Abstract
Deregulation of vascular endothelial growth factor (VEGF) levels leads to retinopathy of prematurity (ROP). Vitamin D (VIT-D) is known to regulate VEGF in an oxygen dependent manner. The purpose of this study was to correlate tear levels of VEGF and VIT-D with different ROP stages in preterm infants. In this prospective cross-sectional study, we enrolled 104 pre-term infants. They were grouped into: Group-1 (Classical ROP) and Group-2 (Aggressive ROP), which were further subdivided into Group-1A (progressing), Group-1B (regressing), Group-2A (pre-treatment), and Group-2B (post-treatment). Tear VEGF and VIT-D levels and their association with different ROP stages were assessed. Stage 1 and stage 2 had higher whereas stage 3 had lower VEGF levels in Group-1B compared to Group-1A. Stage 1 and stage 3 showed higher levels of VIT-D with no difference in stage 2 in Group-1B compared to Group-1A., Group-2B showed higher VEGF and lower VIT-D levels compared to Group-2A. Presence of a positive correlation at an early stage (stage 1) of ROP and a negative correlation at a more advanced stage (stage 3) of ROP with VIT-D and VEGF implies stage-specific distinct signaling crosstalk. These findings suggest that VIT-D supplementation may have the potential to modify the course and outcome of ROP.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) remains the leading cause of bilateral blindness in preterm infants1,2. Although multifactorial in aetiology, unmonitored and concentrated oxygen exposure during the early postnatal period is believed to set up a cascade of vascular events in the retina. This cascade leads to vaso-attenuation in the early stage and vascular proliferation in the late stage of ROP development1,3,4. Several angiogenic factors have been implicated but vascular endothelial growth factor (VEGF) and angiogenin are of paramount importance5,6.
The complexity of ROP development could be driven by low levels of physiologic VEGF in the early stage, and higher levels of pathologic VEGF in the late stage of ROP. Differential oxygen saturation levels in the early and late stages of ROP must also be considered6,7,8. The biological plausibility that restoration of normal levels of physiologic VEGF might prevent the development or progression of ROP is therefore an intuitive assumption. Likewise, the reduction of pathologic levels of VEGF in late stages of ROP may prevent disease progression or worsening. Hence, exploring modalities to replenish ‘deficient’ VEGF levels in the early stage and to dampen, mitigate or annul pathologic VEGF levels in the late stages of ROP might aid in preventing the development and/or progression of ROP.
Pre-term babies are often deficient in micronutrients. Identifying an appropriate nutraceutical with the potential to regulate VEGF levels under various oxygen levels would provide a novel treatment for infants at risk for ROP. Vitamin D (VIT-D) is a nutrient known to modulate inflammation, angiogenesis, oxidative stress, and fibrosis in humans9,10,11. In preterm infants, VIT-D insufficiency has been reported to be associated with respiratory distress syndrome12, while an appropriate dosage of VIT-D supplementation has been shown to promote growth and increase bone density13,14,15. VIT-D binding domains are present in the promoter regions of VEGF16. Thus, VIT-D supplementation might aid in restoring VEGF levels, especially in pathologic conditions secondary to low VEGF levels.
Tears provide an ideal tool for estimating biomarkers and molecular signatures of ocular vascular changes in health and disease17. Our group has shown that at the first visit lower levels of VEGF and corresponding higher levels of angiogenin were detected in tears of infants with ROP compared to infants without ROP5. Additionally, the ratios of angiogenin to birth weight or gestational age or VEGF levels could distinguish preterm infants with from those without ROP5. We have previously shown a positive correlation between VIT-D in serum and tears of adults18. The aim of this study was to investigate tear levels of VEGF and VIT-D and their association with different ROP stages in preterm infants.
Results
Baseline characteristics of preterm infants:
During the 2-year study period (November 2016–December 2018), 116 preterm infants (175 eyes) fulfilled the inclusion criteria. Twelve infants (12 eyes) were used for standardizing the quantification of tear analytes by ELISA and cytometric bead array. Thus, study analyses were performed on the remaining 104 infants (163 eyes): 77 infants (120 eyes) with classical ROP (Group-1), 11 infants (20 eyes) with AROP (Group-2), and 16 control infants (23 eyes) (Fig. 1). In Group-1B infants, ROP was regressing either spontaneously (n = 17) or after laser therapy (n = 8). Compared to controls, all disease groups (i.e. Group-1A, Group-1B, Group-2A, Group-2B) had statistically significant lower GA and BW. Whereas only Group-1A and Group-2A (but not Group-1B and Group-2B) had statistically significant lower postmenstrual age (PMA) at time of tear sample collection (Supplementary Table 1). The median VEGF and VIT-D were apparently low in controls than progressing Group-1A ROP. In regressing Group-1B ROP VEGF and VIT-D were higher than controls though not significant (Supplementary Table 1).
