Abstract
Pressure recovery (PR) is essential part of the post stenotic fluid mechanics and depends on the ratio of EOA/AA, the effective aortic valve orifice area (EOA) and aortic cross-sectional area (AA). In patients with advanced ascending aortic aneurysm and mildly diseased aortic valves, the effect of AA on pressure recovery and corresponding functional aortic valve opening area (ELCO) was evaluated before and after valve-sparing surgery (Dacron graft implantation). 66 Patients with ascending aortic aneurysm (mean aortic diameter 57 +/− 10 mm) and aortic valve-sparing surgery (32 reimplantation technique (David), 34 remodeling technique (Yacoub)) were routinely investigated by Doppler echocardiography. Dacron graft with a diameter between 26 and 34 mm were implanted. EOA was significantly declined after surgery (3.4 +/− 0.8 vs. 2.6 +/− 0.9cm2; p < 0.001). Insertion of Dacron prosthesis resulted in a significant reduction of AA (26.7 +/− 10.2 vs. 6.8 +/− 1.1cm2; p < 0.001) with increased ratio of EOA/AA (0.14 +/− 0.05 vs. 0.40 +/− 0.1; p < 0.001) and pressure recovery index (PRI; 0.24 +/− 0.08 vs. 0.44 +/− 0.06; p < 0.0001). Despite reduction of EOA, ELCO (= EOA corrected for PR) increased from 4.0 +/− 1.1 to 5.0 +/− 3.1cm2 (p < 0.01) with reduction in transvalvular LV stroke work (1005 +/− 814 to 351 +/− 407 mmHg × ml, p < 0.001) after surgery. These effects were significantly better in patients with Yacoub technique than with the David operation. The hemodynamic findings demonstrate a valve-vessel interaction almost entirely caused by a marked reduction in the ascending AA with significant PR gain. The greater hemodynamic benefit of the Yacoub technique due to higher EOA values compared to the David technique was evident and may be of clinical relevance.
Similar content being viewed by others
Introduction
With Doppler ultrasound the maximum pressure drop (4Vmax2) across the aortic valve at the level of the vena contracta can be calculated, neglecting that kinetic energy is lost distally in the aortic root to turbulent flow, but a smaller portion is converted back to potential energy at the sinutubular junction, referred to as pressure recovery (PR)1. However, cardiac catheterization allows assessment of the actual gradient between the left ventricle and the adjacent aorta including pressure recovery based on the pressure readings at both measurement sites1. Both techniques therefore often measure different gradients across the valve and thus demonstrate the metrological differences of pure Doppler measurements. The phenomenon of PR is an integral part of the fluid mechanics across the aortic and pulmonary valve, especially in compromised valve function1,2,3,4,5. To determine the actual pressure gradient across the aortic valve non-invasively, the Doppler Pmax must be corrected with PR to obtain the true gradient as with catheterization. As early as the 1990s, Voelker et al. demonstrated based on fluid-mechanical experiments6,7 that the calculation of PR distal to a stenotic aortic valve depends critically on the ratio of EOA/AA, the effective valve orifice area (EOA) and the downstream aortic cross-section area (AA) adjacent to the sinutubular junction6.
Since the 1990s, aortic root aneurysms have increasingly been treated by valve-sparing procedures with Dacron graft replacement such as root remodeling [Yacoub technique (Y)] or reimplantation of the aortic valve [David technique (D)]8,9. Little attention has been paid to the hemodynamic importance of surgical ascending AA reduction and its specific influence on valve hemodynamics. Therefore, our aim was to use the Dacron graft replacement as a model to capture the vascular component of PR formation, making AA the dominant determinant of the Voelker ratio. Consequently, we first examined the hemodynamics of the entire cohort of 66 patients with advanced root and ascending aortic aneurysm and only mild hemodynamic impairments at the aortic valve before and after surgery without considering the different techniques. In a second approach, the influence of reduced AA on the formation of PR and the functional valve orifice area (ELCO) for the two different valve-sparing techniques (D vs. Y) and the small subset of patients with at most moderate aortic valve stenosis was examined to determine the hemodynamic impact and potential clinical relevance for these subgroups.
