Abstract
The red blood cell distribution width–albumin ratio (RAR) is a prognostic factor for adverse outcomes in various populations. However, whether RAR is associated with renal outcomes remains unclear. Therefore, we aimed to investigate the impact of RAR on the prognosis in patients with chronic kidney disease (CKD). We conducted a retrospective cohort study using 997 CKD patients who were enrolled in the Fukushima Cohort Study. Patients were categorized into tertiles (T1-3) according to the baseline RAR. The associations of RAR with end-stage kidney disease (ESKD) were assessed using Kaplan–Meier curves and multivariable cox regression analyses. Receiver operating characteristic (ROC) curves were performed to test whether significant differences were present between red cell distribution width (RDW) and RAR. The median age was 66, 57% were men, the median eGFR was 47.8 ml/min/1.73 m2, and the median value of RAR was 3.5. The higher RAR group showed an increased risk for ESKD in the Kaplan–Meier curve analysis. Compared to the lowest RAR group, higher RAR groups had a higher risk of ESKD (hazard ratio [HR] 1.37, 95% CI 0.68–2.78 and 2.92, 95% CI 1.44–5.94) for T2 and T3 groups, respectively. ROC curve analysis proved that the discriminating ability of RAR for ESKD was superior to RDW. A higher RAR value was associated with worse renal outcomes in patients with CKD. RAR could be a convenient and useful prognostic marker for renal prognosis.
Similar content being viewed by others
Introduction
The prevalence of chronic kidney disease (CKD) has increased worldwide in the past few decades, and 843.6 million individuals suffered from CKD in 20171. CKD is a major risk factor for end-stage kidney disease (ESKD) and has emerged as one of the most prominent causes of death and morbidity in the twenty-first century2,3. Therefore, the early stratification of the risk of progression to ESKD is crucial in the clinical setting.
The red cell distribution width (RDW), which is known to be a marker of the heterogeneity of red blood cell volume and used for differential diagnosis of anemia4, has been reported to be an independent risk factor for mortality among various populations5,6,7,8. In terms of patients with CKD, a higher RDW was associated with an increased risk of mortality, cardiovascular events, and renal prognosis9,10,11. Generally, serum albumin is an important marker of nutritional status. Several studies have identified low albumin levels as a powerful predictor of mortality in the general population and in patients with CKD12,13.
Recently, the RDW to albumin ratio (RAR), which was a combined index of RDW and serum albumin, has been reported to be provided more accurate risk prediction for adverse outcomes among various populations, including heart failure, acute coronary syndrome, stroke, and sepsis14,15,16,17,18. Furthermore, recent studies have suggested that RAR may yield better results for predicting prognosis than evaluating RDW and albumin separately16,19.
Nevertheless, very few studies have focused on the impact of RAR on CKD progression and prognosis among patients with CKD. Therefore, we aimed to investigate the association between RAR and kidney failure requiring kidney replacement therapy over five years with annual assessments among Japanese patients with non-dialysis-dependent CKD in the Fukushima Cohort Study.
Materials and methods
Study population (Fukushima cohort study)
The Fukushima Cohort Study was a prospective survey and has been described in detail elsewhere20,21,22,23,24. Participants were recruited between March 2012 and July 2014, and a total of 2724 participants registered for the Fukushima Cohort study. We investigate the characteristics and outcomes (ESKD, cardiovascular events, and death) of outpatients who were under the care of specialists in nephrology or diabetology at the Fukushima Medical University Hospital.
The study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) with the study ID, UMIN000040848. The study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent for participation, and the protocol was approved by the ethics committee of Fukushima Medical University (institutional review board approval numbers 1456 and 2001).
The inclusion criteria were as follows: (1) participants aged ≥ 18 years, (2) CKD patients defined as an eGFR of < 60 ml/min/1.73 m2 and/or proteinuria (positive dipstick results ≥ 1 +), with stable kidney function for ≥ 3 months before study entry. The eGFR was calculated using the estimation equation for Japanese patients with CKD25.
Data collection and definition
Information on baseline characteristics and comorbidities (hypertension, diabetes mellitus, and dyslipidemia), as well as a history of cardiovascular disease, were obtained from the electronic medical records or the results of blood examinations at registration. RAR was calculated using the following formula: [RDW (%)/serum albumin (g/dL)]. Body mass index (BMI) was calculated as the ratio of body weight (kg) to height (m2). Blood pressures were measured in the sitting position using a standard sphygmomanometer or an automated device after 5 min of rest. Hypertension was defined as systolic blood pressure of ≥ 140 mmHg, diastolic blood pressure of ≥ 90 mmHg, or the use of antihypertensive medications.
