Abstract
New inclusions of Trichoneura preserved in Upper Cretaceous (Cenomanian) Kachin amber allow the description of a new subgenus, Burmania subgen. nov., and four new species: Trichoneura (Burmania) burmitensis subgen. et sp. nov., Trichoneura (Burmania) chungkuni subgen. et sp. nov., Trichoneura (Burmania) sevciki subgen. et sp. nov. and Trichoneura (Burmania) wangi subgen. et sp. nov. The species differ mainly by the morphology of the hypopygium or wing venation but also the construction of the antenna. Based on a comparison of the wing venation and the morphology of the hypopygium it was possible to describe features which are characteristic of the new subgenus, especially the presence of vein R3+4. Moreover, it was possible to elucidate the evolutionary pattern of Trichoneura with two distinct extant and extinct branches. Trichoneura (Trichoneura) canadensis from Upper Cretaceous Canadian amber is transferred to the new subgenus Burmania.
Similar content being viewed by others
Introduction
Diptera appeared probably in the Triassic, as indicated by the fossil record. The oldest representative, Grauvogelia arzvilleriana Krzemiński, Krzemińska and Papier1, is known from sediments, and was described based on a fossilized wing. The presence of Nematocera in the Triassic fauna is confirmed by inclusions in amber from Italy (the deposits were discovered in the Alps), in which a specimen of a nematoceran fly was found of unspecified taxonomic position2. In the Mesozoic era, there was an evolutionary radiation, which led to increased diversity of Diptera. The oldest representatives of the Tipulomorpha belong to the family Archilimoniidae Krzemiński and Krzemiński3, occurring near the early\middle Triassic boundary. The greatest radiation of these insects was during the Jurassic. The family Limoniidae Speiser4 are known since the Late Triassic, had become numerous among fossils from Europe5,6,7 and Asia8,9 since Early Jurassic (Toarcian). In the Middle Cretaceous, not only Triassic and Jurassic Limoniidae lineages are known, but also the representative of extant genera like Helius Lepeletiere & Sterville10 or Dicranoptycha Osten-Sacken11. Those known from fossil resins and sedimentary deposits of the Cretaceous and early Cenozoic12,13 are relatively numerous. Recently, this family comprised ca. 11.000 species14. Flies belonging to the extant subfamily Limoniinae were already present in the Cretaceous Period.
The genus Trichoneura Loew15 is represented by 19 species (six extinct, 13 extant), divided into four subgenera: Ceratolimnobia Alexander16, Cretalinea Kania-Kłosok, Krzemiński, Kopeć & Arillo17, Trichoneura Loew15 and Xipholimnobia Alexander18. Two of them, Cretalinea and Trichoneura, are known from the fossil record. Cretalinea is known only from one Cretaceous species, Trichoneura (Cretalinea) xavieri Kania-Kłosok, Krzemiński, Kopeć, Arillo17. It was described from the Cretaceous (upper Albian) Peñacerrada I Basque—Cantabrian Basin, near the village of Moraza, Province of Burgos (Spain) and so far has been the oldest representative of the genus. The second species is known from the Cretaceous Period, Trichoneura canadensis Krzemiński et Teskey19, came from Upper Cretaceous amber from an open pit coal mine near Medicine Hat in southern Alberta, Canada and until now was treated as a representative of the subgenus Trichoneura. The subgenus Trichoneura is known mainly from Eocene amber (four species). From the recent fauna only one species, Trichoneura (Trichoneura) umbrosa Alexander20, is known and occurs in Australia and Oceania. The subgenus Ceratolimnobia is represented by two recent species: Trichoneura (Ceratolimnobia) ishigakiensis Kato21 and Trichoneura (Ceratolimnobia) munroi (Alexander16). There are ten recent species within the subgenus Xipholimnobia (Table 1). Ceratolimnobia and Xipholimnobia mainly occur in the Oriental region, but also in Cameroon, Nigeria and Madagascar14. The occurences of Trichoneura in Canadian amber, Baltic amber, Spanish and Kachin amber (described herein) (Fig. 1, Table 2) suggest that they were widely distributed in the past. Although, the subgenus Trichoneura is very rare in recent fauna, though from the fossil record six species are known. The study of new material preseved in Kachin amber provides additional information on the distribution and diversity of species of Trichoneura. The genus Trichoneura was probably not only widely distribiuted in the past but also numerous in species. The differentiated morphology and visible trends (reduction of huge lobe on gonocoxite and atrophy of R3+4). of the changing morphological features indicate two evolutionary branches—one extinct and one extant, but almost relict in the recent fauna. Based only on the morphology of wing venation it is possible to separate these two branches. The Cretaceous Period is very important for understanding the history of life on Earth and the evolution of modern ecosystems. Inclusions in Cretaceous resins, such as Barremian Lebanese amber (120–135 Ma)22,23, upper Albian Spanish amber (105 Ma)24, Albian French amber (Charente-Maritime, SW France)25,26 or younger Cenomanian Kachin amber (98.79 ± 0.62 Ma)27 document diversity and disparity of the World’s terrestrial fauna from over 98 milion years ago. There are other rich Cretaceous deposits, e.g. in China, like Late Cretaceous Xixia of Henan in Henan Province, Upper Cretaceous Jiayin amber in Heilongjiang Province28 or Hailar amber, the oldest known amber in China29, located within the Central Asian Orogenic Belt between the Siberian and North China–Mongolian cratons30. It was proposed that amber discovered from Lower Cretaceous deposits would bridge gaps among several well-known amber deposits, including Lebanese amber and Spain amber, amber from France or from Myanmar29.
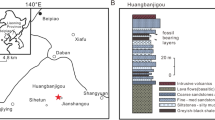
Maps of location of recent amber mining area in the Hukawng Valley, Myitkina Province, Burma. (A) Map of the world with location of Hukawng Valley; (B) Enlarged view of location of Hukawng Valley. (C). Geological setting of the Kachin amber deposits, after Kania41, modified. Maps were built using the map Maps-For-Free (https://maps-for-free.com) and modified with the software programs Corel Draw and Corel Photopaint X7.
Results
Systematic palaeontology.