Methods
Patients
Sixty-six Patients with aortic root and ascending aortic aneurysm (mean diameter 58 +/− 11 mm) and aortic valve-sparing surgery (32 David technique, 34 Yacoub technique) were routinely investigated by echocardiography between 2014 and 2020 (Table 1) and retrospectively analyzed. Dacron grafts with a diameter between 26 and 34 mm were implanted. The decision on procedure (David vs. Yacoub) was at the surgeon's discretion, with a larger intraoperatively measured annulus diameter (< 28–30 mm, depending on BSA) determined with a Hegar dilator being an indicator of a David operation and vice versa (personal communication, Prof. em. Hans-Hinrich Sievers, Clinic of Cardiac and Thoracic Vascular Surgery, University of Lübeck, Germany, who performed all operations). Most patients showed sinus rhythm (n = 63), and a normal LV ejection fraction (EF 56 + − 6%); 44 patients had mild aortic regurgitation (AR I°) according to ASE guidelines10 and 15 patients mild to moderate aortic stenosis (D = 8; Y = 7), but no other severe valvular heart disease. Both subgroups did not differ in their baseline characteristics. Echocardiography (GE Vivid E9) was performed in these patients immediately before and 4–6 weeks after surgery. Participation was on a voluntary basis and has been performed in accordance with the Declaration of Helsinki. All patients gave their written informed consent for the collection and use of the respective data. The study was approved by the local ethics committee of the University of Lübeck (No. 22-250) and was in accordance with relevant guidelines and regulations of the University of Lübeck.
Theoretical background for pressure recovery and energy loss
According to the basic principles of fluid mechanics, conversion of potential energy into kinetic energy occurs downstream to the obstructed aortic valve2. Distal to the vena contracta in the aortic root, blood flow decreases and turbulence results in energy loss (EL) by converting kinetic energy into heat2. In addition, a smaller part of the kinetic energy is converted back into potential energy, which explains the PR phenomenon adjacent behind the sinutubular junction. The sum of kinetic and potential energy is therefore significantly reduced in the proximal aorta compared to the LVOT and is caused by the energy loss (EL). Cardiac catheterization enables direct measurement of the actual pressure gradient between LVOT and ascending aorta1,3. However, the pure Doppler signal (Pmax) cannot capture the PR distal to the measurement point (vena contracta) in the ascending aorta and therefore causes the Doppler gradient to be overestimated compared to invasive catheter measurement. To overcome these Doppler limitations, equations using Doppler-derived parameters were developed to calculate PR and the corresponding “functional valve orifice area”, referred to as energy loss coefficient (ELCO) and means EOA corrected for PR. The problem was solved by developing the Voelker equation (see below) based on the ratio of EOA/AA, the effective orifice area (EOA) and the cross-sectional area of the ascending aorta (AA)1, forming a parabolic function (Fig. 1A).
Definition of parameters
Pmax could be derived from Doppler measurements, where Vmax is the maximum velocity derived from CW Doppler
where EOA is the effective orifice area of the aortic valve when using continuity equation and AA is the cross-sectional area of aorta.
It is calculated by AA = (D/2)2 × π (D = diameter of proximal aorta)
Accordingly, Fig. 1A graphically describes the parabolic function between EOA/AA and the relative pressure recovery (PRI) in the ascending aorta according to Voelker et al.6 Mathematically the summit of PRI of 50% can be attained by EOA/AA of 0.5. Smaller and higher values of EOA/AA result in lower values of PRI indicated by a parabolic function.
Effective orifice area of aortic valve when using the continuity equation indexed to body surface area (BSA): EOAI.