Study Flow chart showing the number of infants (I) and eyes (E)included in this study and grouping of tear samples by clinical stage of ROP on the date of sample collection. AROP, aggressive retinopathy of prematurity; Group-1A, infants/eyes with progression in ROP from its preceding screening visit; Group-1B, infants/eyes with regression in ROP(spontaneous or after laser treatment)from its preceding screening visit; Group-2A, AROP infants/eyes prior to treatment; Group-2B, AROP infants/eyes after laser treatment; ROP, retinopathy of prematurity; S1, stage 1 ROP; S2, stage 2 ROP; S3, stage 3 ROP; VEGF, vascular endothelial growth factor; VIT-D, Vitamin-D.
Following the combined group analysis, the baseline characteristics for each ROP stage in Group-1A and Group-1B infants are detailed in Table 1. Among Group-1A infants, GA and BW were significantly higher in those with stage 1 compared to stage 2 or stage 3 ROP. Comparing infants in Group-1A and Group-2A, PMA was significantly higher in Group-1A infants with stage 1, 2, and 3 ROP whereas GA and BW were significantly higher only among those in Group-1A with stage 1ROP (Table 1). Comparing Group-1B and Group-2B infants, GA was significantly higher in Group-1B infants with ROP stage 1 and 2 (Table 1).
Levels of VEGF and VIT-D in ROP stages
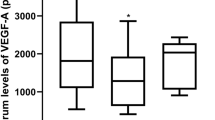
Followed by the clinical characteristics, the molecular factors VEGF and VIT-D were analyzed in each stages of progressing group-1A and regressing group-1B. We observed that tear VEGF levels in progressing stage1 ROP were significantly lower compared to control, progressing stage 2, stage 3 ROP, and Group-2A AROP (Fig. 2A, Supplementary Table 2). Tear VIT-D levels in progressing stage 1 ROP was significantly lower than its progressing ROP S2 and controls (Fig. 2B, Supplementary Table 2). Similarly, we then compared the tear VEGF and VIT-D levels of control with the stages (stage 1, 2, 3) of regressing Group-1B and Group-2B AROP. Tear VEGF levels in the regressing stage 3 ROP was significantly lower than its regressing stage1 and stage2 ROP and Group-2B APROP (Fig. 2C, Supplementary Table 2). In regressing ROP stages the tear VIT-D levels showed no statistical differences among regressing stages (ROP-S1, ROP-S2, ROP-S3) controls and Group-2B AROP (Fig. 2D). The median (minimum and maximum) values of VEGF and VIT-D and the P values comparing the different Groups, stages and controls are shown in Supplementary Table 2.
Comparison of tear vascular endothelial growth factor (VEGF) and Vitamin-D (VIT-D) levels in Control, Group-1 (Classical Retinopathy of Prematurity [ROP]) infants by disease activity (i.e. progressing [Group-1A] and regressing [Group-1B]) and ROP stage, and Group-2 (aggressive ROP [AROP]) infants grouped by those prior to treatment (Group-2A) or post-laser treatment (Group-2B). Scatter plots showing the median tear levels of (A and C), VEGF and (B and D) VIT-D. Comparative difference of median tear levels of (E) VEGF and (F) VIT-D between Group-1A and Group-1B infants by ROP stage shows the median difference *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
We then wanted to find if there was any difference of tear VEGF and VIT-D levels between the stages of progressing Group-1A and stages of regressing Group-1B. Results revealed a 2.09-fold higher ratio (p = 0.0001) among those with stage1 ROP, a 1.16-fold higher ratio (p = 0.0457) among those with stage 2 ROP, and a 0.6-fold lower ratio (p = 0.0183) among those with stage3 ROP (Fig. 2E). Comparison of median tear VIT-D levels revealed a 1.24-fold higher ratio (p = 0.0456) among those with stage 1 ROP and a 1.17-fold higher ratio (p = 0.0345) among those with stage3 (Fig. 2F). Additionally, we also wanted to determine if there existed any difference of tear VEGF and VIT-D levels among pretreatment Group-2A AROP and post laser treated Group-2B AROP. The median tear VEGF levels revealed a 1.64-fold higher ratio (p = 0.0392) (Supplementary Fig. 1A) and of median tear VIT-D levels revealed a 0.87-fold lower ratio (p = 0.0117) (Supplementary Fig. 11) in Group-2B compared to Group-2A.
