Abstract
Chlamydiosis is a significant disease affecting Eastern Australian koala (Phascolarctos cinereus) populations, impacting individual animal welfare and fecundity and therefore influencing population dynamics. The aim of this study was to investigate the effect of a synthetic peptide vaccine based on 4 components of the Chlamydia pecorum major outer membrane protein (MOMP), over an 18-month period in a koala population severely impacted by chlamydiosis. Wild koalas were recruited into a vaccination or a placebo treatment group on a random allocation, then followed through a period of 18 months, with recapture at 6 monthly intervals. Vaccination did not alter clinical disease expression or chlamydial shedding from the ocular or urogenital sites. Vaccination did not stimulate a significant plasma anti-MOMP IgG response, when compared to the placebo group. There was no significant effect of vaccination on IFN-γ and IL-17A mRNA expression of peripheral blood lymphocytes when stimulated with rMOMP. We have demonstrated that a synthetic peptide vaccination against chlamydiosis is not an effective management tool in a koala population with a high prevalence of C. pecorum infection and related disease. The lack of antigenic response found in this study suggests that further research utilising a larger, full-length antigen is an avenue worth investigation if we are to consider vaccination as a part of a management strategy in diseased koala populations.
Similar content being viewed by others
Introduction
Koalas (Phascolarctos cinereus) have been listed as an endangered species in the northern parts of their range in the Australian states of New South Wales, Queensland and the Australian Capital Territory1, as these populations are forecast to decline by approximately 50% over three generations2. One of the major factors contributing to this decline is infection with Chlamydia pecorum3, with some reports of prevalence within free-ranging populations ranging from 58 to 66% within New South Wales4,5. Chlamydiosis, the resultant disease of C. pecorum infection, causes conjunctivitis, cystitis6 and reproductive tract pathology of females and males7,8 which can result in infertility, the main mechanism of population decline9. Infected females often exhibiting paraovarian cysts (up to 63% of cases)10 and males also showing sperm abnormalities11.
Options to address C. pecorum infection in wild koala populations are limited due to the duration and side effects of antibiotic therapy12. Strategic vaccination therefore offers potential as an alternative management tool, to reduce transmission and limit progression of disease. In murine models, vaccination has been shown to induce protection against the development of infertility in female mice infected with Chlamydia muridarum13. Previous studies investigating a C. pecorum vaccine in koalas have shown that vaccination can elicit humoral and cell-mediated immune responses14,15,16,17. Most studies examining vaccine induced immune responses have been in disease free koalas14,15,16,17,18,19,20. Vaccine efficacy has been assessed in wild koalas with low levels of C. pecorum prevelance21,22 and koalas with mild ocular disease23,24,25, however the effect of vaccination in moderate to severe or chronic disease has not been thoroughly investigated. Vaccination may be of limited direct benefit to individuals in chronically diseased populations, as chlamydiosis induces a chronic inflammatory response and structural disease comprising proliferation and fibrosis of the conjunctiva6, fibrosis of the salpingeal ducts, uteri and cervices7, and formation of paraovarian cysts26,27, most of which is expected to be irreversible28. In these populations, however, vaccination may have a role in reducing transmission by reducing chlamydial shedding in infected animals, but its potential benefits need to be further evaluated before widespread implementation.
To determine if vaccination is a valuable tool to address disease in wild koala populations, data on long-term efficacy also needs to be established. The majority of vaccine studies in koalas have monitored responses up to 6 months post vaccination14,18,20,21,23,24,25,29. To be a part of a feasible strategy to address chlamydiosis in wild koala populations, vaccination should provide beneficial effects for greater than 6 months. Specifically, vaccination should aim to reduce chlamydial shedding in infected individuals to reduce transmission events and reduce the development or progression of reproductive pathology. Koala chlamydial vaccine efficacy has been monitored for up to 12 months22 but despite immune responses persisting, beneficial effects including a reduction in chlamydial shedding or prevention of clinical disease progression has not been shown beyond 6 months post vaccination21,22.
Production of the antigen within the existing recombinant protein-based vaccine for widespread use is challenging. An alternative that addresses the difficulties of production is use of synthetic peptide antigens within the vaccine. Peptide based antigens are more cost effective, easier to produce, are stable for longer term storage and have been shown to stimulate immune responses and reduced chlamydial shedding in mice30,31,32. A synthetic peptide vaccination with two epitopes of the major outer membrane protein (MOMP) has been trialled in disease-free koalas. This vaccination showed a synthetic peptide vaccine could stimulated humoral and cell-mediated immune responses18.
To evaluate a synthetic peptide vaccine as a management tool in wild koala populations, this study investigates vaccine efficacy, measured by changes to disease expression, chlamydial shedding and humoral and cell mediated immune responses, over 18-months in a koala population with a high prevalence of chlamydiosis in a blinded randomised placebo-controlled vaccination trial.
Materials and methods
Ethics
All handling and sampling of koalas was performed by experienced veterinarians under the University of Sydney Animal Ethics Approval Number 2019/1547 and NPWS Scientific Licence SL102331. All methods were performed in accordance with the relevant guidelines and regulations.