Energy loss coefficient index (= functional opening area of aortic valve taking PR into account and indexed to BSA):
Valve resistance considering PR:
Transvalvular stroke work:
Transvalvular stroke work considering PR:
Echocardiographic measurements
Transthoracic Doppler echocardiography was carried out with a Vivid 9 system (GE Healthcare, Oslo, Norway). Details of echocardiographic evaluation have been previously described12.
Valvular hemodynamics
Mean and maximal transvalvular pressure gradients (Pmean/Pmax) were assessed by using the CW-Doppler signal across the aorta. Stroke volume was calculated using the diameter of the LVOT as well as pulsed wave Doppler VTI of LVOT. SV = D2/4 × \(\pi\) × VTILVOT where D is the diameter of LVOT. The diameter of the aorta was measured about. 5 mm above the sinutubular junction of the aorta using parasternal long axis view. This localization was recommended by other authors1,13, because at this position the blood flow should be laminar again and thus the pressure recovery should be completed. The ventricular ejection time (t) was measured by assessing the time between the R-wave of ECG and the end of the pulsed-waved Doppler LVOT-VTI signal10. Flow was calculated by Q = SV/t.
Statistics
Continuous variables are presented as mean ± standard deviation. Before starting the statistical analysis, a Kolmogorov–Smirnov test for checking normal distribution of the samples was performed. Changes in hemodynamic parameters were analyzed and compared using paired Student’s t-test, if the data were normally distributed. Otherwise, a Wilcoxon matched-pairs signed rank test was used. Changes in hemodynamic parameters between groups were analyzed and compared using unpaired Student’s t-test, if the data were normally distributed. Pearson correlation was calculated in normally distributed parameters. Binary distributed variables of baseline characteristics of different groups were compared using Fisher’s exact test. p-values < 0.05 were considered to reflect statistically significant differences. Statistical analyses were performed with GraphPad Prism 8.1.2.
Results
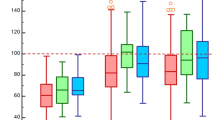
Table 2 displays the Doppler sonographic and calculated hemodynamic values before and after Dacron graft implantation for the entire cohort. Figure 1B–F summarizes the pure Doppler-derived parameters without considering PR. The maximum pressure Pmax is slightly but not relevantly reduced after the operation (Fig. 1B). SV and flow were significantly diminished after valve-sparing procedures (Fig. 1 C, D). Stroke work (SW) across the valve was significantly lower after surgery (Fig. 1E), while valvular resistance (Vr) remained unchanged (Fig. 1F).
In accordance with these results effective orifice area (EOA) was significantly reduced after surgery (Fig. 2A). Insertion of Dacron graft resulted in a significant reduction of the aortic diameter and aortic cross-sectional area (AA) (Fig. 2B,C), so that the ratio EOA/AA increased clearly after surgery (Fig. 2D). This increase is also graphically illustrated in Fig. 3A and B demonstrating the “Voelker parable”—the relationship between PRI and EOA/AA. Preoperatively, most patients are in the ascending limb of the parabola up to a maximum PRI of 0.40, while postoperatively the values are shifted to the summit of the curve and beyond, i. e. values between 0.4 and 0.5. This means that reducing (AA) resulted in an improved EOA/AA ratio at which optimal pressure recovery can be achieved, as shown in Fig. 3C and D. A good linear correlation could only be demonstrated between the aortic diameter and PRI for all pre and post-surgery points, while there was a weak correlation between the PRI and EOA.
Distribution of the PRI points of the investigated patients on the Voelker parabola before (A) and after surgery (B). Correlation of PRI and D-Aorta (C) as well as PRI and EOA (D). Comparison of PR (E) and PRI (F) before and after surgery (n = 66), *p < 0.00001 versus pre surgery, +p < 0.0001 versus pre surgery.