Correlation of tear levels of VEGF and VIT-D in progressing and regressing stages of ROP and AROP
As a next step we wanted to evaluate if there was any correlation of the tear VEGF and VIT-D levels in stages of progressing Group-1A. A significant positive correlation was observed for VEGF and VIT-D in progressing stage 1(p = 0.021) and Stage-3 (exact p = 0.060) ROP (Figure-3 A and C).Stage-2 progressing ROP showed an apparent negative correlation (p = 0.268) (Fig. 3B). In regressing ROP (Group-1B), though not significant ROP-S1 (p = 0.297) and ROP-S2 (p = 0.286) showed a positive correlation and ROP-S3 showed a negative correlation (p = 0.260)(Fig. 3D–F). Controls also showed a positive correlation for VEGF and VIT-D (p = 0.085) (Fig. 3G). The Group-2A (p = 0.222) and Group-2B (p = 0.177) showed a negative correlation (Supplementary Fig. 1C,D).
Tear vascular endothelial growth factor (VEGF) and Vitamin-D (VIT-D) levels and their correlation between progressing Group-1A and regressing Group-1B infants by retinopathy of prematurity (ROP) stage. Spearman’s rank correlation for VEGF and VIT-D (A-C) in progressing stage-1, stage-2 and stage-3 ROP; (D-F) in regressing stage-1, stage-2 and stage-3 ROP;and (G) Control. *p < 0.05.
To determine the relationship of VEGF and VIT-D with GA or BW, we carried out multivariable analysis. In progressing group (Group-1A), the VEGF association with BW and GA showed a statistical significance (P = 0.023), though the odds ratio is near 1 (0.995). Similarly, the VIT-D association with BW and GA showed a similar pattern like VEGF with statistical significance (P = 0.016) and odds ratio near 1(0.83). In regressing ROP (Group-1B) the BW and GA category though showed an odds ration > 1, no significance was observed. This may be due to smaller sample size and wider confidence interval (Supplementary Table 3).
Discussion
ROP is one of the leading causes of blindness in preterm infants. The present treatment paradigm revolves around anti-VEGF and laser treatment. In an effort to explore potential non-invasive mode of disease management, we investigate a correlation of tear VIT-D and VEGF levels for different stages of ROP. In ocular disease management, tear acts as a non-invasive tool for assaying cytokines and growth factors. To our knowledge this is the first clinical study that reports the association between VEGF and VIT-D in tears of premature infants with ROP. Low levels of serum 25(OH) D have been implicated in low BW preterm infants, respiratory morbidity, and development of ROP19,20. The low VIT-D levels in preterm infants may be due to insufficient transfer from maternal source through placenta in the third trimester21,22. The lower levels of VIT-D in Group-1A is also dependent on the mothers VIT-D intake23. The VEGF physiologic and pathologic role in retinal angiogenesis has been reported24. Acting upstream of VEGF regulation, VIT-D and its receptors modulate retinal angiogenesis16,25.
We found that among those with progressing classical ROP (i.e. Group-1A), those with stage 1 ROP had lower tear levels of VEGF and VIT-D levels compared to those with higher stage ROP, APROP, and controls. Interestingly, in the regressing classical ROP (i.e. Group-1B) infants the VIT-D and VEGF levels were higher in stage1 ROP whereas in stage3 ROP, VIT-D levels were higher while VEGF levels were lower compared to progressing classical ROP (i.e. Group-1A). The source for the higher levels of VIT-D in the tears of regressing ROP (Group-1B) of stage-1 and stage-3 infants’ may be from maternal breast milk or formula milk26. This could also be an outcome driven by increase of VIT-D assimilation following intake. It also needs to be stated that in spite of breastmilk or formula milk there exists VIT-D deficiency in the preterm infants. Studies have emphasized the need for supplementation to maternal breastfeed in order to prevent VIT-D deficiency in infants27,28. Supplementation of VIT-D in preterm infants has shown improvement in the linear growth and bone density15. In a mice supplementation of VIT-D promoted vascular invasion and endo-chondral ossification by regulating VEGF and matrix metalloproteinase-929.