Order: Diptera Linnaeus42.
Infraorder: Tipulomorpha Rohdendorf43.
Family: Limoniidae Speiser4.
Subfamily: Limoniinae Speiser4.
Genus: Trichoneura Loew15.
Subgenus: Burmania subgen. nov.
Type-species: Trichoneura (Burmania) burmitensis subgen. et sp. nov.
LSID urn:lsid:zoobank.org:act:79247CE1-D8C3-4123-93EE-848A78002749.
Diagnosis. Vertex smooth, without corniculus; vein R4 separating from R2+3+4 far beyond separation of vein R2 (r-r), and with vein R3 forming sector R3+4.
Etymology. The specific epithet is derived from Burma (Myanmar).
Description. Body 3.08–3.84 mm long, brown, pterostigma sometimes present.
Head with antenna 16-segmented, 0.70–0.98 mm long, shorter than head and thorax combined; scape elongate, cylindrical; pedicel elongate, longer than wide, slightly wider than flagellomeres, flagellomeres oval elongate, at most twice as long as wide; becoming progressively slender toward antennal tip; last flagellomere usually shorter than penultimate one; the length of antennomeres according to: 1/0.10–0.15; 2/0.06–0.10; 3/0.05–0.08; 4/0.04–0.07; 5–16/0.04–0.05). Antenna with two–four moderately elongate setae on each flagellomeres; palpus four-segmented, slender, 0.22–0.31 mm long (1/0.07–0.08; 2/0.04–0.06; 3/0.04–0.06; 4/0.06) first, second and fourth palpomeres not very elongate, at least 3 × as long as wide, second palpomere sometimes widened in distal part, third palpomere sometimes widened in midlength.
Thorax: wing 2.94–5.00 mm long, 0.72–1.15 mm wide; R3 variable in length; R4 from one and a half to twice the length of d-cell; d-cell 0.27–0.46 mm long, approximately twice to twice and a half as long as wide; crossvein r-m usually elongate, equal or longer than basal section of R5; M3 shorter than M1+2, longer than M4; A1 and A2 elongate, usually almost straight, sometimes slighlty curved at the tip. Tergite IX with straight or only slightly indented front edge.
Abdomen with hypopygium 0.40–0.53 mm long; gonocoxite 0.25–0.38 mm long; outer gonostylus 0.07–0.19 mm long, inner gonostylus 0.17–0.22 mm long, aedeagus about 0.31 mm long.
Comparison. In Cretalinea gonocoxite is elongate, over 3 × as long as wide with huge, spoon-shaped lobe at apex measuring approximately 0.5 × the length of gonocoxite; gonostylus measuring less than 0.5 × the length of gonocoxite in Burmania subgen. nov. this lobe does not occur, gonocoxite is differentiated in length, gonostylus measuring more than 0.5 × the length of gonocoxite. Moreover, in Burmania subgen. nov. vein R4 separating from R2+3+4 far beyond separation of vein R2 (r–r), and with vein R3 forming sector R3+4; in Trichoneura, Cretolimnobia and Xipholimnobia vein R4 separates from R2+3+4 before or at the same point of separation of vein R2 (r–r), and R3+4 does not occur. In Ceratolimnobia occur corniculus on vertex and gonostylus is deeply bifid, in Burmania subgen. nov. vertex is smoth and gonostylus is undivided.
New nomenclatoral decision
Trichoneura (Trichoneura) canadiensis Krzemiński and Teskey19 is transfered to the new subgenus Burmania subgen. nov. as Trichoneura (Burmania) canadiensis Krzemiński and Teskey19 comb. nov.
Remark: Such features as smooth vertex, without the cornicuus, presence of vein R3+4 and morphology of hypygium without huge, spoon-shaped lobe on its tip allow to classify this species to the new subgenus.
Trichoneura (Burmania) burmitensis subgen. et sp. nov. (Figs. 2, 3).
LSID urn:lsid:zoobank.org:act:B7E1A8D2-FCF8-48E6-B045-D29487DA559C.
Diagnosis. Tip of Sc situated just before fork of Rs, sc-r at two of its length from the tip of Sc; vein R1 terminates at C opposite approximately 0.8 × common length of R2+3+4 and R3+4, tip of R1 curved; R3+4 slightly longer than R2 (r–r); R5 widely separated from Rs, basal section of R5 equal in length to r-m; m-cu just before midlength of d-cell; d-cell approximately twice as long as wide; tip of Cu beyond d-cell; tip of A1 beyond m-cu; tip of A2 situated opposite approximately half the length of Mb, medial-basal vein; gonocoxite not elongate, at most 2.5 × as long as wide with few, not very elongate setae at apex; outer gonostylus strongly curved, narrow in basal part, widened and sclerotized just before apex, apex of outer gonostylus narrow, pointed, with a brush of very short and coarse bristles at the end; inner gonostylus narrow, slightly sclerotized with narrow, pointed apex, inner gonostylus only approximately 0.3 × longer than outer; aedeagus thick, almost as long as gonocoxite, curved at apex.
Etymology. The specific epithet is derived from the Burmite.
Type material. Holotype No. MP/4365 (male) ISEA PAS; specimen in Kachin amber, Myanmar; Paratypes No. MP/4332 (male), No. MP/4335 (male), No. MP/4336 (male), No. MP/4340 (male) ISEA PAS, specimens in Kachin amber, Myanmar.
Horizon and locality. Lowermost Cenomanian, Hukawng Valley, northern Myanmar. The mining is done at a hill named Noije Bum, near Tanai Village (26° 21′ 33.41″ N, 96° 43′ 11.88″ E).
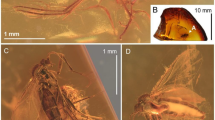
Description. Body (Figs. 2A, 3A–C) brown, 3.16–4.40 (holotype: 3.84) mm long, pterostigma present.