Figure 3E and F demonstrate the significant increase of absolute and relative pressure recovery (PRI) caused by the improved EOA/AA ratio. Therefore, ELCO is significantly increased after surgery (Fig. 4A) although EOA was reduced post-operatively (Fig. 2A). Figure 4C andD compare EOA and ELCO before and after surgery. The mean difference between ELCO and EOA increased significantly after surgery (p < 0.001). Accordingly, the energy loss across aortic valve was significantly reduced after surgery (Fig. 4B), resulting in significantly reduced stroke work (Fig. 4E) and valvular resistance (Fig. 4F) after surgery.
Comparison of ELCO (A) and EL (B) before and after surgery. Comparison of EOA and ELCO before surgery (C) and after surgery (D). Comparison of SWPR (E) and VrPR (F) before and after surgery (n = 66), *p < 0.00001 versus pre surgery, +p < 0.0001 versus pre surgery. SWEL/VrEL = stroke work/valve resistance corrected for energy loss (EL).
Fifteen of the investigated patients showed mild to moderate aortic stenosis based on EOA (mean: 1.6 +/− 0.3 cm2). If the pressure recovery post-surgery was considered in those patients, the ELCO depicted a significant increase in the valve opening area (mean: 2.2 + − 0.4 cm2). The individual changes are shown in Fig. 5A. It is remarkable that patients with initially moderate stenosis only had minor stenoses and patients with initially minor stenosis consistently had no stenoses when pressure recovery was calculated postoperatively. The corresponding increase of PRI in these patients after surgery is demonstrated in Fig. 5A (right side).
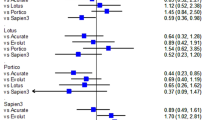
Comparison of EOA and ELCO after surgery (A, left side) and comparison of PRI before and after surgery (A, right side) in patients with mild to moderate aortic valve stenosis. (n = 15). *p < 0.00001 versus pre surgery, +p < 0.0001 versus pre surgery. Comparison of EOA (B), D-Aorta (C), EOA/AA (D), PRI (E) and ELCO (F) in patients treated with David (D) or Yacoub (Y) technique before and after surgery. *p < 0.05 versus pre surgery, #p < 0.05 post surgery D versus Y.
When comparing the remodeling technique (Yacoub) versus the reimplantation technique (David) in our patients (Fig. 5B–D, Table 3), it was striking that the David technique, despite identical preoperative EOA values, resulted in significantly greater gathering of the aortic valve postoperatively with significantly lower EOA in comparison to the Yacoub technique (Fig. 5B). Since both techniques reduced the size of the ascending aorta to the same extent (Fig. 5C), the EOA/AA ratio, however, increased significantly for the Yacoub technique after surgery (Fig. 5D), so that the relative pressure recovery and the functional valve opening area (ELCO) (Fig. 5E, F) were also significantly greater than when using the David technique.
Discussion
The aortic cross-sectional area (AA) as the vascular trigger of PR formation
The PR phenomenon has been demonstrated experimentally14 and clinically using invasive pressure measurements4,15, and can also be precisely calculated by Doppler ultrasound based on Voelker's equation6. The area of the proximal ascending aorta just behind of the sinutubular junction is critical for pressure recovery measurements1,13. This equation allows EOA and AA to be considered separately as the valvular and vascular components of PR formation, depending on the pathological defect, both determinants have different weights for the calculation of PR. So far, the literature has dealt predominantly with studies on EOA in aortic stenosis as the dominant valvular component of PR formation1,2,3. Aortic aneurysm surgery with Dacron graft replacement as a model made it possible to transfer this weight almost entirely to the vessel side. Our aim was therefore, to emphasize the importance of the vascular side (AA) with its special influence on the aortic valve hemodynamics. The hemodynamic findings showed a vessel-valve interaction that leads to a significant PR gain with improved aortic valve functional orifice area (ELCO). The clinical implications of this effect are most evident in the subset of patients with mild to moderate aortic stenosis, while in the subset of patients with different valve-sparing techniques, aortic hemodynamics were slightly better for the Yacoub technique.