Further, in this study postnatally infants were feed with breastmilk or formula milk. The VIT-D levels in human milk (40 IU/L; 15–26 IU/L) and formula milk (400 IU/L) may differ23,30,31,32. For a healthy infant 50 nm/L of standard 25 (OH) D concentration is required. Studies, state that breast fed infants VIT-D may be inadequate to reach the normal value. Moreover, formula fed infants need to take 1 L of milk every day, for an intake of 400 IU/L VIT-D. In newborn infants it is not possible to consume 1 L of milk/day, which also leads to inadequate amount of VIT-D32. Previous study from our group, stated that tear VIT-D levels are higher than serum Vit-D levels, suggesting it may have its own relevance for certain ocular pathogenesis and disease severity18. In this study, tear VIT-D levels might be an outcome of absorption and intake of each infant. This study did not compare with any standard cutoff values. Though breastfed/formula milk VIT- D may be not adequate for all infants, however, it could be one of the main sources of VIT-D in this study. In the regressing Classical ROP (i.e. Group-1B) infants, the increased VEGF in tears of stage1 ROP infants might have resulted from either maternal breast milk or due to the physiological increase with increasing gestational age33, while in the stage3 ROP infants, the lower levels of VEGF can be attributed to laser treatment34.
In our previous study, we demonstrated that hyperoxia-conditioned primary retinal pigment epithelial (RPE) cells secreted low levels of VEGF35. Further we functionally demonstrated an incomplete tube formation in vitro, which supports our current findings of lower tear VEGF levels in stage1 of classical progressing ROP. In our in vitro study, VIT-D supplementation increased VEGF levels in the primary RPE cells and improved endothelial tube formation36. At a molecular level it has been shown that VIT-D has a binding affinity in the promoter region of VEGF and induces the regulation of VEGF16. Further studies investigating the role of VIT-D supplementation on stage-1 ROP disease would possibly provide a newer avenue of ROP disease management. It needs to be explored if laser treatment could have influenced the increase in VIT-D levels. Further, the spearman’s rank correlation analysis for VEGF and VIT-D in Stage-3 ROP belonging to Group-1A, showed an inverse correlation. It is also reported that VIT-D acts as an anti-angiogenic factor in hypoxic conditions such as in cancer by regulating the endothelial cells35,37,38.
The in vitro hyperoxic condition may be ‘closer’ to stage1 ROP and the hypoxic condition akin to stage3 ROP. It is also reported that VIT-D acts as an anti-angiogenic factor in hypoxic conditions like cancer by regulating the endothelial cells35,37,38. Therefore, VIT-D regulating VEGF may be influenced by oxygen saturation levels, as it is acting as a pro-angiogenic factor in hyperoxic conditions and anti-angiogenic in hypoxic conditions.
In this study, stage2 ROP showed variable results and demanding further research. Comparable levels of VEGF in Group-1B ROP stage3 infants further corroborates the possibility that VEGF may play a dual physiological and pathological role depending on the prevailing oxygen saturation condition. Tear analysis of Group-2A infants showed low VEGF and high VIT-D levels compared to Group-2B. This implies that the Group-2B infants need aggressive treatment to reduce the proangiogenic factors. In the present study, we included AROP eyes as a positive control for the VEGF levels. However, further investigations on understanding detailed crosstalk of VIT-D and proangiogenic factors are needed.
Low GA and BW remain the most promising determinants of ROP, that is convincingly restored in patients with resolved ROP39. Additionally, studies have revealed that premature infants with high GA and BW have a low risk of developing ROP40. In our study cohort we also observed that GA and BW showed high mean levels in the regressing (Group-1B) group in all stages of ROP. Though, VIT-D levels might play a crucial role in occurrence and progression of the disease and its resolution, the pertinent role of BW and GA cannot be undermined. Furthermore, studies can be designed to elaborate the implication of VIT-D with GA/BW to understand the underlying mechanisms.
It is well established that along with ROP, pre term infants can have additional non-ocular complications such as respiratory distress syndrome (RDS), necrotizing enterocolitis (NEC) and intraventricular hemorrhages (IVH). In our study, IVH was detected in just one infant. NEC was observed in eight cases, evenly divided between Group-1A and Group-1B, with four instances in each. Respiratory Distress Syndrome (RDS) was notably prevalent in our study, particularly in Group-1A, where a higher prevalence of RDS corresponded to more severe disease groups. Within progressing Group-1A, 15 out of 23 cases (65.2%) in the control group and 15 out of 23 cases (65.2%) in ROP Stage-1 exhibited RDS, with average tear Vitamin D concentrations of 22.5 ng/mL and 19.9 ng/mL, respectively. ROP Stage-2 showed RDS in 20 out of 27 cases (74.0%), while Stage-3 had RDS in 23 out of 30 cases (76.6%), with average tear Vitamin D concentrations of 21.4 ng/mL and 19.6 ng/mL, respectively.