Cohort recruitment
Koalas recruited to this study were captured within a 15 km radius from − 31.159855, 150.118456, south–west of the town of Gunnedah, New South Wales, using the ‘noose and flag’ technique33. Alfaxalone (Jurox), was used to sedate the koalas at a dose rate of 1.8 mg/kg by intramuscular injection and then oxygen and isoflurane was administered to effect through a fitted mask to anaesthetise the koala. The same veterinarian (SJS) assessed and sampled all koalas. At initial assessment, koalas were classified as either diseased or non-diseased, based on external signs of chlamydiosis, (presence of a “wet bottom” or ocular disease)34. These criteria were chosen as these clinical signs can improve with veterinary treatment35 and infection status of individuals was unknown at initial capture. Following this classification, the koala was randomly assigned to one of two treatment groups, vaccination or placebo. The veterinarian who examined the koalas and administered the treatment was blinded to treatment group allocation. VHF collars (transmitter model M3420, ATS Australia) were fitted to the koalas so they could be radio-tracked and visually monitored from a distance at 2-month intervals throughout the study, and recaptured and clinically evaluated at 6, 12 and 18 months following vaccination.
Koala clinical examination
The following procedures were performed at timepoint 0 (recruitment), 6, 12 and 18 months post vaccination. Digital images were taken of the koala’s identification tag, eyes, teeth (for age estimation)36 and perineal region. Koalas were weighed and a body condition score (BCS) ranging from 1 to 5 was recorded37. Both eyes were assessed for ocular discharge, conjunctival proliferation, and conjunctival chemosis, each criterion with a score between 0 and 3 allocated to each eye34. The perineal region was examined for urine leakage, erythema, or the presence of purulent discharge. A wet bottom score was allocated, based on a modification of the Griffith34 scoring system, whereby only scores 0–6 were used because scores 7–9 required prolonged observation, not amenable to field examinations. Ultrasonography was performed with the koala in dorsal recumbency38,39,40. For ultrasonographic examination of the kidneys, a small patch of fur was clipped on the abdomen ventral to the approximate site of each kidney. Both kidneys were identified and the presence of any structural abnormalities such as dilated renal pelvis, dilated ureters or presence of renal calculi were recorded. For females, the transducer was placed inside the pouch and female reproductive structures visualised by scanning the region cranio-lateral to the bladder. If paraovarian cysts were identified, it was noted if the cyst/s were unilateral, bilateral, multiloculated or singular. A paraovarian cyst score was allocated with 0 = no abnormalities, 1 = unilateral paraovarian cysts identified and 2 = bilateral paraovarian cysts identified. If paraovarian cysts were not detected, a thorough scan of the caudal abdomen was performed to confirm their absence. In males, ultrasound was performed to identify the prostate by directing the transducer caudo-ventral from the bladder. Ultrasound was also performed on both testes.
Swabs for detection of C. pecorum DNA were collected from the conjunctiva bilaterally and the female urogenital sinus or male urethra (Copan, Interpath, 160C). For conjunctival sampling, swabs were placed into the conjunctival fornix and rotated five times. In females, swabs were inserted 2–3 cm into the urogenital sinus and rotated five times. In males, the penis was everted, and the swab placed 2 cm into the urethra and rotated five times. All swabs were stored at − 20 °C until processing.
Vaccination
Four peptides from C. pecorum MOMP, were used as the antigens within the vaccine, P1 H-EGMSGDPCDPCATW-OH, P2 H-INYHEWQVGAALSYRLNMLIP-OH, utilised previously18, P3 H-VLQIVSLQINKLKSRKACG-OH, P4 H-KKLLKSAFLSAAFFAG-OH, representative of the fifth conserved region and the leader sequence of MOMP, respectively. All peptides were synthetised by Mimotopes (Melbourne, Australia), at a purity of > 70% determined by high pressure liquid chromatography. Two of these peptides were chosen as they have been shown to stimulate humoral and cell mediated immune responses in healthy koalas following vaccination18. The additional two peptides were selected as they represent identified B-cell vaccine specific epitopes of conserved regions within C. pecorum MOMP that have been recognised by koalas, following MOMP antigen vaccination25. Kyte-Doolittle plots were constructed to determine hydrophobic and hydrophilic regions of each four peptides. The antigen was combined with a three-component adjuvant, containing Poly I:C (250 μg), Host Defence Peptide-Innate Defence Regulator IDR-1002 (500 μg), and Polyphosphazene EP3 (250 μg) (VIDO-Intervac, University of Saskatchewan, Canada). Vaccine preparation involved mixing the Poly I:C and IDR-1002 and incubating for 15 min at room temperature, with gentle rocking. Next, EP3 and the four peptides were added, with the final mixture incubated for a further 15 min at room temperature, with gentle rocking, then stored at 4 °C until use25. Vaccination and placebo injections (Hartmann’s solution) were administered subcutaneously (0.5 ml) into the interscapular region at first capture and then again at 6 months.
Urogenital and ocular swab DNA extraction and qPCR
DNA was extracted from ocular and urogenital swabs using the MagMAX™ CORE Nucleic Acid Purification Kit #A32702. A sterile swab was included as a control to monitor for contamination during the extraction process. A multiplex qPCR assay was used to quantify the following genes from each extracted swab sample, P. cinereus beta actin (HEX), 23S Chlamydia (ROX) and C. pecorum ompB (FAM); primers adapted from Hulse et al.41. PCR reactions were made to a final volume of 20 µl consisting of 10 µl of SensiFAST™ Probe, 400 nM of each primer, 200 nM of each probe, 4.4 µl of dH2O and 2 µl of DNA. The cycling conditions included initial denaturation for 2 min at 98 °C, followed by 40 cycles of denaturation for 15 s at 98 °C and a combined annealing and extension step for 30 s at 58 °C. Samples were determined to be C. pecorum positive if the CT value of C. pecorum ompB was ≤ 34. Samples were determined to be suspect C. pecorum positive if the CT value of C. pecorum ompB was 34–40 and the 23S Chlamydia was 34–40. qPCR assay was used to quantify P. cinereus beta actin and C. pecorum ompB genes.
