residents in Shandong and Henan provinces. Int. J. Greenh. Gas Control 84, 54–61 (2019).
Meng, X. L. Low-carbon development strategy and CCS/CCUS practices of Sinopec. Carbon Manag. Technol. Conf. 2015 Sustain. Econ. CCUS Options C. 2, 1031–1036 (2015).
Garcia, M. D. Maximum carbon dioxide capture, sequestration and maximum carbon dioxide beneficial reuse at the optimal cost efficiency for the power generation sector to address global temperature rise (2021).
Restrepo-Valencia, S. & Walter, A. CO2 capture in a thermal power plant using sugarcane residual biomass. Energies 16, 4570 (2023).
de Haas, J. & van Dril, T. Decarbonisation Options for the Industry Cluster Botlek/Pernis Rotterdam (2022).
Cioli, M., Schure, K. M. & Van Dam, D. Decarbonisation options for the Dutch industrial gases production. Manufacturing Industry Decarbonisation Data Exchange Network Decarbonisation options for the Dutch industrial gases production (2021).
Oliveira, C. & Schure, K. M. Decarbonisation options for the Dutch dairy processing industry. PBL Netherlands Environ. Assess. Agency; TNO 63 (2020).
Joseph, E. T. et al. Carbon sequestration: Capture, storage and utilization of CO2 emissions from anthropogenic sources. AIP Conf. Proc. 2521 (2023).
Page, B. et al. The Global Status of CCS 2020: Vital to Achieve Net Zero (2020).
Hägg, M. B., Lindbråthen, A., He, X., Nodeland, S. G. & Cantero, T. Pilot demonstration-reporting on CO2 capture from a cement plant using hollow fiber process. Energy Procedia 114, 6150–6165 (2017).
Adriana, R.-L., Gardarsdottir, S. O., Biermann, M., Lundqvist, K. & Harvey, S. Potential impact of the Preem CCS project. 11th Trondheim Conf. Carbon Capture, Transp. Storage 10–18 (2021).
Viljoen, J. H. A., Stapelberg, F. D. J. & Cloete, M. Technical Report on the Geological Storage of Carbon Dioxide (2010).
Chabangu, N. et al. The investigation of CO2 storage potential in the Algoa basin in South Africa. Energy Procedia 63, 2800–2810 (2014).
Viebahn, P., Vallentin, D. & Höller, S. Integrated assessment of carbon capture and storage (CCS) in South Africa’s power sector. Energies 8, 14380–14406 (2015).
Viebahn, P. CCS Global final report—Part 4: Country study South Africa (2012).
Cloete, M. ATLAS on geological storage of carbon dioxide in South Africa 1–61 (2010).
Hendriks, C., Graus, W. & van Bergen, F. Global carbon dioxide storage potential and costs. Ecofys. Utr. 64 (2004).
Viebahn, P., Vallentin, D. & Höller, S. Prospects of carbon capture and storage (CCS) in China’s power sector—An integrated assessment. Appl. Energy 157, 229–244 (2015).
Viebahn, P., Vallentin, D. & Höller, S. Prospects of carbon capture and storage (CCS) in India’s power sector—An integrated assessment. Appl. Energy 117, 62–75 (2014).
European Commission. Consultative Communication on The Future of Carbon Capture and Storage in Europe (2013).
Zhu, L. & Fan, Y. A real options–based CCS investment evaluation model: Case study of China’s power generation sector. Appl. Energy 88, 4320–4333 (2011).
Qian, C. & Rennings, K. Low carbon scenarios vs. clean coal scenarios in China: How to close the carbon gap? In Applied Mechanics and Materials, vols. 556–562 841–848 (Trans Tech Publications Ltd, 2014).
Von Hirschhausen, C., Herold, J. & Oei, P. Y. How a ‘low carbon’ innovation can fail-tales from a ‘lost decade’ for carbon capture, transport, and sequestration (CCTS). Econ. Energy Environ. Policy 1, 115–123 (2012).
Viebahn, P., Daniel, V. & Samuel, H. Integrated assessment of carbon capture and storage (CCS) in the German power sector and comparison with the deployment of renewable energies. Appl. Energy 97, 238–248 (2012).
Osman, K., Coquelet, C. & Ramjugernath, D. Review of carbon dioxide capture and storage with relevance to the South African power sector. S. Afr. J. Sci. 110, 1–12 (2014).
Chabangu, N. et al. The investigation of CO2 storage potential in the Zululand Basin in South Africa. Energy Procedia 63, 2789–2799 (2014).
Vincent, C. J. et al. The proposed CO2 Test Injection project in South Africa. Energy Procedia 37, 6489–6501 (2013).
Afolayan, B. A., Mackay, E. & Opuwari, M. 3D static modeling and CO2 static storage estimation of the hydrocarbon-depleted Charis Reservoir, Bredasdorp Basin, South Africa. Nat. Resour. Res. 32, 1021–1045 (2023).
Saffou, E. et al. Full-scale simulation of a nearly depleted gas field in South Africa for carbon utilisation and storage. Greenh. Gases Sci. Technol. 12, 486–507 (2022).
