Abstract
The Heuchera genus, a member of the Saxifragaceae family, encompasses a wide array of varieties and hybrids, serving both traditional medicinal and ornamental purposes. However, a significant knowledge gap persists in achieving efficient mass propagation of diverse Heuchera cultivars creating a substantial market void. To address this, our study focuses on expedited seedling regeneration by investigating leaf cutting and tissue culture techniques to offer novel insights to cultivators. Herein, we successfully rooted thirteen distinct cultivars from the Heuchera and Heucherella (Heuchera × Tiarella) genera through cutting. Moreover, in vitro culture experiments led to the successful induction of calli and shoots from petiole samples. Notably, variations in measured parameters were observed across cultivars in both cutting and tissue culture methodologies. When petiole explants were exposed to cytokinin 6-benzylaminopurine (BA) at concentrations of 0.5, 1.0, and 2.0 mg/L along with auxin α-naphthaleneacetic acid (NAA) at 0.5 mg/L, shoots were produced either directly or indirectly during the primary culture. Exposure to darkness and the application of 2,4-dichlorophenoxyacetic acid (2,4-D) did not promote shoot formation but were beneficial for callus stimulation. Interestingly, a negative correlation was observed between the ease of initiating cutting recovery and inducting tissue culture regeneration, suggesting that cultivars that easily regenerate through cutting might encounter difficulties during induction by tissue culture. In light of these findings, we devised a streamlined and effective protocol for rapid Heuchera propagation. This protocol involves micropropagation, directly acquiring adventitious shoots from primary cultures supplemented by cutting-based propagation methods.
Similar content being viewed by others
Introduction
The genus Heuchera, a member of the Saxifragaceae family encompasses a wide range of varieties and hybrids, with new additions every year. Heuchera, also known as Alum root or Coral bells, is a perennial herbaceous plant native to North America, capable of enduring temperatures as low as – 30 °C. While the Heuchera has historically been employed medicinally for various applications such as reducing tissues in nosebleeds, sore throats, ulcers, diabetes, and hemorrhoids1, its numerous hybrid cultivars are now extensively utilized across North America, Europe, Africa, and Asia for their ornamental properties2. Heuchera displays ornamental value with its incredible variety of foliage colors and shapes. The foliage and venation come in colors like purple, rose, lime green, and gold, and the foliage shapes can be rounded, lobed, and hairy. These plants are grown in woodlands, rock gardens, containers, borders, and ground covers. Heuchera cultivars are in high demand worldwide as ornamental foliage plants, but they are short-lived perennials that may die out in just a few years if not divided regularly. Therefore, ensuring the preservation of Heuchera genetic resources and market supply is a major challenge for breeders and plant geneticists.
While conventional methods of propagation involve splitting and sowing, Heuchera can theoretically be propagated through sowing, cutting, dividing, and tissue culture. Nevertheless, certain propagation techniques come with inherent limitations. Seed propagation, for instance, is a cost-effective approach but is hindered by low seed production and substantial genetic variation post-sowing. Similarly, division propagation’s pace is insufficient for large-scale reproduction, yielding 10–15 plantlets annually from a mother plant3. Furthermore, numerous varieties exhibit limited ramet production potential, and division can detrimentally impact root systems, diminishing survival rates. Cutting and tissue culture provide viable alternatives to retain distinctive phenotypic attributes and generate numerous offspring. Among them, tissue culture culture provides a basic tool for many applied technologies. However, only handful of Heuchera varieties have been previously studied in vitro, such as Red Spangles3, Caramel4, Silver Scrolls5, etc.
Despite the abundance of Heuchera cultivars and their widespread popularity, information on effective propagation methods remains limited. The present study sought to help producers obtain seedlings in the shortest regeneration period, thereby minimizing production costs. We examined clonal propagation methods like cutting and in vitro culture, which depend on cell totipotency. Given this shared dependency, we questioned whether cultivars that easily initiate cutting propagation also readily initiate in vitro culture. However, the correlation between these methods has not been empirically studied before. Thus, we investigated cutting propagation and histological differentiation on 13 diverse Heuchera and Heucherella (Heuchera × Tiarella) cultivars with varying foliage colors and venation patterns.
Materials and methods
The study complied with local and national regulations.
Cultivars and cutting propagating by leaf cuttings
This research was conducted at the Plant Tissue Culture Factory of the Agricultural Science and Technology Innovation Center (Latitude: 29.8045° N, Longitude: 106.2932° E), situated in Bishan National Agricultural Science Park, Chongqing, China. The mother plants of Heuchera and Heucherella under investigation were sourced from TianMu Agriculture Co., Ltd. (Chongqing, China) and were bred by Terra Nova Nurseries, INC. (Oregon, USA); their traits are detailed in Table 1. Leaf cuttings, each containing intact petioles, were obtained from one-year-old mother plants cultivated in a greenhouse adjacent to the tissue culture laboratory. All leaf cuttings were placed in 50-cell plug trays (measuring 4.8 cm in top diameter, 1.8 cm in bottom diameter, and 8.5 cm in height), filled with a mixture of peat soil and perlite (3:1), and were grown in the greenhouse. During the study period, a fertilizer (Stanley Fertilizer Co., LTD, Shandong, China) was sprayed onto the leaves twice weekly.