Plasma anti-major outer membrane protein (MOMP) IgG ELISA
All concentrations of reagents, blocking and incubation conditions were determined by optimisation experiments using chequerboard titrations. In the optimised assay, 96 well flat bottom plates (Grenier #0030125150) were coated with 2 µg of recombinant MOMP serovar G (produced utilising methods previously described)14 in carbonate-bicarbonate buffer (Sigma-Aldrich #C3041) at 100 µl/well at 4 °C overnight. Wells were emptied and then blocked with 300 µl of 5% skim milk powder in PBS with Tween (0.05%) (PBST) at 37 °C for 1 h. Wells were emptied and koala plasma was applied to the wells at a concentration of 1:400 at 100 µl/well and incubated at 37 °C for 1 h. Wells were then emptied and washed five times with PBST with a microplate washer (Biorad model 1575). An in-house sheep anti-koala-IgG antibody18 100 µl was added to each well at a dilution of 1:8000 and incubated at 37 °C for 1 h. Following incubation, wells were washed as described above. 100 µl of rabbit anti-sheep IgG HRP conjugated (Abcam #ab6747) was added to each well (1:20,000 dilution) and incubated at 37 °C for 1 h. Following incubation, wells were washed as described above, using PBS only. Finally, 100 µl of 3,3′,5,5′Tetramethylbenzidine (Sigma-Aldrich #T5525) was added to each well and allowed to develop at room temperature, in the dark, for 20 min before 100 µl of 1 M H2SO4 was added to each well. Optical density (OD) was read at 450 nm. Each plate contained a blank well (no antigen or plasma), the same negative control from a captive, C. pecorum negative koala and a standard curve based on doubling dilutions of a known strong positive sample, selected during optimisation, from a C. pecorum positive koala. The inter-assay coefficient of variation was < 15% and the intra-assay coefficient of variation was accepted if it was < 10%. Each sample was run in two wells coated with antigen and two without antigen (four wells total). All samples were run in duplicate individual wells, with one well coated with antigen and one without antigen, and the mean was calculated from these replicates. All OD values were blank adjusted; the OD value of the no-antigen well was subtracted from the OD value of the antigen and plasma well for each sample. The highest concentration standard was given a nominal value of 32. The other values were calculated relative to that standard, based on dilution. The OD values of the samples were compared to the standard curve using a 4-parameter logistic curve.
Lymphocyte stimulations
Three millilitres RPMI medium (Sigma-Aldrich #R7388), was incubated for 30 min at 37 °C. The same volume of heparanised blood from each koala was centrifuged at 3000 g for 5 min and the leukocyte fraction (buffy coat) was aspirated and suspended in the pre-incubated media. In duplicate, 5 µg of rMOMP genotype G in 25 µl of foetal calf serum (FCS) or, in the case of negative controls FCS 25 µl, was added to 220 µl of the buffy coat solution. Suspensions were then incubated for 12 h at 37 °C then 750 µl of RNA later (Sigma-Aldrich #R0901) was added to each sample and the sample stored at room temperature for 24 h, then frozen at − 20 °C until processing.
RNA extraction and cDNA synthesis
RNA was extracted from the leukocyte suspensions using the RiboPure—Blood kit (Invitrogen #AM1928) according to the manufacturer’s protocol. Extracts were treated with RNAse-free DNAse, (Thermofisher #EN0521) and cDNA synthesis was then performed using the RevertAid First Strand cDNA Synthesis Kit (K1622) according to the manufacturer’s protocol.
IFNγ and IL-17A expression
A qPCR assay was used to estimate expression of the following genes: GAPDH, IFNγ and IL-17A42,43. Reactions were made to a final volume of 20 µl consisting of 10 µl of SsoAdvanced™ Universal SYBR Green Supermix (BioRad), 0.5 µM of each primer with 6 µl of H2O (GAPDH) and 0.3 µM of primer with 6.8 µl of H2O (IFNγ and IL-17A) and 2 µl of DNA. Cycling conditions for the different genes were applied as previous42,43. All samples were run in duplicate with negative controls at each step. IFNγ and IL-17A expression for each koala for each time point were normalised to GAPDH by the 2−ΔΔCT method, where ΔΔCT = (CT of target − CT of GAPDH) − (CT of target—CT of GAPDH) and presented as a fold change in relation to the unstimulated sample.
Statistical analysis
All data analyses were performed using the R Statistical Environment (Version 3.6.1)44. Data were initially assessed using the ‘ggplot’ and ‘ggdensity’ functions from the ‘ggplot2’45 and ‘ggpubr’46 packages respectively. Shapiro–Wilk tests were used to check for normality. Data that did not conform to normality were log transformed47.
Spearman’s Rank Correlations were used to test for relationships between immunological markers, chlamydial shedding, and disease variables. A variable was removed if a pair of variables had a correlation coefficient > 0.5 (or < –0.5)48. To normalise C. pecorum CT against swab yield (koala beta-actin DNA) from the same swab sample, ΔCT values were generated (ΔCT = CT of C. pecorum CT of beta-actin). Chlamydial shedding is expressed as the inverse of C. pecorum ΔCT values because, as chlamydial shedding decreases, C. pecorum ΔCT values increase.