Subjects with diabetes were identified by a fasting plasma glucose concentration of ≥ 126 mg/dL, glycated hemoglobin (HbA1c) value of ≥ 6.5% (National Glycohemoglobin Standardization Program), or those who used insulin or oral antidiabetic medications. Dyslipidemia was defined as triglyceride concentration ≥ 150 mg/dL, low-density lipoprotein cholesterol concentration ≥ 140 mg/dL, high-density lipoprotein cholesterol concentration < 40 mg/dL, or use of antihyperlipidemic medications.
Exposure and outcome
The exposure of interest in this study was RAR. We categorized CKD patients into tertile groups according to the baseline RAR values described as T1, T2, and T3. The primary outcome was ESKD requiring kidney replacement therapy, and secondary outcomes were all-cause mortality and cardiovascular events, including fatal or nonfatal myocardial infarction, angina pectoris, congestive or acute heart failure, arrhythmias, cerebrovascular disorder, chronic arteriosclerosis obliterans, aortic disease, and sudden death. Information on kidney replacement therapy, deaths, and cardiovascular events were obtained from the medical record by attending physicians.
Statistical analyses
Variables were expressed as medians with interquartile ranges or frequency (percent). Non-parametric trend tests (Jonckheere–Terpstra and Cochran–Armitage trend test) were used to evaluate the differences in baseline characteristics among the three groups26,27,28,29.
Kaplan–Meier curves and the log-rank test were used to compare the event-free survivals of patients by RAR levels. Then we used the Cox proportional hazards model to examine the association between the RAR and the incidence of ESKD, all-cause death, and cardiovascular events. Schoenfeld residuals were used to test the proportional hazard assumption. Additionally, receiver operating characteristic (ROC) curve analyses were performed to predict the prognostic efficiency of RAR and RDW. The following adjustments were used in these analyses: (1) Model 1, which was adjusted for age and sex; (2) Model 2, which included the above variables plus BMI, comorbidities (hypertension, diabetes, dyslipidemia), history of cardiovascular disease, and smoking; and (3) Model 3, which included the variables in Model 2 plus eGFR, proteinuria, and hemoglobin.
As secondary analyses, evaluating nonlinear associations between RAR and the incidence of ESKD, all-cause deaths, and cardiovascular events, we used Model 3-adjusted restricted cubic spline functions with four knots at the 5th, 35th, 65th, and 95th percentiles30,31. Also, Fine-Gray proportional hazard models were used to assess the cumulative incidence of ESKD and CV events in the presence of competing risks because of deaths before ESKD and cardiovascular events act as competing risks in CKD patients. The frequencies of missing hemoglobin, BMI, and smoking history were 0.4%, 2.7%, and 5.6%, respectively. Hence, we used multiple imputation with 20 datasets for all the analyses. All p-values < 0.05 were considered statistically significant; we used STATA MP, version 15.1 (Stata Corp, College Station, TX, USA) for all analyses.
Results
Participants characteristics
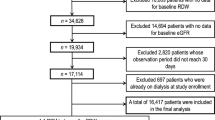
A total of 2,724 participants were registered in the Fukushima Cohort Study, and 997 patients with CKD who fulfilled the study criteria were included in this study (Fig. 1). The median age was 66 years, 57% were men, the median eGFR was 47.8 ml/min/1.73 m2, and the median value of RAR was 3.5. Most patients had hypertension (88%), dyslipidemia (87%), and almost half of the patients had diabetes (49%). Patients in the high RAR group were older and more likely to have diabetes. In addition, they had lower eGFR, a higher proportion of proteinuria, lower hemoglobin, and lower serum albumin than those with a low RAR group (Table 1).
RAR and the incidence of ESKD
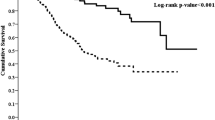
During the median observational period of 4.6 years, 115 patients reached ESKD requiring kidney replacement therapy. Kaplan–Meier curve showed that the higher RAR group had a significantly increased risk for ESKD (Fig. 2a). Using the Cox regression model, patients with the highest RAR group (T3) showed a significantly higher risk of ESKD than those with lower RAR when we used the lowest RAR group (T1) as a reference. Although these associations were attenuated after additional adjustment, they remained significant in Model 3. The adjusted hazard ratios (HRs) were 1.37 (95% confidence interval [CI] 0.68–2.78) and 2.92 (95% CI 1.44–5.94) for T2 and T3 groups, respectively (Table 2). Similar results were observed when we used Fine-Gray proportional hazards models to assess the cumulative incidence of ESKD in the presence of competing risks (Supplemental Table).