Head (Fig. 2A) with antenna (Figs. 2B, 3A) 0.97 mm (holotype) long (1/0.12; 2/0.10; 3/0.08; 4/0.07; 5/0.05–16/0.05), shorter than head and thorax combined; scape elongate, cylindrical, narrower than other segments of antenna; pedicel elongate, longer than wide, slightly wider than flagellomeres, flagellomeres oval elongate, approximately twice as long as wide; becoming progressively slender toward antennal tip; last flagellomere shorter than penultimate one. Antenna with three moderately elongate setae on each flagellomere, two on one side and one on the opposite side of each member; palpus (Figs. 2B, 3B) 0.22 mm (holotype) long (1/0.08; 2/0.04; 3/0.04; 4/0.06) first, second and fourth palpomeres not very elongate, approximately 3 × as long as wide, second palpomere widened in distal part, third palpomere widened in midlength.
Thorax (Fig. 2A): wing 2.94–5.00 mm long, 0.91–1.15 mm wide (holotype: 4.01 mm long, 1.00 wide) (Figs. 2A,C, 3C); tip of R3 beyond half the length of R4; R4 approximately twice the length of d-cell; d-cell 0.27–0.44 mm long (holotype), approximately twice as long as wide; crossvein r-m rather elongate, equal in length to basal section of R5; M3 0.82 mm long (holotype), shorter than M1+2, longer than M4; A1 and A2 elongate, almost straight.
Abdomen (Fig. 2A): hypopygium wide (Figs. 2A,D, 3D), 0.41–0.46 mm long (holotype); gonocoxite 0.25–0.33 mm long (holotype); outer gonostylus 0.16 (holotype) − 0.19 mm long, inner gonostylus 0.19 mm long (holotype), aedeagus 0.31 mm long.
Comparison. In Trichoneura (Burmania) burmitensis subgen. et sp. nov. the tip of Sc is situated just before the fork of Rs, and sc-r at two of its length from the tip of Sc, in T. (B.) wangi the tip of Sc is situated opposite approximately 0.8 × before the fork of Rs, and vein sc-r at one of its length from the tip of Sc. Moreover, vein R1 in T. (B.) burmitensis terminates at C opposite approximately 0.8 × common length of R2+3+4 and R3+4, tip of R1 is curved, R3+4 is slightly longer than R2 (r–r), while in T. (B.) wangi vein R1 terminates at C opposite approximately 0.9 × common length of R2+3+4 and R3+4, tip of R1 is straight, R3+4 is slightly shorter than R2 (r-r). In T. (B.) burmitensis R5 is widely separated from Rs, basal section of R5 is equal in length to r-m, in T. (B.) wangi R5 is narrowly separated from Rs, basal section of R5 is shorter in length to r-m. There are also some differences in the position of tips Cu, A1 and A2. In T. (B.) burmitensis tip of Cu is situated beyond d-cell level, tip of A1 beyond m-cu level and tip of A2 opposite approximately half the length of Mb, in T. (B.) wangi tip of Cu is situated at d-cell level, tip of A1 before m-cu level and tip of A2 opposite approximately 0.3 × the length of Mb. But, the main differences are visible in the shape of outer gonostylus: in T. (B.) burmitensis subgen. et sp. nov. this structure is narrow at the basal part, widened just before apex, in T. (B.) wangi outer gonostylus is wide along its entire length, tiped at apex. In contrast to T. (B.) chungkuni, in T. (B.) burmitensis gonocoxite is not elongate, at most 2.5 × as long as wide with only few, not very elongate setae at apex, aedeagus is thick, almost as long as gonocoxite, curved at apex, in T. (B.) chungkuni gonocoxite is elongate, at least 3.5 × as long as wide, aedeagus is distinctly shorter than gonocoxite, approximately 0.6 × of its length. In T. (B.) sevciki, aedeagus is longer than gonocoxite, outer gonostylus is rather narrow and inner gonostylus is elongate and lobe shaped, widened at apex, in T. (B.) burmitensis outer gonostylus is strongly curved, widened and sclerotized just before apex, apex of outer gonostylus is narrow, pointed, inner gonostylus is narrow, slightly sclerotized with narrow, pointed apex. From Canadian amber T. (B.) canadensis comb. nov. inner gonostylus is long, twice longer than outer gonostylus, in T. (B.) burmitensis inner gonostylus is shorter.
Trichoneura (Burmania) chungkuni subgen. et sp. nov.
LSID urn:lsid:zoobank.org:act:4BF9D26F-2A2E-4AB8-AEB4-144B8362FEB3. (Figures. 4, 5).
Trichoneura (Burmania) chungkuni subgen. et sp. nov. No. MP/4334, holotype (male) (ISEA PAS): (A) antenna; (B) palpus; (C) wing; (D) hypopygium, ventral view. Abbreviations as in Fig. 3.
Diagnosis. Tip of Sc situated just before fork of Rs, sc-r at two of its length from the tip of Sc; vein R1 terminates at C opposite at fork of R3+4, tip of R1 almost straight; R3+4 shorter than R2 (r–r); R5 widely separated from Rs, basal section of R5 equal in length to r-m; m-cu at midlength of d-cell; d-cell approximately twice as long as wide; tip of Cu beyond d-cell level; tip of A1 before m-cu level; tip of A2 situated just before half the length of Mb, medial-basal vein; gonocoxite very elongate, at least 3.5 × as long as wide with numerous, dense, elongate and thick setae especially concentrated at apex; outer gonostylus tiny, pointed and curved at apex, twice longer than inner, inner gonostylus narrow, thick with brush of setae at apex, aedeagus tiny, not very elogate, 0.6 × the length of gonocoxite, not divided.
Etymology. The specific epithet is dedicated to Prof. Chungkun Shih (Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing 100,048, China), the eminent specialist on fossil and recent insects.
Type material. Holotype MP/4334 (male) ISEA PAS; specimen in Kachin amber, Myanmar. Horizon and locality. Lowermost Cenomanian, Hukawng Valley, northern Myanmar. The mining is done at a hill named Noije Bum, near Tanai Village (26° 21′ 33.41″ N, 96° 43′ 11.88″ E).
Description. Body (Fig. 4A) brown, 3.84 mm long.