Characteristics of aortic valve-vessel interaction when dominated by AA reduction
The entire cohort of patients after valve-sparing surgery showed a slight reduction in stroke volume, flow and EOA with unchanged Pmax and Vr, which can be explained by the replacement of the aneurysm with the Dacron graft and simultaneous correction of functional aortic regurgitation by gathering of the aortic valve9. Flow dependent reduction of EOA may also aggravate this effect16. When PR is considered, which is flow independent6, two interesting aspects stand out in the data presented.
First, despite the advanced aortic aneurysm, the vessel already showed an unexpected preoperative PR with a significant increase in ELCO and a decrease in EL compared to the pure Doppler-derived measurements of EOA and Pmax. These results demonstrate that PR is even present in the ascending aortic aneurysm with a mean diameter of 5.7 cm (mean AA of 26,7cm2) and an EOA of 3.4 cm2 resulting in a preoperative ratio of EOA/AA of 0.14, which increased to 0.40 postoperatively. Therefore, our study extends the energy loss principle for AA in a wider range of applicability. So far it has been postulated that no relevant pressure recovery can be achieved with an ascending aortic diameter of more than 3 cm (AA > 7cm2) in the case of high-grade aortic valve stenosis1. The extension of aortic diameter for PR formation is made possible by a larger EOA (e. g. normal aortic valve), because it shifts the EOA/AA ratio to the right on the “Voelker parabola” towards higher PRI values (Fig. 3A, B). In the past, studies in literature have only focused on the aortic valve opening area as the primary determinant of EOA/AA ratio3,7,17. Accordingly, Voelker et al. found no dependence of PR on the valve shape by varying the opening area of the aortic valve, but they did not vary the cross-sectional area of the aorta in their used pulsatile flow model6.
Second, our postoperative results clearly demonstrated the importance of reducing the cross-sectional area of the ascending aorta not only to prevent vascular rupture but also to improve aortic valve hemodynamics. These results underscore the functional interaction of the aortic valve and root, initiating valve-vessel “crosstalk”18. Even though the aortic valves showed reduced EOA after surgery, ELCO increased significantly while EL, VrPR and SWPR decreased as expected. This effect was caused by the significantly increased absolute and relative PR, the latter effected by a rightward shift towards the summit of the Völker parabola with a mean PRI value of 0.44 close to the optimum. This shift is mainly triggered by the reduction of aortic cross-sectional area, which is also confirmed by the good correlation between PRI and AA, but not for EOA. Accordingly, clinically relevant PR is more common in pediatric patients and young adults, with downstream vessel diameters typically < 3 cm compared to the larger downstream vessels in adults1,17,19. Similarly, improved pressure recovery could be shown for the smaller pulmonary artery compared to the aorta4.
The reduction of aortic cross-sectional area and its clinical perspective
The hemodynamic benefit of normal or smaller diameters of the ascending aorta and pulmonary artery increases with the degree of upstream valve obstruction4,17. This effect was clearly demonstrated in a subgroup of patients with mild to moderate aortic stenosis after Dacron graft implantation. Due to the smaller Dacron graft diameter, the increased pressure recovery led to a reclassification of the postoperative aortic stenosis from moderate to mild and from mild to no stenosis. In addition to treating the risk of vessel rupture, the improved PR in these patients could be clinically relevant to increase the free interval for possible future valve surgery.
Further considerations appear to be important as to whether a particular surgical technique should be given preference in aneurysm surgery. If one compares the valve- sparing techniques of David and Yacoub, it is striking that in the studied cohort the Yacoub procedure achieves a larger anatomical aortic orifice area, which is reflected in an enlarged Doppler EOA postoperatively compared to the David technique with the same reduction in AA. Therefore, Yacoub surgery leads to a better EOA/AA ratio post-operatively probably due to less valve tightening, which results in a better PRI, a higher functional valve orifice area (ELCO) and more unloading of the LV compared to the David technique. The anatomical background of these findings can be explained by clinical and in vitro echocardiographic studies showing better root compliance and almost physiological cusp movement when the Yacoub technique was used20,21. From the early postoperative results it could be concluded that patients treated with the Yacoub technique may have improved hemodynamic and possibly clinical advantages over those treated with the David technique, but this needs to be confirmed by long-term hemodynamic and clinical studies.