The comparative analysis of ROC curves revealed that the discriminating ability of the RAR was superior to RDW (Fig. 3a). The area under the ROC curves (AUCs) for RAR and RDW were 0.713 (95%CI: 0.667–0.759) and 0.601 (95% CI: 0.544–0.658), respectively (p < 0.001). The restricted cubic spline curve showed a similar association between RAR and the incidence of ESKD (Fig. 4a).
Receiver operator characteristic (ROC) curves of RAR and RDW for predicting the primary and secondary outcomes. (a) ESKD, (b) All-cause mortality, (c) Cardiovascular events. RAR red blood cell distribution width to albumin ratio, RDW red cell distribution width, ESKD end-stage kidney disease, AUC area under the curve.
Distributions and Model 3 adjusted restricted cubic splines comparing the relationship of RAR with primary and secondary outcomes. Solid line represent adjusted hazard ratio estimates and dashed lines represent 95% CIs, respectively. (a) ESKD, (b) All-cause mortality, (c) Cardiovascular events. Model 3: adjusted for age, sex, BMI, comorbidities (hypertension, diabetes, and dyslipidemia), history of cardiovascular disease, smoking history, eGFR, proteinuria, and hemoglobin. RAR red blood cell distribution width to albumin ratio, ESKD end-stage kidney disease, BMI body mass index, eGFR estimated glomerular filtration rate.
RAR and all-cause death, cardiovascular events
During the observational period, 103 patients died, and 142 developed cardiovascular events.
Similar to the incidence of ESKD, higher RAR groups showed significantly worse prognoses for all-cause death and cardiovascular events in Kaplan–Meier analyses (Fig. 2b,c).
Cox regression analyses showed that patients with higher RAR groups had significantly higher risks of all-cause death and cardiovascular events compared to the lowest group (T1). The adjusted hazard ratios (HRs) of T2 and T3 groups for all-cause death were 1.33 (95% CI 0.69–2.56) and 3.38 (95% CI 1.81–6.30); for cardiovascular events, were 1.63 (95% CI 1.00–2.64) and 2.27 (95% CI 1.36–3.78) (Table 2).
Although no significant difference was found between RAR and RDW by comparative analysis of ROC curves for all-cause death (p = 0.053) and cardiovascular events (p = 0.490), both showed similar AUC values (Fig. 3b,c). In addition, consistent trends were observed in all-cause mortality and cardiovascular events in restricted cubic spline analyses (Fig. 4b,c).
Discussion
In this observational study of Japanese patients with CKD, we clarified that higher RAR was associated with an elevated risk for ESKD requiring kidney replacement therapy, all-cause death, and cardiovascular events even after adjustment for confounding variables. Moreover, the ROC curve analyses revealed that RAR had a stronger ability to predict ESKD in patients with CKD than RDW. Our findings suggest that the combination of RDW and serum albumin might be a useful marker for early risk stratification of the risk for ESKD, death, and cardiovascular events among patients with CKD.
Both RDW and serum albumin have been reported to be independent prognostic markers for various populations, including patients with CKD. Several studies reported that, high RDW levels were associated with poor renal outcomes10,11. Whereas the underlying mechanisms of the associations between high RDW and poor renal prognosis have still not been fully elucidated, it seems likely that these results are due to inflammation and oxidative stress. Inflammation impairs bone marrow function and iron metabolism, inhibiting erythrocyte synthesis and leading to increasing RDW levels32. Also, oxidative stress damages erythrocytes directly and shortens erythrocyte survival, resulting in elevated RDW levels33,34. Since the increased levels of inflammatory biomarkers and oxidative stress markers were associated with a faster decline in eGFR and ESKD after adjusting for known risk factors among patients with CKD, a high RDW level was likely to be associated progression of CKD. However, the inflammation and oxidative stress markers were not available in this study. Thus, we could not assess whether these markers affect RAR levels. Serum albumin is not only a nutritional marker as well an inflammation marker. Malnutrition and inflammation both reduce albumin concentration by decreasing its synthesis, and inflammation is associated with a greater fractional catabolic rate and increased transfer of albumin out of the vascular compartment12. Decreased serum albumin has been reported as an independent predictor of the progression of CKD, even though the slight decrease is within the normal range13,35.