The effect of treatment (vaccination or placebo), time since vaccination, their interaction and sex, was tested with different linear mixed effects models (LMM), constructed using the ‘lme4’ package49, with individual koala as the random factor. Dependant variables examined included clinical disease variables, specifically ocular disease scores, wet bottom score and paraovarian cyst score (females only), chlamydial shedding (ocular and urogenital ΔCT values), anti-MOMP plasma IgG values (log transformed) and cytokine expression (log transformed fold change).
Results
Cohort recruitment
Fifty-two koalas were recruited into the study (Table 1). Post vaccination data were measured for all koalas at 6 months, 41 koalas at 12 months and 28 koalas at 18 months. The decrease in sample size was due to koalas dying from natural causes. The average tooth wear class at the beginning of the study was pre-molar four being “flat” with molar one having some wear which signifies an estimated average age of 9 years and above36. C. pecorum infection prevalence of the koalas sampled at the beginning of the study was 79% (Table 1).
Ultrasonography
Repeated ultrasonography showed that the presence of paraovarian cysts can change over time. Unilateral or bilateral paraovarian cysts of five female koalas were not detected subsequently. The maximum size of any paraovarian cystic structure that was not identified on subsequent ultrasound was 55 mm in diameter. Four additional female koalas showed fluctuations over 18 months, whereby paraovarian cysts would be detected, not detected, and then detected again, sometimes on opposing sides.
Vaccination response
No correlations were sufficiently strong to warrant exclusion of variables from the model (Supplementary Table S1), in supplementary material.
Clinical disease
There was no significant effect of vaccination treatment (F1,53 = 0.19, P = 0.665), time (F3,113 = 2.14, P = 0.09), sex (F1,73 = 0.39, P = 0.529) or the interaction of time and treatment (F3,113 = 0.42, P = 0.733) on ocular disease scores. There was also no significant effect of vaccination treatment (F1,51 = 0.07, P = 0.783), time (F3,115 = 1.13, P = 0.337) or their interaction (F3,115= 0.99, P = 0.397) on wet bottom score disease scores. Sex had a significant effect on wet bottom score, with females having a higher wet bottom score compared to males (F1,56 = 7.71 P = 0.007). In females, there was no significant effect of vaccination treatment (F1,28 = 0.63, P = 0.432), time (F3,64 = 0.48, P = 0.696) or their interaction (F3,64 = 0.51, P = 0.674) on paraovarian cyst score.
Chlamydial shedding
Ocular site
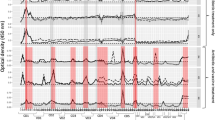
There was no significant effect of vaccination treatment (F1,55 = 1.99, P = 0.163), sex (F1,61 = 0.71, P = 0.401) or the interaction of time and treatment (F3,119 = 0.59, P = 0.620) on C. pecorum ocular ∆CT values (Fig. 1). There was a significant effect of time (F3,118 = 9.49, P = 0.001): both vaccinated and placebo groups had higher ocular ∆CT values (i.e., less chlamydial shedding) at time zero.
C. pecorum ∆CT values at the ocular site of vaccinated and placebo koalas, 0–18 months following vaccination (n = 52 at 0 and 6 months, n = 41 at 12 months and n = 28 at 18 months). Note: ∆CT reflects C. pecorum CT—β-Actin CT (normalising gene) whereby an increase in ∆CT reflects a decrease in chlamydial shedding.
Urogenital site
There was no significant effect of vaccination treatment (F1,55 = 1.58, P = 0.213), time (F3,119 = 0.61, P = 0.603), sex (F1,59 = 1.54, P = 0.218) or the interaction of time and treatment (F3,119 = 0.36, P = 0.776) on C. pecorum urogenital ∆CT values (Fig. 2). Vaccination did not prevent the detection of new C. pecorum shedding at the urogenital site, as three vaccinated koalas that were negative on initial sampling, became positive over the 18 months. Four female koalas ceased shedding, however this seemed unrelated to vaccine status as two were vaccinated and two were not.
C. pecorum ∆CT values at the urogenital site of vaccinated and placebo koalas, 0–18 months following vaccination (n = 52 at 0 and 6 months, n = 41 at 12 months and n = 28 at 18 months). ∆CT reflects C. pecorum CT—β-Actin CT (normalising gene) whereby an increase in ∆CT reflects a decrease in chlamydial shedding.
Plasma anti-major outer membrane protein (MOMP) IgG ELISA
There was no significant effect of vaccination treatment (F1,39 = 0.05, P = 0.820), time (F2,79 = 0.09, P = 0.906), or the interaction of time and treatment (F2,79 = 1.25, P = 0.291) on anti-MOMP IgG levels (Fig. 3). Sex had a significant effect on anti-MOMP IgG values (F1,39 = 3.86, P = 0.050) with females having lower anti-MOMP IgG values compared to males.
IFNγ and IL-17A mrNA expression from rMOMP stimulated peripheral blood lymphocytes
IFNγ
There was no significant effect of vaccination treatment (F1,73 = 2.90, P = 0.09), sex (F1,73 = 2.06, P = 0.155, or the interaction of time and treatment (F1,73 = 1.35, P = 0.247) on IFNγ mRNA expression from peripheral blood lymphocytes (Fig. 4). Time had a significant effect on IFNγ expression (F1,73 = 6,44, P = 0.013) where expression was higher 12 months post vaccination.