Saffou, E. et al. Geomechanical characterization of CO2 storage sites: A case study from a nearly depleted gas field in the Bredasdorp Basin, South Africa. J. Nat. Gas Sci. Eng. 81, 103446 (2020).
Roelf, W. South Africa aims to bring pilot carbon capture project online in 2023 | Reuters. Reuters (2021).
Surridge, A. et al. CCUS progress in South Africa. SSRN Electron. J. https://doi.org/10.2139/ssrn.3820378 (2021).
Perkins, R. Denmark to end all North Sea oil, gas production by 2050, bans new exploration. https://www.spglobal.com/commodityinsights/en/market-insights/latest-news/natural-gas/120420-denmark-to-end-all-north-sea-oil-gas-production-by-2050-bans-new-exploration (2020).
State of Green. Denmark set to end all new oil and gas extraction in the North Sea by 2050. https://stateofgreen.com/en/news/denmark-set-to-end-all-new-oil-and-gas-extraction-in-the-north-sea-by-2050/ (2020).
Delprat-Jannaud, F. et al. Sitechar-methodology for a fit-for-purpose assessment of CO2 storage sites in Europe. Oil Gas Sci. Technol. 70, 531–554 (2015).
Orlic, B. Geomechanical effects of CO2 storage in depleted gas reservoirs in the Netherlands: Inferences from feasibility studies and comparison with aquifer storage. J. Rock Mech. Geotech. Eng. 8, 846–859 (2016).
Orlic, B., Heege, J. T. & Wassing, B. Assessing the integrity of fault- and top seals at CO2 storage sites. Energy Procedia 4, 4798–4805 (2011).
Vilarrasa, V. & Carrera, J. Geologic carbon storage is unlikely to trigger large earthquakes and reactivate faults through which CO2 could leak. Proc. Natl. Acad. Sci. U.S.A. 112, 5938–5943 (2015).
Siriwardane, H. J., Gondle, R. K., Varre, S. B., Bromhal, G. S. & Wilson, T. H. Geomechanical response of overburden caused by CO2 injection into a depleted oil reservoir. J. Rock Mech. Geotech. Eng. 8, 860–872 (2016).
Arts, R. J. et al. The feasibility of CO2 storage in the depleted P18–4 gas field offshore the Netherlands (the ROAD project). Int. J. Greenh. Gas Control 11, 10–20 (2012).
Song, Y. et al. Geomechanical challenges during geological CO2 storage: A review. Chem. Eng. J. 456, 140968 (2023).
Sander, R. Compilation of Henry’s law constants, version 3.99. Atmos. Chem. Phys. Discuss 14, 29615–30521 (2014).
Li, Y. & Nghiem, L. X. Phase equilibria of oil, gas and water/brine mixtures from a cubic equation of state and Henry’s law. Can. J. Chem. Eng. 64, 486–496 (1986).
Harvey, A. H. Semiempirical correlation for Henry’s constants over large temperature ranges. AIChE J. 42, 1491–1494 (1996).
Machida, H., Ando, R., Esaki, T., Yamaguchi, T. & Norinaga, K. Modelling of CO2 solubility in phase separation solvent composed of amine/ether/water system for CO2 capture. J. Mol. Liq. 292, 111411 (2019).
Wang, G., Pickup, G., Sorbie, K. & Mackay, E. Numerical modelling of H2 storage with cushion gas of CO2 in subsurface porous media: Filter effects of CO2 solubility. Int. J. Hydrog. Energy 47, 28956–28968 (2022).
Opuwari, M. et al. Petrophysical core-based zonation of OW oilfield in the Bredasdorp Basin South Africa. Sci. Rep. 12, 1–19 (2022).
Bennion, B. & Bachu, S. Relative permeability characteristics for supercritical CO2 displacing water in a variety of potential sequestration zones. In SPE Annual Technical Conference and Exhibition (OnePetro, 2005).
Huang, J., Jin, T., Chai, Z., Barrufet, M. & Killough, J. Compositional simulation of three-phase flow in mixed-wet shale oil reservoir. Fuel 260, 116361 (2020).
Churchwell, L., Radhakrishnan, A. & DiCarlo, D. Measurements of three-phase relative permeability as a function of fluid composition. In Proc. SPE Symp. Improv. Oil Recover. 2020-August (2020).
Churchwell, L. & Dicarlo, D. Oil and gas relative permeability as a function of fluid composition. In Proc. SPE Symp. Improv. Oil Recover. 2022-April (2022).
Zhou, X., Al-Otaibi, F. & Kokal, S. Relative permeability characteristics and wetting behavior of supercritical CO2 displacing water and remaining oil for carbonate rocks at reservoir conditions. Energy Fuels 33, 5464–5475 (2019).
Adibifard, M., Talebkeikhah, M., Sharifi, M. & Hemmati-Sarapardeh, A. Iterative Ensemble Kalman Filter and genetic algorithm for automatic reconstruction of relative permeability curves in the subsurface multi-phase flow. J. Pet. Sci. Eng. 192, 107264 (2020).