In recent years, RAR has been reported to be a novel and useful prognostic indicator among patients with inflammatory diseases due to reflecting the pathological status of hematopoietic dysfunction and hypoalbuminemia at the same time. Furthermore, several studies have shown that RAR had more predictive power than using RDW and albumin separately15,16. In our study, the discriminating ability of RAR for ESKD requiring kidney replacement therapy was significantly superior to RDW. Although there was no superiority in predictive power between RAR and RDW in all-cause death and cardiovascular events, a similar area under the curve values was observed. These findings imply that RAR reflects a higher effect of low albumin on ESKD and a relatively small effect on all-cause mortality and cardiovascular events. Furthermore, the RAR can be easily obtained in laboratory tests with certain advantages of simplicity in clinical practice. Given its availability, cost-effectiveness, and high predictive value, the RAR has significance for clinicians to make quick risk stratification on patients with CKD.
The strength of this study was using a relatively large-scale population database that received specialist care for the prevention of CKD. However, we should acknowledge several limitations. First, owing to the nature of an observational study, the associations described may not reflect a cause-and-effect relationship between RAR and the progression of CKD as well as all-cause mortality and cardiovascular events. Second, RAR was assessed only at a one-time point at baseline, and changes over time were not accounted for in this study. A single measurement of the value of RAR might have led to some misclassification in this population. Third, since our study was limited to Japanese, the results cannot be generalized to all individuals with CKD.
In conclusion, we demonstrated a significant association between higher RAR and an increased risk of CKD progression, all-cause mortality, and cardiovascular events in Japanese patients with CKD. RAR can be measured at no additional cost in daily clinical practice; therefore, this variable might be convenient for identifying patients at a high risk of poor renal outcomes. However, further studies are still warranted to clarify whether aggressive interventions on RAR can improve the renal outcomes of patients with high RAR.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jager, K. J. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 96(5), 1048–1050. https://doi.org/10.1016/j.kint.2019.07.012 (2019).
Kovesdy, C. P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 12(1), 7–11. https://doi.org/10.1016/j.kisu.2021.11.003 (2022).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351(13), 1296–1305. https://doi.org/10.1056/NEJMoa041031 (2004).
Salvagno, G. L., Sanchis-Gomar, F., Picanza, A. & Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 52(2), 86–105. https://doi.org/10.3109/10408363.2014.992064 (2015).
Felker, G. M. et al. Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 50(1), 40–47. https://doi.org/10.1016/j.jacc.2007.02.067 (2007).
Patel, K. V. et al. Red cell distribution width and mortality in older adults: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 65(3), 258–265. https://doi.org/10.1093/gerona/glp163 (2010).
Kim, C. H. et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care. 17(6), R282. https://doi.org/10.1186/cc13145 (2013).
Abrahan, L. L. T., Ramos, J. D. A., Cunanan, E. L., Tiongson, M. D. A. & Punzalan, F. E. R. Red cell distribution width and mortality in patients with acute coronary syndrome: A meta-analysis on prognosis. Cardiol. Res. 9(3), 144–152. https://doi.org/10.14740/cr732w (2018).
Vashistha, T. et al. Red cell distribution width and mortality in hemodialysis patients. Am. J. Kidney Dis. 68(1), 110–121. https://doi.org/10.1053/j.ajkd.2015.11.020 (2016).
Yonemoto, S. et al. Red cell distribution width and renal outcome in patients with non-dialysis-dependent chronic kidney disease. PLoS ONE 13(6), e0198825. https://doi.org/10.1371/journal.pone.0198825 (2018).
Saito, H. et al. Hematological parameters of anemia and prognosis of non-dialysis-dependent chronic kidney disease: The Fukushima CKD cohort study. Clin. Exp. Nephrol. 27(1), 55–65. https://doi.org/10.1007/s10157-022-02282-1 (2023).
Don, B. R. & Kaysen, G. Poor nutritional status and inflammation: Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 17(6), 432–437. https://doi.org/10.1111/j.0894-0959.2004.17603.x (2004).
Amano, H. et al. A slight decrease in the serum albumin level is associated with the rapid progression of kidney dysfunction, even within the normal range. Intern. Med. https://doi.org/10.2169/internalmedicine.4466-20 (2020).
Ni, Q., Wang, X., Wang, J. & Chen, P. The red blood cell distribution width–albumin ratio: A promising predictor of mortality in heart failure patients: A cohort study. Clin. Chim. Acta 527, 38–46. https://doi.org/10.1016/j.cca.2021.12.027 (2022).
Li, D., Ruan, Z. & Wu, B. Association of red blood cell distribution width–albumin ratio for acute myocardial infarction patients with mortality: A retrospective cohort study. Clin. Appl. Thromb. Hemost. 28, 10760296221121286. https://doi.org/10.1177/10760296221121286 (2022).