Head (Fig. 4A) with antenna (Figs. 4A,B, 5A) 0.70 mm long (1/0.10; 2/0.08; 3/0.07; 4–16/0.05), shorter than head and thorax combined; scape elongate, cylindrical, as narrow as other segments of antenna; pedicel elongate, longer than wide, widened in midlength, flagellomeres oval, not very elongate, approximately twice as long as wide; becoming progressively slender toward antennal tip; last flagellomere only slightly shorter than penultimate one. Antenna with four moderately elongate setae on each flagellomeres, two on one side and two on the opposite side of each flagellomeres; palpus (Figs. 4A, B 5B) 0.31 mm long (1/0.07; 2–4/0.06) four-segmented, palpomeres not very elongate, but the last one as long as two penultimate; few setae on each palpomeres.
Thorax (Fig. 4A): wing 3.80 mm long, 0.94 mm wide (Figs. 4A,D, 5C); vein R1 elongate, ending opposite fork of R3+4 on R3 and R4; tip of R3 beyond half the length of R4; R4 approximately twice the length of d-cell; d-cell 0.46 mm long, approximately twice as long as wide; crossvein r-m rather elongate, equal in length to basal section of R5; M3 0.81 mm long, shorter than M1+2, longer than M4; A1 and A2 elongate, A1 almost straight, A2 curved at the tip.
Abdomen (Fig. 4A): hypopygium narrow (Figs. 4A,C, 5D), 0.49 mm long; gonocoxite 0.35 mm long, 0.07 mm wide; outer gonostylus 0.11 mm long, inner gonostylus 0.22 mm long.
Comparison. The main difference between the Trichoneura (Burmania) chungkuni subgen. et sp. nov. and other species of Burmania subgen. nov. known from Cretaceous is the morphology of hypopygium. Hypopygium of T. (B.) chungkuni is narrow, with elongate, at least 3.5 × as long as wide. Gonocoxite with dense, elongated setae, especially at the tip of gonocoxite with aedeagus not very elongate, reaching at most 0.6 × the length of gonocoxite and lobe shaped inner gonostylus. In other species of Burmania the hypopygium is wide with not very elongate gonocoxite, similarly to these species known from Baltic amber, aedeagus is almost as long as gonocoxite or longer and inner gonostylus is narrow and tipped. Some differences are well visible in wing venation. R1 in T. (B.) chungkuni it is ending opposite fork of R3+4 on R3 and R4, while in other species of Burmania is also elongate, but always ended before this bifurcation.
Trichoneura (Burmania) sevciki subgen. et sp. nov.
LSID urn:lsid:zoobank.org:act:0BAC90FF-9210-4AE9-90A2-9E72D3FD6AF9. (Figures. 6, 7).
Trichoneura (Burmania) sevciki subgen. et sp. nov. No. NIGP177895, holotype (male) (A–C): (A) body, lateral view; (B) head with antenna and palpus visible; (C) hypopygium, ventral view; No. 52/2019, paratype (male) (ISEA PAS), paratype (male) (D–F): hypopygium, ventral view; (E) antenna; (F) body, lateral view.
Trichoneura (Burmania) sevciki subgen. et sp. nov. No. NIGP177895, holotype (male) (NIGP). (A) antenna, reconstruction; (B) palpus; (C) wing; (paratype No. 52/2019, paratype (male) (ISEA PAS); (D) hypopygium, ventral view. Abbreviations as in Fig. 3.
Diagnosis. Vein R1 terminates at C opposite approximately 0.8 × common length of R2+3+4 and R3+4, tip of R1 curved; R3+4 longer than R2 (r–r); R5 widely separated from Rs, basal section of R5 shorter than r-m; m-cu before midlength of d-cell; d-cell approximately twice as long as wide; tip of Cu far beyond d-cell level; tip of A1 before m-cu level; tip of A2 situated opposite approximately 0.5 × the length of Mb, medial-basal vein; gonocoxite elongate, approximately 3 × as long as wide, with few, not very elongate setae at apex; aedeagus with extension and curved, elongate appendix at apex, longer than gonocoxite; inner gonostylus elongate, lobe shaped, only slightly sclerotized, widened in distal part, rounded, outer gonostylus tiped at apex, rather straight, arrange 0.5 × length of inner gonostylus.
Etymology. The specific epithet is dedicated to Dr. Jan Ševčík Department of Biology and Ecology, Faculty of Science, University of Ostrava, the eminent specialist on fossil and recent insects.
Type material. Holotype No. NIGP177895 (male) NIGP, coll. B. Wang, specimen in Kachin amber, Myanmar; Paratype No. 52/2019 (male) ISEA PAS, coll. J. Ševčík.
Horizon and locality. Lowermost Cenomanian, Hukawng Valley, northern Myanmar. The mining is done at a hill named Noije Bum, near Tanai Village (26° 21′ 33.41″ N, 96° 43′ 11.88″ E).
Description. Body (Fig. 6A,F) brown, 3.08–3.72 (holotype) mm long.
Head (Fig. 6A,B) with antenna (Figs. 6A,B,E, 7A) 0.64 mm long, shorter than head and thorax combined; scape elongate, narrow, cylindrical, longer than pedicel, wider than other segments of antenna; pedicel elongate, longer than wide, widened, approximately as long as first flagellomere; flagellomeres wide, approximately as wide as long, but becoming progressively slender toward antennal tip; first flagellomere elongate, longer than the rest, approximately 4 × as long as wide; last flagellomere longer than penultimate one. Antenna with two moderately elongate setae on each flagellomeres, one on one side and one on the opposite side of each member; palpus (Figs. 6A,B, 7B) four-segmented, first, second and fourth palpomeres narrow, sleder, third palpomere widened distally, last palpomere longer than rest, all palpomeres with few, rather not very elongate setae, shorter than segments bearing them.
Thorax (Fig. 6A,F): wing (Figs. 6F, 7C) 3.57 mm long, 0.89 mm wide; vein R1 rather elongate, ending opposite 0.5 × length of vein R3+4; d-cell approximately twice as long as wide; A1 and A2 elongate, slightly curved at the tip.
Abdomen (Figs. 6A,F, 7D,C): hypopygium wide (Figs. 6C,D, 7D), 0.40–0.52 mm long (holotype).