IL-17A
There was no significant effect of vaccination treatment (F1,37 = 0.09, P = 0.762), time (F1,38 = 0.4, P = 0.530), sex (F1,35 = 1.91, P = 0.175, or the interaction of time and treatment (F1,38 = 2.09, P = 0.155) on IL-17A mRNA expression from peripheral blood lymphocytes (Fig. 5).
Discussion
Immunological markers, along with the degree of chlamydial shedding and clinical response parameters in this blinded randomised placebo-controlled vaccination trial, indicate that this synthetic peptide vaccination regime is not an effective management tool to manage chlamydiosis within a high disease prevalence koala population. At 6, 12 and 18 months post vaccination, there was no significant difference in chlamydial shedding, disease expression, plasma anti-MOMP IgG levels or antigen stimulated IFNγ or IL-17A expression between vaccinated and placebo koalas. Additional findings include apparent vaccine-independent resolution of paraovarian cysts and the low prevalence of chlamydial shedding among koalas with clinical ocular disease.
This synthetic peptide vaccine provided no identifiable reduction in chlamydial shedding or disease as measured by ocular, urogenital and paraovarian cyst scoring. The inability to demonstrate a reduction in chlamydial shedding or disease is likely a result of no identifiable vaccine-induced cell-mediated or humoral immune stimulation. The lack of response found in this study contrasts studies utilizing full length MOMP as a vaccine antigen in mice50, humans51 and koalas21,22. The antigen used in this study comprised of four synthetic peptides of the MOMP protein that ranged from 16 to 21 amino acids in length. There is evidence that synthetic peptide vaccines utilising MOMP peptide components can stimulate immune responses and reduced chlamydial shedding in mice31,32,52. Our contrasting results may be the result of the size of peptides; the peptides examined in murine studies were larger and thus likely to be a more immunogenic antigen31. An alternate possibility is that the antigens chosen did not represent immunogenic epitopes or that the peptide was not conformationally consistent with that of the full MOMP protein. We hypothesise that either of these two scenarios would result in a lack of immune recognition of the C. pecorum elementary body (EB) when the koala is infected. It is possible that a response occurred to the vaccine peptides but was not detected in our assays as the full length recombinant MOMP was used as an antigen in the ELISA and lymphocyte stimulations as it is this response that we hypothesised is needed for vaccine efficacy. Another alternative is that there was a transient response prior to first sampling at 6 months; previous synthetic peptide vaccine trials examined vaccine response over weeks rather than months31. Tifrea et al.31 detected an elevation in plasma anti-MOMP IgG and an increase in plasma IFN-γ at 4 weeks following vaccination, however, did not measure immune responses beyond this time. Therefore, it remains possible that the synthetic peptide vaccination provided a positive effect over the short term (weeks) but, as our study aimed to evaluate longer-term responses to build evidence on the use of widespread vaccination programs, short term vaccine response was out of scope of this study. While the vaccine protocol within this study included a single booster, it is plausible that the observed lack of response could stem from the need for additional booster vaccines. In studies involving other marsupials, multiple booster vaccinations have shown a tenfold rise in antibody levels53. However, Nyari et al.18 demonstrated that a single dose of a vaccine comprised of two synthetic peptides of the MOMP antigen stimulated a plasma anti-MOMP IgG response in healthy koalas. Therefore, it is likely that the characteristics of our population impacted our ability to demonstrate a vaccine induced immune response.
The demographics of this population may have contributed to the lack of development of vaccine induced immune responses. There is evidence of reduced fecundity within our study population, with only one breeding female observed within this cohort across two breeding seasons. This decline in fertility has coincided with an increase in C. pecorum prevalence within the population5. As a consequence of the reduced fecundity over a period of years, the population is geriatric, with the average estimated age from tooth class wear being 9 years and above36. Immunosenescence is well documented in humans and refers to an age-related dysfunction of immune cells54. One example of immunosenescence is the reduced antibody production and cell mediated responses (IFNγ) in geriatric patients following influenza vaccination54. In koalas, increased age, specifically 9 years and older, has been associated with a decrease in CD4 and CD8β responses55. The lack of immune response development in this study may be in part, therefore, a result of our study population demographics.
Koala populations with a high degree of pathology attributable to chronic inflammation may not be a suitable population to assess vaccine responses and vaccination may not be a suitable tool to address individual animal health effects however may provide a suitable tool to reduce chlamydial shedding and therefore reduce transmission events. Urogenital fibrotic inflammation that results from C. pecorum infection is likely somewhat irreversible7. In humans, severe persistent conjunctival inflammation following C. trachomatis infection, is associated with scarring and fibrosis56 and can persist despite medical intervention57. Histopathological assessment of koalas with severe ocular disease demonstrates conjunctival fibrosis6, which is unlikely to resolve with medical treatment. At the beginning of this study, 5% of the males and females showed evidence of severe conjunctival proliferation and 73% of the females had evidence of genital tract pathology (paraovarian cysts) attributable in some part to chronic inflammatory process such as fibrosis of the salpingeal ducts. This chronic pathology was previously thought to be a permanent structural change and therefore unlikely to be altered by medical treatment alone, including vaccination7. In populations severely impacted by chlamydiosis, vaccination may therefore be ineffective at altering disease outcomes. The results of this study suggest that reducing transmission events by sterilisation or culling of infected individuals may offer better potential as management tools in these severely affected populations58.