Weng, Y. et al. The ratio of red blood cell distribution width to albumin is correlated with all-cause mortality of patients after percutaneous coronary intervention: A retrospective cohort study. Front. Cardiovasc. Med. 9, 869816. https://doi.org/10.3389/fcvm.2022.869816 (2022).
Zhao, N. et al. The red blood cell distribution width–albumin ratio: A promising predictor of mortality in stroke patients. Int. J. Gen. Med. 14, 3737–3747. https://doi.org/10.2147/ijgm.S322441 (2021).
Xu, W. et al. Association between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: A retrospective cohort study. Front. Nutr. 9, 1019502. https://doi.org/10.3389/fnut.2022.1019502 (2022).
Hong, J. et al. Impact of red cell distribution width and red cell distribution width/albumin ratio on all-cause mortality in patients with type 2 diabetes and foot ulcers: A retrospective cohort study. Cardiovasc. Diabetol. 21(1), 91. https://doi.org/10.1186/s12933-022-01534-4 (2022).
Kimura, H. et al. Association of polypharmacy with kidney disease progression in adults with CKD. Clin. J. Am. Soc. Nephrol. 16(12), 1797. https://doi.org/10.2215/CJN.03940321 (2021).
Tanaka, K. et al. Status of anemia according to underlying renal disease in chronic kidney disease: The Fukushima CKD cohort. Ann. Clin. Epidemiol. 3(1), 27–35. https://doi.org/10.37737/ace.3.1_27 (2021).
Oda, A. et al. Association between serum inorganic phosphorus levels and adverse outcomes in chronic kidney disease: The Fukushima CKD cohort study. Intern. Med. 61(11), 1653–1662. https://doi.org/10.2169/internalmedicine.7870-21 (2022).
Saito, H. et al. Xanthine oxidase inhibitors are associated with reduced risk of cardiovascular disease. Sci. Rep. 11(1), 1380. https://doi.org/10.1038/s41598-020-80835-8 (2021).
Nakajima, A. et al. Blood pressure control in chronic kidney disease according to underlying renal disease: The Fukushima CKD cohort. Clin. Exp. Nephrol. 24(5), 427–434. https://doi.org/10.1007/s10157-019-01838-y (2020).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53(6), 982–992. https://doi.org/10.1053/j.ajkd.2008.12.034 (2009).
Jonckheere, A. R. A distribution-free k-sample test against ordered alternatives. Biometrika 41(1/2), 133–145. https://doi.org/10.2307/2333011 (1954).
Terpstra, T. J. The asymptotic normality and consistency of kendall’s test against trend, when ties are present in one ranking. Indag. Math. 14(3), 327–333 (1952).
Cochran, W. G. Some methods for strengthening the common χ2 tests. Biometrics 10(4), 417–451. https://doi.org/10.2307/3001616 (1954).
Armitage, P. Tests for linear trends in proportions and frequencies. Biometrics 11(3), 375–386. https://doi.org/10.2307/3001775 (1955).
Durrleman, S. & Simon, R. Flexible regression models with cubic splines. Stat. Med. 8(5), 551–561. https://doi.org/10.1002/sim.4780080504 (1989).
Marrie, R. A., Dawson, N. V. & Garland, A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J. Clin. Epidemiol. 62(5), 511–7.e1. https://doi.org/10.1016/j.jclinepi.2008.05.015 (2009).
Weiss, G. & Goodnough, L. T. Anemia of chronic disease. N. Engl. J. Med. 352(10), 1011–1023. https://doi.org/10.1056/NEJMra041809 (2005).
Tsuboi, S. et al. Impact of red blood cell distribution width on long-term mortality in diabetic patients after percutaneous coronary intervention. Circ. J. 77(2), 456–461. https://doi.org/10.1253/circj.CJ-12-0730 (2013).
Friedman, J. S. et al. SOD2-deficiency anemia: Protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood 104(8), 2565–2573. https://doi.org/10.1182/blood-2003-11-3858 (2004).
Amdur, R. L. et al. Inflammation and progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 11(9), 1546 (2016).
Acknowledgements
The authors would like to thank Ayumi Kanno for her assistance with data collection. We would like to thank Editage (www.editage.com) for the English language editing.
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
Research idea and study design: H.K.; data acquisition: H.K., K.T., H.S., T.I., J.J.K.; data analysis: H.K.; data interpretation: all authors; supervision or mentorship: K.T., J.J.K. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kimura, H., Tanaka, K., Saito, H. et al. Impact of red blood cell distribution width–albumin ratio on prognosis of patients with CKD. Sci Rep 13, 15774 (2023). https://doi.org/10.1038/s41598-023-42986-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42986-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.