There are no studies implying tear Vitamin D levels with RDS. All the studies have correlated the serum Vitamin D levels and RDS. There being a positive correlation between the tear and serum Vitamin D levels18, it could be assumed that although the control and Stage-1 groups had similar percentages of RDS cases, controls had a higher concentration of Vitamin D compared to ROP Stage-1, which might have acted as a preventive factor in controls. The lower Vitamin D concentration in Stage-1 could potentially contribute to disease progression. However, in Stage-3, where RDS was present in a higher percentage, Vitamin D concentration was lower compared to Stage-2 and equal to Stage-1. The systemic arterial oxygen saturation index might also play a role in regulating Vitamin D levels for development of RDS.
In regressing Group-1B, RDS was prevalent in higher numbers, nearly identical across all groups when compared to controls. Specifically, Group-1B ROP Stage-1 had RDS in 10 out of 11 cases (90.9%), Stage-2 had RDS in 15 out of 20 cases (75%), and Stage-3 had RDS in 7 out of 9 cases (77.7%), with respective Vitamin D concentrations of 21.4 ng/mL, 20.45 ng/mL, and 22.27 ng/mL. A discernible pattern of Vitamin D levels corresponding to RDS was observed in Group-1B. In Group-2A, AROP had RDS in 10 out of 14 cases (71.4%), while Group-2B had RDS in 4 out of 6 cases (66.6%).
A meta-analysis study demonstrates an association between vitamin D levels within 24 h of birth and the risk of bronchopulmonary dysplasia41. In a recent study vitamin-D deficiency below 30 ng/mL was associated with increased risk for respiratory distress syndrome42. A clinical study demonstrated that vitamin D supplementation significantly reduces serum inflammation factors and the incidence of bronchopulmonary dysplasia in preterm infants43. In 2018, Yang et al.44 validated that vitamin D deficiency was associated with a higher risk of necrotizing enterocolitis in premature infants. In animal models, Vitamin D has been shown to promote proliferation, inhibit apoptosis, reduce inflammation, and protect the intestinal injury of necrotizing enterocolitis45,46. Further, an increased risk of intraventricular hemorrhages is associated with low vitamin-D levels of preterm infants with recommendations for supplementation47.
A limitation of this study is lack of longitudinal follow-up, which would have provided patient specific information on the interaction of VEGF and VIT-D over time. Oxygen saturation is a crucial component during retinal vasculature development. Due to ethical and technical limitations, we have not investigated the oxygen saturation in affected eyes. Studies in animal models would provide insight into the role of oxygen in the interaction between VEGF and VIT-D. These findings could alter the treatment course of ROP by providing a better understanding of the interplay between VEGF, VIT-D, and retinal status. For example, VIT-D supplementation during stage 1 ROP might halt ROP progression by regulating VEGF and promoting physiological vascular growth towards the ora serrata. Similarly, VIT-D supplementation in laser-treated stage3 ROP eyes may further help regression of severe ROP (i.e. pathologic vascular growth) and promote physiologic vascular growth. Possible routes of VIT-D administration for better bioavailability need to be investigated. We hope that our findings serve as a foundation to explore possible therapeutic potentials of VIT-D supplementation in premature infants at risk for ROP.
Methods
Study design
This cross-sectional study was carried out in the Department of Pediatric Retina, Narayana Nethralaya Eye Institute, Bangalore, India between November 2016 and December 2018. This study used the International Classification of ROP (ICROP) revisited and Early Treatment for ROP (ETROP) guidelines for screening, staging and treatment of infants48,49,50. All preterm infants screened for this study followed the methodology of the Karnataka Internet assisted Diagnosis for ROP (KIDROP) program51,52. Tear samples were collected from infants following the guidelines of Narayana Nethralaya Ethics Committee and abiding by the tenets of the Declaration of Helsinki. This study was performed with the approval of Narayana Nethralaya Institutional Review Board. Written informed consent was obtained from the parents of all infants included in the study.
Study population
Preterm infants with a birth weight (BW) of ≤ 2000 g and gestational age (GA) of ≤ 34 weeks were recruited for the study according to the prevailing Indian National ROP screening guidelines53 and the inclusion criteria of the KIDROP program51,52,54,55. We collected demographic details including BW, GA, gender and postnatal factors. As a part of the KIDROP screening program, wide-field imaging was performed on all preterm infants using the RetCam Shuttle (Natus, CA, USA), or the 3Nethra Neo Camera (Forus Health, India). In this study, we collected tears at one ROP screening visit from preterm infants who presented to the headquarters during any visit of ROP screening and whose parents consented for the study.