The sonographic resolution or change in paraovarian cysts in nine koalas, independent of vaccination, and monitored by a single operator, further supports the notion that, in at least some koalas, cyst formation is less likely to be primarily due to fibrotic occlusions and may have a more dynamic pathogenesis than previously thought27. Historically, due to strong associations between the two, studies investigating the pathogenesis of paraovarian cysts in koalas have hypothesised that paraovarian cysts develop due to fibrosis and occlusion of the salpingeal lumen and were presumed to be irreversible7,59. More recently, Pagliarani et al.27 demonstrated paraovarian cyst development in the absence of fibrotic inflammatory occlusions and hypothesised disruptions in epithelial homeostasis of the ovarian bursa due to C. pecorum infection was the main factor resulting in the accumulation of fluid in cystic ovarian bursae. Our sonographic findings further support the evidence that paraovarian cysts have a dynamic pathogenesis that may not be solely associated with fibrosis of the reproductive tract, and yet were still not influenced by the vaccine treatment in this study.
Within this population there was a disparity between clinical ocular disease and infection status, measured by qPCR. At the commencement of this study 3% of this population tested positive for C. pecorum at the ocular site despite 46% demonstrating clinical ocular disease. Within this population, higher disease scores correlated to severe proliferation of the conjunctiva, which likely represents the chronic stages of inflammation. The low sensitivity is likely a result of the fact that chronic inflammation and clinical signs can persist despite clearance of infection. This finding is consistent with humans with clinical trachomatis60 and the demonstration of persistent clinical signs despite clearance of infection following antibiotic therapy61. Another possibility for this low sensitivity is the incorrect classification of signs such as mild ocular discharge classified to be solely as a result of C. pecorum infection, rather than other pathogenesis.
We have demonstrated that a synthetic peptide vaccination against chlamydiosis is not an effective management tool in an aging koala population with a high prevalence of C. pecorum infection and related disease. Vaccination failed to achieve a reduction in chlamydial shedding and therefore is unlikely to reduce transmission of infection in this setting. The lack of antigenic response found in this study suggests that further research utilising a larger, full-length antigen warrants further investigation if we are to consider vaccination as a part of a management strategy in diseased populations. Although the demographics and chronic pathology exhibited by our population may have impacted the assessment of vaccine efficacy, the scenario is, nonetheless, typical of many koala populations for which vaccination might be considered desirable as a management option. Evaluation of future full length antigen vaccines should include investigation of longer-term efficacy, surpassing 6 months of vaccination, in wild koala populations less severely affected by chlamydiosis. Once a viable vaccine is developed, disease and demographic status of the population should be considered, and context-specific efficacy evaluated, prior to incorporating vaccine into population wide disease management plans.
Data availability
The data that support the findings of this study is available from the corresponding author upon request. There are no restrictions on data availability. This study is reported in accordance with ARRIVE guidelines.
References
Australia, C.o. List of Threatened Species Amendment (Phascolarctos cinereus (combined populations of Queensland, New South Wales and the Australian Capital Territory) (280)) Instrument 2022. (Department of Agriculture, W.a.t.E., editor, 2022).
Conservation advice for the Phascolarctos cinereus (Koala). (Department of Agriculture, W.a.t.E., editor. Canberra; 2022).
7, P.C.N. Koala populations and habitat in New South Wales, 3 edn. )Department of Planning and Environment, New South Wales Government, 2020)
Kollipara, A. et al. Genetic diversity of Chlamydia pecorum strains in wild koala locations across Australia and the implications for a recombinant C. pecorum major outer membrane protein based vaccine. Vet. Microbiol. 167, 513–522 (2013).
Fernandez, C. M. et al. Genetic differences in Chlamydia pecorum between neighbouring sub-populations of koalas (Phascolarctos cinereus). Vet. Microbiol. 231, 264–270 (2019).
Hemsley, S. & Canfield, P. Histopathological and immunohistochemical investigation of naturally occurring chlamydial conjunctivitis and urogenital inflammation in koalas (Phascolarctos cinereus). J. Comp. Pathol. 116, 273–290 (1997).
Higgins, D. P., Hemsley, S. & Canfield, P. J. Immuno-histochemical demonstration of the role of Chlamydiaceae in Renal, uterine and salpingeal disease of the koala, and demonstration of Chlamydiaceae in novel sites. J. Comp. Pathol. 133, 164–174 (2005).
Palmieri, C. et al. Chlamydia pecorum infection in the male reproductive system of koalas (Phascolarctos cinereus). Vet. Pathol. 56, 300–306 (2019).
Fabijan, J. et al. Chlamydia pecorum prevalence in South Australian koala (Phascolarctos cinereus) populations: Identification and modelling of a population free from infection. Sci. Rep. 9, 6261 (2019).
Robbins, A., Hanger, J., Jelocnik, M., Quigley, B. L. & Timms, P. Longitudinal study of wild koalas (Phascolarctos cinereus) reveals chlamydial disease progression in two thirds of infected animals. Sci. Rep. 9, 1–9 (2019).
Hulse, L. et al. The effect of Chlamydia infection on koala (Phascolarctos cinereus) semen quality. Theriogenology 167, 99–110 (2021).
Booth, R. & Nyari, S. Clinical comparison of five anti-chlamydial antibiotics in koalas (Phascolarctos cinereus). PLoS ONE 15, e0236758 (2020).
Pal, S. et al. Vaccination with the recombinant major outer membrane protein elicits long-term protection in mice against vaginal shedding and infertility following a Chlamydia muridarum genital challenge. NPJ Vaccines 5, 90 (2020).
Khan, S. A. et al. Antibody and cytokine responses of koalas (Phascolarctos cinereus) vaccinated with recombinant chlamydial major outer membrane protein (MOMP) with two different adjuvants. PLoS ONE 11, e0156094 (2016).