Comparison. The most characteristic feature which separates Trichonerua (Burmania) sevciki subgen. et sp. nov. from all known Cretaceous species of Trichoneura is morphology of gonostyles and aedeagus, aedeagus is very elongate, longer than gonocoxite with extension, curved, elongate appendix at apex, lobe shaped and only slightly sclerotized inner gonostylus.
Trichoneura (Burmania) wangi subgen. et sp. nov.
LSID urn:lsid:zoobank.org:act:5985861F-7EE9-4722-9FAF-E35A1BDC4A00. (Figs. 8, 9).
Trichoneura (Burmania) wangi subgen. et sp. nov., No. MP/4337, holotype (male) (ISEA PAS): (A) antenna; (B) palpus; (C) wing; (D) hypopygium, ventral view. Abbreviations as in Fig. 3.
Diagnosis. Tip of Sc situated just before fork of Rs, sc-r at one of its length from the tip of Sc; vein R1 terminates at C opposite approximately 0.9 × common length of R2+3+4 and R3+4, tip of R1 curved; R3+4 shorter than R2 (r–r); R5 narrowly separated from Rs, basal section of R5 shorter than r-m; m-cu in midlength of d-cell; d-cell approximately twice as long as wide; tip of Cu at d-cell level; tip of A1 before m-cu level; tip of A2 situated opposite approximately 0.3 × the length of Mb, medial-basal vein; gonocoxite with few, not very elongate setae at apex; outer and inner gonostyles almost equal in length, outer gonostylus broad and strongly curved at apex, pointed, slightly sclerotized, inner gonostylus narrow, pointed at apex, slightly folded, longer than outer gonostylus; aedeagus thick, elogate, curved at apex only slightly shorter than gonocoxite.
Etymology. The specific epithet is dedicated to Prof. Bo Wang (State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, China), the eminent specialist on fossil and recent insects.
Type material. Holotype MP/4337 (male) ISEA PAS; specimen in Kachin amber, Myanmar.
Horizon and locality. Lowermost Cenomanian, Hukawng Valley, northern Myanmar. The mining is done at a hill named Noije Bum, near Tanai Village (26° 21′ 33.41″ N, 96° 43′ 11.88″ E).
Description. Body (Fig. 8A) brown, 3.34 mm long.
Head (Fig. 8A) with antenna (Figs. 8A, B, 9A) 0.78 mm long (1/0.15; 2/0.06; 3/0.05; 4–16/0.04), shorter than head and thorax combined; scape elongate, cylindrical, longer than other segments of antenna; pedicel buble-like, flagellomeres cylindrical, elongate, approximately 2 × as long as wide; becoming progressively slender toward antennal tip; last flagellomere tinner than penultimate one, but of similar length. Antenna with two moderately elongate setae on flagellomeres 1–6 and four on flagellomeres 7–13; palpus (Fig. 9B) four-segmented, palpomeres first, second and fourth not very elongate, approximately 3 × as long as wide, rather narrow only third palpomere widened in midlength.
Thorax (Fig. 8A): wing 3.72 mm long, 0.98 mm wide (Figs. 8A,D, 9C); vein R1 elongate, ending opposite approximately 0.9 × length of vein R3+4; tip of R3 at the midlength of R4; R4 1.5 × the length of d-cell; d-cell 0.27. mm long, 2.5 × as long as wide; crossvein r-m short, shorter than basal section of R5; M3 0.70 mm long, shorter than M1+2, longer than M4; A1 and A2 elongate, slightly waved.
Abdomen (Figs. 8A): hypopygium wide, 0.50 mm long; gonocoxite rather short; outer and inner gonostylus almost the same length (Figs. 8A,C, 9D).
Comparison. See comparison of Trichoneura (Burmania) burmitensis subgen. et sp. nov. above. Moreover, in contrast to T. (B.) chungkuni, in T. (B.) wangi gonocoxite is not elongate, at most 2.5 × as long as wide with only few, not very elongate setae at apex, in T. (B.) chungkuni gonocoxite is elongate, at least 3.5 × as long as wide. In contrast to T. (B.) sevciki, in T. (B.) wangi aedeagus is thick, not very elogate, no longer than gonocoxite, while in T. (B.) sevciki aedeagus is longer than gonocoxite. In contrast to T. (B.) canadensis comb. nov. where inner gonostylus is long, twice longer than outer gonostylus, in T. (B.) wangi inner gonostylus is shorter.
Key to species of Burmania subgen. nov.
-
1.
Aedeagus shorter than gonocoxite, inner gonostylus narrow … 2.
- Aedeagus longer than gonocoxite, inner gonostylus lobe shaped … Trichoneura (Burmania) sevciki subgen. et sp. nov. (Figs. 6C,D 7D).
-
2.
Gonocoxite at most 2 × as long as wide, not very elongate setae on apex of gonocoxite shorter than half of its length; R1 always ended before fork of R3+4 on R3 and R4 … 3.
- Gonocoxite at least 3.5 × as long as wide, very elongate setae on apex of gonocoxite longer than half of its length; R1 ending opposite fork of R3+4 on R3 and R4 … Trichoneura (Burmania) chungkuni subgen. et sp. nov. (Figs. 4C, 5D).
-
3.
Vein sc-r at two of its length from the tip of Sc; vein R1 terminates at C opposite approximately 0.8 × common length of R2+3+4 and R3+4; R3+4 slightly longer than R2 (r-r); R5 widely separated from Rs; m-cu just before midlength of d-cell; tip of Cu beyond d-cell level; tip of A1 beyond m-cu level; tip of A2 situated opposite approximately half the length of Mb; inner gonostylus narrow, lobe shaped, rounded at apex, slightly folded … Trichoneura (Burmania) burmitensis subgen. et sp. nov. (Figs. 2A,C, 3C).
Vein sc-r at one of its length from the tip of Sc; vein R1 terminates at C opposite approximately 0.9 × common length of R2+3+4 and R3+4; R3+4 shorter than R2 (r-r); R5 narrowly separated from Rs; m-cu in midlength of d-cell; tip of Cu at d-cell level; tip of A1 before m-cu level; tip of A2 situated opposite approximately 0.3 × the length of Mb; gonocoxite with few, not very elongate setae at apex; outer gonostylus broad in distal part and strongly curved at apex, pointed, strongly sclerotized, inner gonostylus narrow, lobe shaped, pointed at apex; … Trichoneura (Burmania) wangi subgen. et sp. nov. (Figs. 8C,D, 9C).