Pham, T. H. V., Maast, T. E., Hellevang, H. & Aagaard, P. Numerical modeling including hysteresis properties for CO2 storage in Tubåen formation, Snøhvit field, Barents Sea. Energy Procedia 4, 3746–3753 (2011).
Pan, I., Babaei, M., Korre, A. & Durucan, S. Artificial neural network based surrogate modelling for multiobjective optimisation of geological CO2 storage operations. Energy Procedia 63, 3483–3491 (2014).
Eiken, O. et al. Lessons learned from 14 years of CCS operations: Sleipner, In Salah and Snøhvit. Energy Procedia 4, 5541–5548 (2011).
Chiaramonte, L., White, J. A. & Trainor-Guitton, W. Probabilistic geomechanical analysis of compartmentalization at the Snøhvit CO2 sequestration project. J. Geophys. Res. Solid Earth 120, 1195–1209 (2015).
Ringrose, P. & Sæther, Ø. CO2 injection operations: Insights from Sleipner and Snøhvit. in SPE CCUS Conference 1–14 (2020).
Liang, R., Fan, X., Luo, X., Sun, S. & Zhu, X. Improved IMPES scheme for the simulation of incompressible three-phase flows in subsurface porous media. Energies 14, 2757 (2021).
Løvoll, G., Méheust, Y., Måløy, K. J., Aker, E. & Schmittbuhl, J. Competition of gravity, capillary and viscous forces during drainage in a two-dimensional porous medium, a pore scale study. Energy 30, 861–872 (2005).
Lassen, R. N. et al. Effects of geologic heterogeneity on migration of gaseous CO2 using laboratory and modeling investigations. Int. J. Greenh. Gas Control 43, 213–224 (2015).
Qiao, Y. & Evje, S. A compressible viscous three-phase model for porous media flow based on the theory of mixtures. Adv. Water Resour. 141, 103599 (2020).
Khan, M. Y. & Mandal, A. Analytical model for gravity segregation in WAG displacement recovery of inclined stratified reservoirs. J. Pet. Sci. Eng. 186, 106722 (2020).
Aghabozorgi, S. & Sohrabi, M. Generalised model for simulation of two- and three-phase cycle-dependent hysteresis in sandstones. Fuel 310, 122328 (2022).
Nygard, J. I. & Andersen, P. O. Simulation of immiscible water-alternating-gas injection in a stratified reservoir: Performance characterization using a new dimensionless number. SPE J. 25, 1711–1728 (2020).
Black, J. R., Carroll, S. A. & Haese, R. R. Rates of mineral dissolution under CO2 storage conditions. Chem. Geol. 399, 134–144 (2015).
Chaves, G. Simulation of CO2 Sequestration in Deep Saline Aquifers Simulation of CO2 Sequestration in Deep Saline Aquifers (New Mexico Institute of Mining and Technology, 2011).
Lindeberg, E. Escape of CO2 from aquifers. Energy Convers. Manag. 38, S235–S240 (1997).
Brunet, J.-P.L., Li, L., Karpyn, Z. T. & Huerta, N. J. Fracture opening or self-sealing: Critical residence time as a unifying parameter for cement–CO2–brine interactions. Int. J. Greenh. Gas Control 47, 25–37 (2016).
Huerta, N. J., Hesse, M. A., Bryant, S. L., Strazisar, B. R. & Lopano, C. Reactive transport of CO2-saturated water in a cement fracture: Application to wellbore leakage during geologic CO2 storage. Int. J. Greenh. Gas Control 44, 276–289 (2016).
Jian, X. & Xiaofeng, G. Assessing a potential site for offshore CO2 storage in the Weixinan Sag in the northwestern Beibu Gulf Basin (BGB), northern South China Sea. Greenh. Gases Sci. Technol. https://doi.org/10.1002/ghg.2199 (2022).
Zhang, C., Oostrom, M., Wietsma, T. W., Grate, J. W. & Warner, M. G. Influence of viscous and capillary forces on immiscible fluid displacement: Pore-scale experimental study in a water-wet micromodel demonstrating viscous and capillary fingering. Energy Fuels 25, 3493–3505 (2011).
Acknowledgements
The Petroleum Agency of South Africa (PASA) is greatly appreciated for providing data and well reports for the study. The authors also thank the Computer Modelling Group (CMG) for providing GEM software used for data analysis. Also, thanks to the Community of Practice in Oil and Gas of South Africa for travel support.
Author information
Authors and Affiliations
Contributions
B.A., E.M., and M.O. conceptualized the research. B.A. did the formal analysis and wrote the manuscript. E.M. and M.O. reviewed and edited the manuscript and figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afolayan, B., Mackay, E. & Opuwari, M. Dynamic modeling of geological carbon storage in an oil reservoir, Bredasdorp Basin, South Africa. Sci Rep 13, 16573 (2023). https://doi.org/10.1038/s41598-023-43773-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43773-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.