Waugh, C. A. et al. Comparison of subcutaneous versus intranasal immunization of male koalas (Phascolarctos cinereus) for induction of mucosal and systemic immunity against Chlamydia pecorum. Vaccine 33, 855–860 (2015).
Khan, S. A. et al. Vaccination of koalas (Phascolarctos cinereus) with a recombinant chlamydial major outer membrane protein adjuvanted with poly I: C, a host defense peptide and polyphosphazine, elicits strong and long lasting cellular and humoral immune responses. Vaccine 32, 5781–5786 (2014).
Kollipara, A. et al. Antigenic specificity of a monovalent versus polyvalent MOMP based Chlamydia pecorum vaccine in koalas (Phascolarctos cinereus). Vaccine 31, 1217–1223 (2013).
Nyari, S. et al. Vaccination of koalas (Phascolarctos cinereus) against Chlamydia pecorum using synthetic peptides derived from the major outer membrane protein. PLoS ONE 13, e0200112 (2018).
Carey, A. J. et al. A multi-subunit chlamydial vaccine induces antibody and cell-mediated immunity in immunized koalas (Phascolarctos cinereus): comparison of three different adjuvants. Am. J. Reprod. 63, 161–172 (2010).
Kollipara, A. et al. Vaccination of healthy and diseased koalas (Phascolarctos cinereus) with a Chlamydia pecorum multi-subunit vaccine: evaluation of immunity and pathology. Vaccine 30, 1875–1885 (2012).
Desclozeaux, M. et al. Immunization of a wild koala population with a recombinant Chlamydia pecorum major outer membrane protein (MOMP) or polymorphic membrane protein (PMP) based vaccine: New insights into immune response, protection and clearance. PLoS ONE 12, e0178786 (2017).
Waugh, C. et al. A prototype recombinant-protein based Chlamydia pecorum vaccine results in reduced chlamydial burden and less clinical disease in free-ranging koalas (Phascolarctos cinereus). PLoS ONE 11, e0146934 (2016).
Nyari, S., Booth, R., Quigley, B. L., Waugh, C. A. & Timms, P. Therapeutic effect of a Chlamydia pecorum recombinant major outer membrane protein vaccine on ocular disease in koalas (Phascolarctos cinereus). PLoS ONE 14, e0210245 (2019).
Waugh, C., Austin, R., Polkinghorne, A. & Timms, P. Treatment of Chlamydia-associated ocular disease via a recombinant protein based vaccine in the koala (Phascolarctos cinereus). Biologicals 44, 588–590 (2016).
Phillips, S. et al. Vaccination of koalas during antibiotic treatment for Chlamydia-induced cystitis induces an improved antibody response to Chlamydia pecorum. Sci. Rep. 10, 10152 (2020).
Pagliarani, S. et al. Chlamydia spp. infection and male and female reproductive pathology in koalas Phascolarctos cinereus. J Comp Pathol 166, 104 (2019).
Pagliarani, S., Johnston, S. D., Beagley, K. W., Hulse, L. & Palmieri, C. Chlamydiosis and cystic dilatation of the ovarian bursa in the female koala (Phascolarctos cinereus): Novel insights into the pathogenesis and mechanisms of formation. Theriogenology 189, 280–289 (2022).
Hafner, L. M. Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections. Contraception 92, 108–115 (2015).
Kollipara, A., Polkinghorne, A., Beagley, K. W. & Timms, P. Vaccination of koalas with a recombinant Chlamydia pecorum major outer membrane protein induces antibodies of different specificity compared to those following a natural live infection. PLoS ONE 8, e74808 (2013).
Tu, J. et al. A multi-epitope vaccine based on Chlamydia trachomatis major outer membrane protein induces specific immunity in mice. Acta. Biochim. Biophys. Sin. 46, 401–408 (2014).
Tifrea, D. F., Pal, S., Fairman, J., Massari, P. & De la Maza, L. M. Protection against a chlamydial respiratory challenge by a chimeric vaccine formulated with the Chlamydia muridarum major outer membrane protein variable domains using the Neisseria lactamica porin B as a scaffold. NPJ Vaccines 5, 37 (2020).
Su, H., Parnell, M. & Caldwell, A. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine 13, 1023–1032 (1995).
Madani, G. F., Ashman, K. R., Mella, V. S. A. & Whisson, D. A. A review of the ‘noose and flag’method to capture free-ranging koalas. Aust. Mammal. 42, 341–348 (2020).
Griffith, J.E. Studies into the diagnosis, treatment and management of chlamydiosis in koalas. Ph.D. thesis, (The Unviersity of Sydney Sydney, 2010).
Govendir, M. et al. Plasma concentrations of chloramphenicol after subcutaneous administration to koalas (Phascolarctos cinereus) with chlamydiosis. J. Vet. Pharmacol. Ther. 35, 147–154 (2012).
Gordon, G. Estimation of the age of the Koala, Phascolarctos cinereus (Marsupialia: Phascolarctidae), from tooth wear and growth. Aust. Mammal. 14, 5–12 (1991).
Blanshard, W., Bodley, K., Vogelnest, L. & Woods, R. Medicine of Australian Mammals (CSIRO, 2008).
Larkin, R., Palmieri, C., Oishi, M., Hulse, L. & Johnston, S. D. Ultrasonographic assessment of the male koala (Phascolarctos cinereus) reproductive tract. Res. Vet. Sci. 117, 219–223 (2018).