Discussion
The oldest representatives of Trichoneura—T. (C.) xavieri is from Lower Cretaceous Spanish amber. This species exhibits a unique morphology of its hypopygium characterized by a huge lobe on the gonocoxite17. The newly described herein new subgenus Burmania subgen. nov. (Table 2) characterize by the absence of lobe on gonocoxite, while wing venation of these insects indicates a close relationship with Cretalinea, both characterize by the well presented R3+4. Interestingly, in Eocene representatives of Trichoneura and those that occur in the recent fauna, vein R2 (r–r) is shifted toward the apex of wing, and is connected with fork of R3+4 on R3 and R4 or is even positioned beyond this fork. The hypopygium in the Eocene species of subgenus Trichoneura is rather wide (this feature is well visible). A huge lobe which occurred on the gonocoxite of upper Albian (Lower Cretaceous) T. (C.) xavieri was probably subsequently reduced and the apex of the gonocoxite was shortened, as seen in Eocene and recent species of this genus. In Burmania subgen. nov. the huge lobe on the gonocoxite is not presented. The Cretaceous line with a huge lobe on the gonocoxite or elongate gonocoxite is completely extinct (Fig. 10).
Analysis of morphological features of craneflies of the genus Trichoneura shows that two evolutionary branches were probably separated at the early stage of evolution of these insects. In the recent fauna this group is almost relict, represented by only 13 species within three subgenera. In the evolution of this group the evolutionary tendencies are visible, especially in the morphology of the wing venation (Fig. 10), whereby the radial vein R1 was gradually shortened. In Cretaceous representatives we can observe an elongate R1 which terminates far beyond half the length of Rs. Also, in Cretaceous representatives, such as T. (C.) xavieri from Spanish amber and in those described herein from Burmese amber under the new subgenus Burmania occurs a short vein R3+4, whereas in Eocene species vein R3+4 doesn’t occur.
The Trichoneura genus, dynamically developing in the Mesozoic, evolved mainly in what was then Laurasia.
Evidence of its widespread occurrence in this subcontinent is found in the fossil resins of Europe15,17,19,41, Asia (the species described herein) and North America40 (Table 2). Unfortunately, we have no fossil evidence of the presence of representatives of Trichoneura from Gondwana in the Mesozoic. In the modern fauna only in Africa we find four representatives of the Trichoneura genus and several species in the Australian/Oceanian Region (Fig. 11). In modern fauna, representatives of the subgenus Xipholimnobia are more numerous in species than Ceratolimnobia, species belonging to both subgenera occur at similar latitiudes, e.g. T. (C.) ishigakiensis is found in Japan, as is T. (X.) japonica21, and T. (C.) munroi occur in Madagascar similarly to T. (X.) madagascariensis14. The presence of these modern, relict species may indicate the occurrence of representatives of the Trichoneurana genus also in Gondwana in past geological epochs.
Geographical distribution of fossil and recent species of the genus Trichoneura. Points indicate fossil localities of Trichoneura, colour shadow—widespread of recent species of the genus. Map was built using the map Maps-For-Free (https://maps-for-free.com) and modified with the software programs Corel Draw and Corel Photopaint X7.
Limoniidae are highly variable regarding their ecology and biology, their larvae are found in a wide spectrum of habitats, ranging from running waters, through still and stagnant ones, bottom sediments, to terrestrial habitats such as soils, litter, and detritus44,45,46,47. Unlike larvae, imagines of Limoniidae are more habitats restricted and usually present in shady and moist places, often near the shores and banks of waters, feeding on nectar and plant juices exuded on their surface48,49,50,51.
Palaeoentomological investigations on fossil Limoniinae demonstrated the existence of obstacles and needs to reinterpretation of biogeographic opinions concerning these flies. A better knowledge of fossil Limoniinae had enabled to provide palaeohabitats reconstructions and ecological interpretations of the past environments, in which these insects existed.
Material and methods
The study was based on 10 inclusions of the genus Trichoneura (Limoniidae: Limoniinae) preserved in Cretaceous Kachin amber, aged on 98.79 ± 0.62 Ma, (Upper Cretaceous, Cenomanian)2,52,53. The specimens were found as inclusions in the deposits located at the Hukawng Valley in the northern Myanmar, Myitkyina and Upper Chindwin districts (Myanmar)27,54 (Fig. 1) and are housed in Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Kraków (ISEA PAS) (eight specimens) and in State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, China, coll. B. Wang (one specimen) (Table 2). The specimens were examined using a Nikon SMZ 1500 stereomicroscope equipped with a Nikon DS-Fi1 camera, and the measurements were taken with NIS-Elements D 3.0 software in University of Rzeszów. Measurements of individual parts of the body were given only when the measured morphological structures were not distorted. The length of the vein M3 was given from the point of its connection with the crossvein m-m to the margin of wing, the length of the discal cell was given from its posterior edge to the point of connection of vein m-m with vein M3. The length of hypopygium was measured from the posterior margin of tergite IX to the apex of gonocoxite. Drawings were made based on specimens and the photographs. Drawings and photographs were made by Iwona Kania-Kłosok. The wing venation nomenclature and the designation of the hypopygium is followed by Kania41. Maps were built using the map Maps-For-Free (https://maps-for-free.com) and modified with the software programs Corel Draw and Corel Photopaint X7.
Statement The specimen (NIGP177895) involved in this study were collected in 2015. These specimens are now deposited in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences. The collection and storage process of these specimens were in full compliance with the regulations of fossils specimen procurement of the institute. Access is free to all scientist permanently. The specimens (MP/4365, MP/4332, MP/4334, MP/4335, MP/4336, MP/4337, MP/4340) reported in this study were donated to the Natural History Museum of Institute of Systematics and Evolution of Animals Polish Academy of Sciences by the collector Mr. Jacek Serafin in 2001, the specimen No. 52/2019 by dr. Jan Ševčík. They will be permanently deposited in Natural History Museum of Institute of Systematics and Evolution of Animals Polish Academy of Sciences, Kraków, Poland (ISEA PAS).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Krzemiński, W., Krzemińska, E. & Papier, F. Grauvogelia arzvilleriana sp. N.—the oldest Diptera species (Lower/Middle Triassic of France). Acta Zool. Crac. 37(2), 95–99 (1994).