ROP group classification for this study
Based on the clinical stage of ROP on the date of sample collection, tear samples were grouped into two disease groups (Group-1 [Classical ROP], Group-2 [Aggressive ROP (AROP)]) and a control group. Samples in Group-1 were subdivided into ‘progressing’ (Group-1A) or ‘regressing’ disease (Group-1B) depending on the clinical findings prior to and subsequently after the date of tear collection which was ascertained from a retrospective chart review. ‘Progression’ (Group-1A) was defined by an increase or worsening of the disease severity with respect to stage or plus component of the disease compared to the previous screening visit. Infants in progression group were treatment naive up to the date of tear sample collection. ‘Regression’ (Group-1B) was defined as decrease in disease severity or improvement in disease severity with respect to stage or plus component of the disease compared to the previous screening visit which occurred either spontaneously or after laser therapy. Samples in Group-2 were subdivided into pretreatment eyes with AROP (Group-2A) and post laser-treated eyes (Group-2BThe control group had preterm infants who did not develop any ROP throughout their screening visits and whose retinal vessels had reached the ora serrata.
For this study, preterm infants were excluded if they had any of the following: stage 4 or 5 ROP, anti-vascular endothelial growth factor (anti-VEGF) treatment, BW > 2000 g, screened after 4 weeks of birth, or who did not fulfill the criteria of the national screening guidelines.
Tear sample collection and processing
Tear samples were collected from preterm infants at one ROP screening visit using Schirmer strips (Contacare Ophthalmics and Diagnostics, Gujarat, India) before any topical medication. The strips were placed on the conjunctival fornices and removed on wetting of 15 mm or more. The removed strips were then stored in sterile eppendorf tubes at − 80 °C freezer. For the estimation of analytes, the Schirmer’s strips were cut, agitated and incubated for 2 h in phosphate buffered saline (PBS) in sterile Eppendorf tubes at 4 °C. The samples were centrifuged at 8.6 g for 10 min and the supernatant was analyzed for VEGF and VIT-D levels5.
Vitamin-D measurement
Tear VIT-D (25-hydroxyvitamin-D2-D3[25OH-D2 and 25OH-D3]) levels were estimated using a competitive Enzyme-Linked Immuno-sorbent Assay (ELISA; DIA Source, BY, Germany). The tear samples were diluted 1:10 times for the absorbance value to be within the standard range. The standards and samples were analyzed per the manufacturer’s instructions. The substrate reaction product was read with a microplate reader (Bio-Rad, CA, USA) at a wavelength of 540 nm with the reference filter at 670 nm.
VEGF measurement
Tear VEGF-A levels were quantified using a cytometric bead array (BD biosciences, NJ, USA). The assay was carried out per the manufacturer’s instructions. A 1:5 dilution factor was used for each tear sample. A single bead analyte bead position was acquired, and the beads were read out in a FACS Caliber machine (BD, NJ, USA), using the FCAP array™ software (version 3.0).
Statistical analysis
GraphPad Prism 6 software was used for all statistical analyses (GraphPad Software, Inc., La Jolla, CA). Mann–Whitney U-test was used to determine the significant difference of VEGF or VIT-D levels by ROP stage. Spearman’s Rank correlation test was conducted to evaluate associations between tear VEGF and VIT-D levels in each stage. The results were considered statistically significant a P ≤ 0.05.
Data availability
The data supporting the study findings are available on request from the corresponding author.
References
Dogra, M. R., Katoch, D. & Dogra, M. An update on retinopathy of prematurity (ROP). Indian J. Pediatr. 84, 930–936 (2017).
Hong, E. H., Shin, Y. U. & Cho, H. Retinopathy of prematurity: A review of epidemiology and current treatment strategies. Clin. Exp. Pediatr. 65, 115–126 (2022).
Shah, P. K., Narendran, V. & Kalpana, N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch. Dis. Child. Fetal. Neonatal Ed. 97, F371-375 (2012).
Owen, L. A. & Hartnett, M. E. Current concepts of oxygen management in retinopathy of prematurity. J. Ophthalmic Vis. Res. 9, 94–100 (2014).
Vinekar, A. et al. Tear fluid angiogenic factors: Potential noninvasive biomarkers for retinopathy of prematurity screening in preterm infants. Invest. Ophthalmol. Vis. Sci. 62, 2 (2021).
Ryu, J. New Aspects on the treatment of retinopathy of prematurity: Currently available therapies and emerging novel therapeutics. Int. J. Mol. Sci. 23, 8529 (2022).
Mintz-Hittner, H. A., Kennedy, K. A., Chuang, A. Z., Group B-RC. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 364, 603–615 (2011).
Jasani, B., Nanavati, R. & Kabra, N. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 368, 1161–1162 (2013).
Kim, D. H., Meza, C. A., Clarke, H., Kim, J. S. & Hickner, R. C. Vitamin D and endothelial function. Nutrients 12, 575 (2020).