Stalder, K. et al. Sonographic characterisation of the urogenital tract of the Koala (Phascolarctos cinereus) for standardised investigations of urogenital pathology. CVE Control Ther. Ser. 282, 41–48 (2016).
Marschner, C., Flanagan, C., Higgins, D. P. & Krockenberger, M. B. Validation of ultrasonography in detecting structural disease of the urogenital tract of the koala Phascolarctos cinereus. Aust. Vet. J. 92, 177–178 (2014).
Hulse, L. S. et al. Development and application of two multiplex real-time PCR assays for detection and speciation of bacterial pathogens in the koala. J. Vet. Diagn. 30, 523–529 (2018).
Maher, I. E., Griffith, J. E., Lau, Q., Reeves, T. & Higgins, D. P. Expression profiles of the immune genes CD4, CD8β, IFNγ, IL-4, IL-6 and IL-10 in mitogen-stimulated koala lymphocytes (Phascolarctos cinereus) by qRT-PCR. PeerJ 2, e280 (2014).
Mathew, M., Waugh, C., Beagley, K. W., Timms, P. & Polkinghorne, A. Interleukin 17A is an immune marker for chlamydial disease severity and pathogenesis in the koala (Phascolarctos cinereus). Dev. Comp. Immunol. 46, 423–429 (2014).
Team, R.C. R: a language and environment for statistical computing computer program, version 3.6. 1. R J (2019).
Wickham, H. An introduction to ggplot: An implementation of the grammar of graphics in R (Springer, 2016).
Kassambara, A. & Kassambara, M.A. Package ‘ggpubr’. R package version 0.1 6, 1–52 (2020).
Ives, A. R. For testing the significance of regression coefficients, go ahead and log-transform count data. Methods Ecol. Evol. 6, 828–835 (2015).
Booth, G. D. et al. Identifying proxy sets in multiple linear regression: an aid to better coefficient interpretation Vol. 470–476 (US Department of Agriculture, Forest Service Intermountain Research Station, 1994).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823 (2014).
O’Meara, C. P. et al. Induction of partial immunity in both males and females is sufficient to protect females against sexual transmission of Chlamydia. Mucosal Immunol. 9, 1076–1088 (2016).
Abraham, S. et al. Safety and immunogenicity of the Chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 19, 1091–1100 (2019).
Olsen, A. W., Rosenkrands, I., Holland, M. J., Andersen, P. & Follmann, F. A Chlamydia trachomatis VD1-MOMP vaccine elicits cross-neutralizing and protective antibodies against C/C-related complex serovars. NPJ Vaccines 6, 58 (2021).
Rowlands, D. T. The immune response of adult opossums (Didelphis virginiana) to the bacteriophage f2. Immunology 18, 149 (1970).
Murasko, M. et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp. Gerontol. 37, 427–439 (2002).
Chen, J. et al. Animal age affects the gut microbiota and immune system in captive koalas (Phascolarctos cinereus). Microbiol. Spectr. 11, e04101-04122 (2023).
Guzey, M. et al. A survey of trachoma: the histopathology and the mechanism of progressive cicatrization of eyelid tissues. Ophthalmol. 214, 277–284 (2000).
Burton, M. J. et al. A randomised controlled trial of azithromycin following surgery for trachomatous trichiasis in the Gambia. Br. J. Ophthalmol. 89, 1282–1288 (2005).
Wilson, D. P., Craig, A. P., Hanger, J. & Timms, P. The paradox of euthanizing koalas (Phascolarctos cinereus) to save populations from elimination. J. Wildl. Dis. 51, 833–842 (2015).
Obendorf, D. L. Pathology of the female reproductive tract in the koala, Phascolarctos cinereus (Goldfuss), from Victoria. Aust. J. Wildl. Dis. 17, 587–592 (1981).
Baral, K. et al. Reliability of clinical diagnosis in identifying infectious trachoma in a low-prevalence area of Nepal. Bull. World Health Organ. 77, 461 (1999).
Holm, S. O. et al. Comparison of two azithromycin distribution strategies for controlling trachoma in Nepal. Bull. World Health Organ. 79, 194–200 (2001).
Acknowledgements
We thank all the landowners that have granted permission to catch koalas on their properties, in particular Robert Frend and his family. We thank Alana Kidd, Andrea Casteriano and the staff at the Koala Health Hub, The University of Sydney, for their technical help. We would also like to thank Ben Richardson and George Madani for catching koalas and Candice Skelton for her dedication to radio-tracking. We would like to thank Bonnie Quigley, David Amos and Martine Moran for their support. The Koala Health Hub is supported by a grant from Wildlife Information Rescue and Education Service (WIRES). This work was supported by the NSW Government through the NSW Koala Research Strategy (Project number KR2019_07).
Author information
Authors and Affiliations
Contributions
S.S. veterinary examinations, field work, laboratory work, data analysis and writing. D.H. project design, veterinary examinations, field work, data analysis and review. P.T. vaccine design, project design and review. V.M. project design, field work, data analysis and review. M.C. project design, field work, data analysis and review. C.F. field work, laboratory work and review. C.M. project design, field work, data analysis and review. S.P. vaccine design and review. M.K. project design, veterinary examinations, field work, data analysis and review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simpson, S.J., Higgins, D.P., Timms, P. et al. Efficacy of a synthetic peptide Chlamydia pecorum major outer membrane protein vaccine in a wild koala (Phascolarctos cinereus) population. Sci Rep 13, 15087 (2023). https://doi.org/10.1038/s41598-023-42296-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42296-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.