Smith, R. D. A. & Ross, A. J. Amberground pholadid bivalve borings and inclusions in Burmese amber: Implications for proxi-mity of resin-producing forests to brackish waters, and the age of the amber. Earth Environ. Sci. Trans. R. Soc. Edinb. 107, 239–247 (2017).
Krzemiński, W. & Krzemińska, E. Triassic Diptera: Review, revisions and descriptions. Acta Zool. Crac. 46(suppl. – Fossil Insects), 153–184 (2003).
Speiser, P. 4 Orthoptera. Orthoptera Nematocera. Wissenschaftliche Ergebnisse der Schwedischen Zoologische Expededition nach Kilimandjaro. Meru 10 (Diptera), 31–65 (1909).
Handlirsch, A. Die fossilen Insekten und die Phylogenie der rezenten Formen. Ein Handbuch für Paläontologen und Zoologen. Engelmann, Leipzig, 481–640 (1906).
Tillyard, R. J. The panorpoid complex in the British Rhaetic and Lias. Fossil insects no. 3. Br. Mus. (Nat. Hist.) Lond., 79 (1933).
Krzemiński, W. & Zessin, W. The Lower Jurassic Limoniidae from Grimmen (GDR) (Diptera, Nematocera). Dtsch. Entomol. Z. (N. F.) 37, 39–43 (1990).
Kalugina N.S., Kovalev V.G. Dipterous insects of Jurassic Siberia. Paleontological Institute, Akademia Nauk, Moscow 1985, 198 pp. [In Russian.]
Rhodendorf, B. B. Historical development of dipterous insects. Trudy Paleontol. Inst. 100, 1–311 (1964) (In Russian).
Lepeletier, A. L. M. & Serville, J. G. A. Entomologie, ou histoire naturelle des crustacés, des arachnides et des insectes. Encycl. Methodique Hist. Nat. 10, 345–833 (1828).
Osten Sacken, C. R. New gemera and species of North American Tipulidae with short palpi, with an attempt at a new classification of the tribe. Proc. Acad. Natl. Sci. Phila. 1860, 197–256 (1859).
Evenhuis, N. L. Catalogue of the fossil flies of the world (Insecta: Diptera) 1–600 (Backhuys, 1994).
Evenhuis N.L. Catalog of the fossil flies of the world (Insecta: Diptera) website. Version. 2.0. Last update 18 November 2014 Available at: http://hbs.bishopmuseum.org/fossilcat/
Oosterbroek, P. Catalogue of the Crane-flies of the World. (Diptera, Tipuloidea: Pediciidae, Limoniidae, Cylindrotomidae, Tipulidae), (2023). https://ccw.naturalis.nl/ Last updated 27 July 2023. Accessed 12 August 2023.
Loew, H. Über den Bernstein Und die Bernsteinfauna. Program der Keiserischen Realschule Meseritz, 1–44 (1850).
Alexander, C. P. New or little-known Tipulidae (Diptera) III Ethiopian species. Ann. Mag. Nat. Hist. 9(5), 465–472 (1920).
Kania-Kłosok, I., Krzemiński, W., Kopeć, K. & Arillo, A. The oldest evolutionary lineage of Trichoneura Loew, 1850 (Diptera, Limoniidae) and the first evidence of this genus in Cretaceous Spanish Amber. Insects 12, 411 (2021).
Alexander, C. P. New or little-known Tipulidae (Diptera). IV. Ethiopian species. Ann. Mag. Nat. Hist. 9(7), 305–322 (1921).
Krzemiński, W. & Teskey, H. J. New taxa of Limoniidae (Diptera: Nematocera) from Canadian amber. Can. Entomol. 119, 887–892 (1987).
Alexander, C. P. New or little-known Tipulidae (Diptera). LXXVIII. Oriental-Australasian species. Ann. Mag. Nat. Hist. 11(14), 256–280 (1948).
Kato, D. & Tachi, T. The first records of the tribe Lechriini (Diptera: Limoniidae) in Japan, with descriptions of three new species. Eur. Entomol. J. 19(5), 273–280 (2020).
Azar, D. Lebanese amber. Meganeura 1, 26–27 (1998).
Azar, D. Les ambres mésozoïques du Liban. Doctoral thesis. Université Paris XI Orsay, 164 (2000).
Corral, C. J., López del Valle, R. & Alonso, J. El ámbar Cretácico de Álava (Cuenca Vasco-Cantábrica, Norte de España) su colecta y preparación. Estudios Museo Ciencias Nat Álava 14(2), 7–21 (1999).
Néraudeau, D. et al. Un nouveau gisement à ambre insectifère et à végétaux (Albien terminal probable): Archingeay (Charente-Mar-itime, France). Geobios 35, 233–240 (2002).
Perrichot, V. Early Cretaceous amber from south-western France: Insight into the Mesozoic litter fauna. Geol. Acta 2(1), 9–22 (2004).
Cruikshank, R. D. & Ko, K. Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 21, 441–455 (2003).
Wang, B., Zhang, H. & Azar, D. The first Psychodidae (Insecta: Diptera) from the Lower Eocene Fushun amber of China. J. Palaeontol. 85, 1154–1159 (2011).
Chang, S.-C., Li, Y. & Zheng, D. Dating amber: Review and perspective. Minerals 13(948), 1–15 (2023).
Ji, Z. et al. Geodynamic evolution of flat-slab subduction of Paleo-Pacific Plate: Constraints from Jurassic adakitic lavas in the Hailar Basin, NE China. Tectonics 38, 4301–4319 (2019).
Alexander, C. P. New or little-known Tipulidae from the Philippines (Diptera) XVII. Philippine J. Sci. 53, 429–468 (1934).