Limitations
The present study was retrospective, observational and unicentric and focused only on the hemodynamics of the patient valves examined and therefore could not make any significant statement about the clinical relevance of the various groups. Our main goal was to explain the principle of functional valve-vessel interaction emphasizing the importance of the vascular side through the reduced AA. The presented study lacks any invasive data, all parameters were calculated by Doppler echocardiographically-derived measurements. Nevertheless these parameters parameters were invasively validated and clinically checked in literature1,2,6,17,22.
Conclusion
In patients with advanced root and adjacent ascending aortic aneurysm the influence of reduced aortic cross-sectional area on improved pressure recovery, larger corresponding functional aortic valve orifice area (ELCO), and more LV unloading was demonstrated after implantation of the Dacron graft. These results demonstrate a functional interaction of the aortic valve and ascending aorta that initiates valve-vascular “crosstalk” that is primarily determined from the vascular side. Postoperatively, the Yacoub technique showed improved valve hemodynamics compared to the David technique and patients with aortic stenosis were reclassified, which may be of clinical relevance.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AA :
-
Cross-sectional area of aorta ascendens adjacent to the sinotubular junction
- BSA:
-
Body surface area
- D/Y:
-
David/Yacoub operation
- Ea :
-
Effective arterial elastance
- EOA:
-
Effective valve orifice area (continuity equation)
- EL:
-
Energy loss
- ELCO:
-
Energy loss coefficient (= EOA corrected for PR and is also referred to as “functional valve orifice area”)
- EF:
-
Ejection fraction
- LV:
-
Left ventricle
- LVOT:
-
Left ventricular outflow tract
- LVSP:
-
Left ventricular systolic peak pressure
- PR:
-
Pressure recovery
- PRI:
-
Pressure recovery index (relative PR)
- SV:
-
Stroke volume
- SW:
-
Stroke work
- TAPSE:
-
Tricuspid annular plane systolic excursion
- Vr:
-
Valve resistance
- VTI:
-
Velocity time integral
References
Baumgartner, H., Stefenelli, T., Niederberger, J., Schima, H. & Maurer, G. “Overestimation” of catheter gradients by Doppler ultrasound in patients with aortic stenosis: A predictable manifestation of pressure recovery. J. Am. Coll. Cardiol. 33(6), 1655–1661 (1999).
Akins, C. W., Travis, B. & Yoganathan, A. P. Energy loss for evaluating heart valve performance. J. Thorac. Cardiovasc. Surg. 136(4), 820–833 (2008).
Pibarot, P., Garcia, D. & Dumesnil, J. G. Energy loss index in aortic stenosis: From fluid mechanics concept to clinical application. Circulation 127(10), 1101–1104 (2013).
Singh, G. K., Mowers, K. L., Marino, C., Balzer, D. & Rao, P. S. Effect of pressure recovery on pressure gradients in congenital stenotic outflow lesions in pediatric patients-clinical implications of lesion severity and geometry: A simultaneous doppler echocardiography and cardiac catheter correlative study. J. Am. Soc. Echocardiogr.Off. Publ. Am. Soc. Echocardiogr.. 33(2), 207–217 (2020).
Reil, J. C. et al. Impact of pressure recovery on the assessment of pulmonary homograft function using Doppler ultrasound. Physiol. Rep. 10(23), e15432 (2022).
Voelker, W., Reul, H., Stelzer, T., Schmidt, A. & Karsch, K. R. Pressure recovery in aortic stenosis: an in vitro study in a pulsatile flow model. J. Am. Coll. Cardiol. 20(7), 1585–1593 (1992).