Liu, W. et al. The anti-inflammatory effects of vitamin D in tumorigenesis. Int. J. Mol. Sci. 19, 2736 (2018).
Pfeffer, P. E. et al. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS One 13, e0200040 (2018).
Boskabadi, H., Mamoori, G., Khatami, S. F. & Faramarzi, R. Serum level of vitamin D in preterm infants and its association with premature-related respiratory complications: a case-control study. Electron Phys. 10, 6208–6214 (2018).
Thorisdottir, B., Gunnarsdottir, I., Steingrimsdottir, L., Palsson, G. I. & Thorsdottir, I. Vitamin D intake and status in 12-month-old infants at 63–66 degrees N. Nutrients 6, 1182–1193 (2014).
Abrams, S. A. Vitamin D in preterm and full-term infants. Ann. Nutr. Metab. 76(Suppl 2), 6–14 (2020).
Anderson-Berry, A. et al. Randomized trial of two doses of vitamin D3 in preterm infants <32 weeks: Dose impact on achieving desired serum 25(OH)D3 in a NICU population. PLoS One 12, e0185950 (2017).
Cardus, A. et al. 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis 204, 85–89 (2009).
Hagan, S., Martin, E. & Enriquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 7, 15 (2016).
Sethu, S. et al. Correlation between tear fluid and serum vitamin D levels. Eye Vis. 3, 22 (2016).
Kim, I. et al. Association between vitamin D level at birth and respiratory morbidities in very-low-birth-weight infants. Korean J. Pediatr. 62, 166–172 (2019).
Kabatas, E. U. et al. Relationship between serum 25-hydroxy vitamin D levels and retinopathy of prematurity. Scott. Med. J. 62, 129–135 (2017).
Thomas, S. D., Fudge, A. N., Whiting, M. & Coates, P. S. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ Open 1, e000236 (2011).
Burris, H. H. et al. Vitamin D status among preterm and full-term infants at birth. Pediatr. Res. 75, 75–80 (2014).
Choi, Y. J., Kim, M. K. & Jeong, S. J. Vitamin D deficiency in infants aged 1 to 6 months. Korean J. Pediatr. 56, 205–210 (2013).
Ferrara, N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 94, 209–231 (2005).
Jamali, N., Wang, S., Darjatmoko, S. R., Sorenson, C. M. & Sheibani, N. Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3. PLoS One 12, e0190131 (2017).
Balasubramanian, S. Vitamin D deficiency in breastfed infants & the need for routine vitamin D supplementation. Indian J. Med. Res. 133, 250–252 (2011).
Haggerty, L. L. Maternal supplementation for prevention and treatment of vitamin D deficiency in exclusively breastfed infants. Breastfeed Med. 6, 137–144 (2011).
Saadi, H. F. et al. Effect of combined maternal and infant vitamin D supplementation on vitamin D status of exclusively breastfed infants. Matern. Child. Nutr. 5, 25–32 (2009).
Lin, R. et al. 1Alpha,25-dihydroxyvitamin D3 promotes vascularization of the chondro-osseous junction by stimulating expression of vascular endothelial growth factor and matrix metalloproteinase 9. J. Bone Miner. Res. 17, 1604–1612 (2002).
Chong, H. Y. et al. Exploring the potential of human milk and formula milk on infants’ gut and health. Nutrients 14, 3554 (2022).
Morgan, D. W. & Bailey, C. M. Current management of choanal atresia. Int. J. Pediatr. Otorhinolaryngol. 19, 1–13 (1990).
Kim, Y. J. Comparison of the serum vitamin D level between breastfed and formula-fed infants: Several factors which can affect serum vitamin D concentration. Korean J. Pediatr. 56, 202–204 (2013).
Ma, I. T. et al. VEGF mRNA and protein concentrations in the developing human eye. Pediatr. Res. 77, 500–505 (2015).
Li, Z. et al. Comparison of efficacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in Type-1 and threshold retinopathy of prematurity (ROP). BMC Ophthalmol. 18, 19 (2018).
Murugeswari, P. et al. Vitamin-D3 (alpha-1, 25(OH) 2D3) protects retinal pigment epithelium from hyperoxic insults. Invest. Ophthalmol. Vis. Sci. 61, 4 (2020).
Ben-Shoshan, M. et al. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 6, 1433–1439 (2007).
Jiang, Y., Zheng, W. & Teegarden, D. 1alpha, 25-Dihydroxyvitamin D regulates hypoxia-inducible factor-1alpha in untransformed and Harvey-ras transfected breast epithelial cells. Cancer Lett. 298, 159–166 (2010).