Alexander, C. P. Undescribed crane flies from Formosa and Luzon (Tipulidae, Diptera). Philippine J. Sci. 22, 467–481 (1923).
Boardman, P. Twenty-one new species of craneflies (Diptera: Tipulidae and Limoniidae), and a new fold-wing cranefly (Diptera: Ptychopteridae) from Mount Kupe, Cameroon, with notes on eighteen other species new to the country from the same location. Entomol. Mon. Mag. 156, 163–206 (2020).
Alexander, C. P. New or little-known Tipulidae from eastern Asia (Diptera) XXIX. Philippine J. Sci. 59, 225–257 (1936).
Alexander, C. P. Tipulidae nouveaux ou peu connus de Madagascar. VIII. (Diptera). Mem. de l’Inst. Sci. de Madag. (E) 12, 207–235 (1961).
Alexander, C. P. New and little-known Indian craneflies (Diptera: Tipulidae) III. Orient. Insects 4, 77–88 (1970).
Brunetti, E. Revision of the Oriental Tipulidae with descriptions of new species, Part 2. Rec. Indian Mus. 15, 255–344 (1918).
Alexander, C. P. New or little-known Tipulidae (Diptera). LXXXII. Oriental-Australasian species. Ann. Mag. Nat. Hist. 12(1), 639–663 (1949).
Alexander, C. P. Crane-flies of the Baltic amber (Diptera). Bernstein-Forschung 2, 1–135 (1931).
Krzemiński, W. Fossil Tipulomorpha (Diptera, Nematocera) from Baltic amber (Upper Eocene): introductory part: subfamily Lechriinae (Limoniidae). Polskie Pismo Entomol. 60, 177–194 (1990).
Kania, I. Subfamily Limoniinae Speiser, 1909 (Diptera, Limoniidae) from Baltic amber (Eocene): the genus Trichoneura Loew, 1850. Acta Zool. Crac. 58(1), 1–19 (2015).
Linnaeus, C. Systema nature per regna tria naturae, secundum classes, ordines, genera, species, cum caracteribus, differentiis, synonymi, locis. Tomus I. Editio decima, reformata. L. Salvii, Holmiae [= Stockholm], 824 (1758).
Rohdendorf, B. B. The oldest infraorders of Diptera from the Triassic of Middle Asia. Palaeontol. Zhurnal 2, 90–100 (1961).
Brindle, A. The larvae and pupae of the British Cylindrotominae and Limoniinae (Diptera, Tipulidae). Trans. Soc. Br. Entomol. 17(7), 151–216 (1967).
Savchenko, E. N. Komari-limonijidi [Limoniid-flies], (subfamily Eriopterinae). Fauna Ukrajiny 14(3), 1–335 (1982) (in Ukrainian).
Savchenko, E. N. Komary-limoniidy [Limoniid-flies]. Subfamily Limoniinae Fauna Ukrainy 14(4), 1–180 (1985) (Iin Russian).
Savchenko, E. N. Komary-limoniidy [Limoniid-flies]. (Gen. Descr. Subfamilies Pediciinae Hexatominae) Fauna Ukrainy 14(2), 1–380 (1986) (In Russian).
Alexander, C. P. The biology of the North American crane-flies (Tipulidae, Diptera). V. The genus Dicranoptycha Osten Sacken. Pomona Coll. J. Entomol. Zool. 12, 67–74 (1919).
Lindner, E. Beiträge zur Kenntnis der Larven der Limoniidae. Zeitschrift für Morphologie und Ökologie der Tiere 48, 209–319 (1959).
Young, C. W. A revision of the crane fly genus Dicranoptycha in North America. Kans. Univ. Sci. Bull. 1987(53), 215–274 (1987).
Hynes, C. D. The immature stages and biology of the craneflies Toxorhina caledonica and Elephantomyia garrigouana (Diptera: Limoniidae). Pan Pac. Entomol. 73, 93–99 (1997).
Poinar, G. Jr. & Brown, A. Hymenaea mexicana sp. Nov. (Leguminosae: Caesalpinioideae) from Mexican amber indicates Old World connections. Bot. J. Linn. Soc. 139(2), 125–132 (2002).
Shi, G. et al. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 37, 155–163 (2012).
Zherikhin, V. V. & Ross, A. J. A review of the history, geology and age of Burmese amber (Burmite). Bull. Br. Mus. (Nat. Hist.) Geol. Ser. 56(1), 3–10 (2000).
Acknowledgements
We would like to acknowledge Prof. Bo Wang (State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, No. 39, East Beijing Road, Nanjing 210008, PR China) and Dr. Jan Ševčík Department of Biology and Ecology, Faculty of Science, University of Ostrava, Chittussiho 10, 71000 Ostrava, Czech Republic) for giving the material for our disposal. We would like to thank two Anonymus Reviewers for valuable comments and suggestions. The publication was made within the projects of Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Kraków, Poland DS 2022.II1.25 and National Science Centre of Poland, grant number 2016/23/B/NZ8/00936.
Funding
Open Access funding enabled and organized by Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Kraków, Poland DS 2022.II1.25 and National Science Centre of Poland, grant number 2016/23/B/NZ8/00936.
Author information
Authors and Affiliations
Contributions
K.K.K. shared the museum material, coordinated the administration of the project, conceived and designed the study, lead and performed the data analysis, were responsible for taxonomic decisions, interpretations and analysis, writing and corrections of the manuscript; I.K.-K. conceived and designed the study, lead and performed the data analysis, were responsible for taxonomic decisions, interpretations and analysis, writing and corrections of the manuscript, originator and performer graphic ilustrations, was the corresponding author and coordinated the correction of the manuscript; W.K. conceived and designed the study, lead and performed the data analysis, were responsible for taxonomic decisions, interpretations and analysis, writing and corrections of the manuscript; Q.Z. shared the scientific material, corrections of the manuscript. All authors reviewed manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kopeć, K., Kania-Kłosok, I., Zhang, Q. et al. New fossil data reveal evolutionary pathways within the genus Trichoneura Loew, 1850 (Diptera, Limoniidae). Sci Rep 13, 16794 (2023). https://doi.org/10.1038/s41598-023-43468-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43468-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.