Schöbel, W. A., Voelker, W., Haase, K. K. & Karsch, K. R. Extent, determinants and clinical importance of pressure recovery in patients with aortic valve stenosis. Eur. Heart J. 20(18), 1355–1363 (1999).
Tian, D., Rahnavardi, M. & Yan, T. D. Aortic valve sparing operations in aortic root aneurysms: Remodeling or reimplantation?. Ann. Cardiothorac. Surg. 2(1), 44–52 (2013).
David, T. E., David, C. M., Feindel, C. M. & Manlhiot, C. Reimplantation of the aortic valve at 20 years. J. Thorac. Cardiovasc. Surg. 153(2), 232–238 (2017).
Zoghbi, W. A. et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J. Am. Soc. Echocardiogr. Off. Publi. Am. Soc. Echocardiogr. 30(4), 303–371 (2017).
Garcia, D., Pibarot, P., Dumesnil, J. G., Sakr, F. & Durand, L. G. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation 101(7), 765–771 (2000).
Reil, J. C. et al. CaMKII activity contributes to homeometric autoregulation of the heart: A novel mechanism for the Anrep effect. J. Physiol. 598(15), 3129–3153 (2020).
Garcia, D., Pibarot, P. & Durand, L. G. Analytical modeling of the instantaneous pressure gradient across the aortic valve. J. Biomech. 38(6), 1303–1311 (2005).
Heinrich, R. S. et al. Experimental analysis of fluid mechanical energy losses in aortic valve stenosis: Importance of pressure recovery. Ann. Biomed. Eng. 24(6), 685–694 (1996).
Laskey, W. K. & Kussmaul, W. G. Pressure recovery in aortic valve stenosis. Circulation 89(1), 116–121 (1994).
Kadem, L., Rieu, R., Dumesnil, J. G., Durand, L. G. & Pibarot, P. Flow-dependent changes in Doppler-derived aortic valve effective orifice area are real and not due to artifact. J. Am. Coll. Cardiol. 47(1), 131–137 (2006).
Niederberger, J., Schima, H., Maurer, G. & Baumgartner, H. Importance of pressure recovery for the assessment of aortic stenosis by Doppler ultrasound. Role of aortic size, aortic valve area, and direction of the stenotic jet in vitro. Circulation 94(8), 1934–1940 (1996).
El-Hamamsy, I., Warnes, C. A. & Nishimura, R. A. The ross procedure in adults: The ideal aortic valve substitute?. J. Am. Coll. Cardiol. 77(11), 1423–1425 (2021).
Kobayashi, D. & Humes, R. A. Pressure recovery in congenital aortic stenosis. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 28(12), 1418–1419 (2015).
Fries, R. et al. In vitro comparison of aortic valve movement after valve-preserving aortic replacement. J. Thorac. Cardiovasc. Surg. 132(1), 32–37 (2006).
Leyh, R. G., Schmidtke, C., Sievers, H. H. & Yacoub, M. H. Opening and closing characteristics of the aortic valve after different types of valve-preserving surgery. Circulation 100(21), 2153–2160 (1999).
Bahlmann, E. et al. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 127(10), 1149–1156 (2013).
Acknowledgements
We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.C.R. conception of the study, performing echoes, interpreting data, drafting the manuscript. C.M. Statistical analysis, drafting the figures, collection of data, conception of the study critically reviewing the final manuscript. C.B. critically reviewing the final manuscript. M.I. critically reviewing the final manuscript, V.R. critically reviewing the final manuscript. A.A. Interpreting data, critically reviewing the final manuscript. S.E. critically reviewing the final manuscript. H.J.S. critically reviewing the final manuscript. U.S. conception of the study, performing echoes, interpreting data, critically reviewing the final manuscript. G.H.R.conception of the study, interpreting data, drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reil, JC., Marquetand, C., Busch-Tilge, C. et al. Functional interaction of aortic valve and ascending aorta in patients after valve-sparing procedures. Sci Rep 13, 15340 (2023). https://doi.org/10.1038/s41598-023-42068-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42068-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.