Tabrizi, R. et al. The effects of vitamin D supplementation on endothelial activation among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 15, 85 (2018).
Lundgren, P. et al. Low birth weight is a risk factor for severe retinopathy of prematurity depending on gestational age. PLoS One 9, e109460 (2014).
Kim, S. J. et al. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv. Ophthalmol. 63, 618–637 (2018).
Park, H. W. et al. Association between vitamin D level and bronchopulmonary dysplasia: A systematic review and meta-analysis. PLoS One 15, e0235332 (2020).
Kim, Y. J. et al. Association between vitamin D level and respiratory distress syndrome: A systematic review and meta-analysis. PLoS One 18, e0279064 (2023).
Ge, H. et al. Effects of early vitamin D supplementation on the prevention of bronchopulmonary dysplasia in preterm infants. Pediatr. Pulmonol. 57, 1015–1021 (2022).
Yang, L. R., Li, H. & Zhang, T. Zhao RC [Relationship between vitamin D deficiency and necrotizing enterocolitis in preterm infants]. Zhongguo Dang Dai Er Ke Za Zhi 20, 178–183 (2018).
Shi, Y. et al. Vitamin D ameliorates neonatal necrotizing enterocolitis via suppressing TLR4 in a murine model. Pediatr. Res. 83, 1024–1030 (2018).
Lyu, C., Jiang, S., Kong, M., Chen, X. & Zhang, L. Vitamin D protects against necrotising enterocolitis in newborn mice by activating the ERK signalling pathway. Mol. Med. Rep. 22, 2107–2114 (2020).
Boskabadi, H., Zakerihamidi, M. & Faramarzi, R. The vitamin D level in umbilical cord blood in premature infants with or without intra-ventricular hemorrhage: A cross-sectional study. Int. J. Reprod. Biomed. 16, 429–434 (2018).
Early Treatment For Retinopathy Of Prematurity Cooperative. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 121, 1684–1694 (2003).
International Committee for the Classification of Retinopathy of P. The international classification of retinopathy of prematurity revisited. Arch. Ophthalmol. 123, 991–999 (2005).
Good, W. V. Early treatment for retinopathy of prematurity cooperative. G Final results of the Early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 102, 233–248 (2004).
Vinekar, A. et al. The KIDROP model of combining strategies for providing retinopathy of prematurity screening in underserved areas in India using wide-field imaging, tele-medicine, non-physician graders and smart phone reporting. Indian J. Ophthalmol. 62, 41–49 (2014).
Vinekar, A., Jayadev, C., Mangalesh, S., Shetty, B. & Vidyasagar, D. Role of tele-medicine in retinopathy of prematurity screening in rural outreach centers in India - a report of 20,214 imaging sessions in the KIDROP program. Semin. Fetal Neonatal. Med. 20, 335–345 (2015).
India PHFo. Project Operational Guidelines-Prevention of Blindness from Retinopathy of Prematurity in Neonatal Care Units. In: Guidelines P.O. (ed). 1–56 (Public Health Foundation of India, 2018)
Quinn, G. E., e ROPCG. Need for telemedicine in retinopathy of prematurity in middle-income countries-reply. JAMA Ophthalmol. 133, 361–362 (2015).
Vinekar, A. et al. Outcomes of a protocol-based management for zone 1 retinopathy of prematurity: The Indian twin cities ROP screening program report number 2. Am. J. Ophthalmol. 152, 712 (2011).
Acknowledgements
The authors would like to thank Narayana Nethralaya Foundation and DBT Biocare for providing the financial support to conduct this study. The authors would also like to thank Dr. P. Narendra and Dr. K Bhujang Shetty for their administrative support needed for this study. The authors would like to thank Mr. N. Krishnan, for the immense technical support in collecting tear samples from the infants.
Author information
Authors and Affiliations
Contributions
P.M., A.V., A.G., G.K., R.S., A.G., D.D. conceptualized and designed the study, P.M., A.V., S.G.P., V.R.A., M.S., T.A.V., A.P.N performed the experiments and analyzed the results. P.M., A.V., S.G.P., C.J., R.S., G.K., A.G. provided the tissue samples and edited the manuscript. S.G.P., C.J., provided critical analysis for the manuscript. P.M., A.V., D.D. wrote the manuscript and analyzed all the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murugeswari, P., Vinekar, A., Prakalapakorn, S.G. et al. Correlation between tear levels of vascular endothelial growth factor and vitamin D at retinopathy of prematurity stages in preterm infants. Sci Rep 13, 16175 (2023). https://doi.org/10.1038/s41598-023-43338-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43338-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.