Explant materials and sterilization
Petiols explants were taken from healthy mother plants of the cultivars listed in Table 1. The selected petiole sections were thoroughly washed under running tap water to remove dirt and dust; then surface moisture was removed using filter paper. The cleaned petioles were surface-sterilized by soaking in 75% ethanol for 30–60 s, followed by 2% sodium hypochlorite for 10–20 min, and finally rinsed three times with sterile water. Both ends of the sterilized petioles were then removed and discarded. The remaining petiole sections were cut into small segments (1–2 cm) for explant inoculation. Three to five of these segments were inoculated into each culture bottle. The experiment was repeated three times, with over 80 explants of each variety inoculated in each repeat.
Primary culture medium and conditions
The culture media used were based on MS (Murashige and Skoog, 1962)6, supplemented with 3% sucrose and 6.5% agar, and adjusted to a pH of 6.0 ± 0.2. Various combinations of plant growth regulators (PGRs) were incorporated, including 6-benzylaminopurine (BA) as a cytokinin and α-naphthaleneacetic acid (NAA) as an auxin. The specific PGR combinations included concentrations of BA (0.5, 1.0, 2.0, and 4.0 mg/L) along with 0.5 mg/L NAA. The prepared media were autoclaved at 121 °C for 20 min. Cultures were maintained in a culture room with conditions set at 25 ± 1 °C and a 16 h light/8 h dark photoperiod. A dark pre-culturing step was introduced for cultivars exhibiting callus induction rates below 80% with the aforementioned PGR combinations after 30 days of primary culture. Specifically, Blonde, Electra, Electronic Lime, Forever Red, Lime Ricky, Sweet Tart, and Sweet Tea cultivars underwent dark culture. Dark culture was implemented for 0, 5, 10, and 15 days to identify the optimal period for improving callus induction rates. The cultivars were transferred back to the previously mentioned culture conditions after dark treatment. PGRs at 0.5 mg/L BA plus 0.5 mg/L NAA were utilized for dark culture.
Furthermore, the aforementioned screened cultivars were subjected to in another primary culture experiment. Based on the outcomes of the prior primary culture, attempts were made to enhance induction rates by adjusting the ratio of NAA or by substituting NAA with 2,4-D (2,4-dichlorophenoxyacetic acid). The PGR combinations involved 0.5 mg/L BA + 0.5 mg/L NAA, 0.5 mg/L BA + 1.0 mg/L NAA, 0.5 mg/L BA + 0.5 mg/L 2,4-D, and 0.5 mg/L BA + 1.0 mg/L 2,4-D.
Statistical analysis
For the leaf cutting experiments, we recorded the rooting rate, root length, and number of roots after 50 days of growth. For the tissue culture experiments, we first observed the time of callus initiation, then recorded the callus induction rate on day 30 and shoot regeneration frequency on day 60 after inoculation. All data were analyzed and graphed using R software (version 4.3.1). ANOVA was performed using the ‘stats’ package with a fitted ANOVA model. Mean comparisons were conducted using Duncan's new multivariate range test at a 5% significance level via the ‘agricolae’ package. Correlation analysis between individual cutting and tissue culture parameters was performed using Pearson’s correlation in the ‘PerformanceAnalytics’ package.
Results
Initiation of cutting and tissue culture
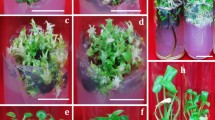
Numerous cultivars from the Heuchera genus including Blonde, Electra, Electric Lime, Forever Red, Lime Marmalade, Lime Ricky, Paris, Plum Pudding, Rio, Shanghai, Sweet Tart, along with cultivars form the Heucherella genus including Sweet Tea and Tapestry, were effectively initiated both through cutting propagation (Fig. 1) and in vitro culture (Fig. 2).
Rooting responses of different cultivars to leaf cutting after 50 days of growth. (A) Rooting status of each cultivar after cutting. (B) Dot plot illustrating the index for each cultivar and its leaf cutting regeneration potential. The distribution of points from the bottom left to the top right indicates increasing regeneration potential. The dot color represents the quantile calculated based on root number, length, and rooting rate.
Rooting of leaf cuttings
Although all 13 cultivars successfully initiated rooting through cutting (Fig. 1), notable variations in rooting responses were observed among the cultivars. Following the cutting procedure, Lime Ricky, Blonde, Sweet Tart, Electra, and Sweet Tea exhibited rooting rates of 96%, 88%, 80%, 78%, and 64%, respectively, while the remaining cultivars exhibited rates below 60% (Fig. 1B). Lime Ricky, Blonde, and Sweet Tart displayed more than ten roots, while the remaining cultivars had fewer roots (Fig. 1B). Among the cultivars, five displayed roots longer than 1.0 cm, while the rest exhibited shorter roots (Fig. 1B). To assess relative regeneration potential, the three indices were normalized and averaged to obtain scores for each cultivar. Based on the quantile of these scores, the sequence of cutting regeneration potential was: Lime Ricky, Blonde, Sweet Tart > Electra, Sweet Tea > Electric Lime, Lime Marmalade, Paris > Rio, Tapestry > Shanghai, Forever Red, Plum Pudding (Fig. 1B).
Responses of cultivars during in vitro culture
During primary in vitro culture, both calli and shoots were observed in each cultivar, with shoots emerging from the incised callus (Fig. 2). Diverse cultivars displayed variable callus induction and shoot formation when exposed to an MS medium containing BA and NAA. Callus initiation spans ranged from 15 to 23 days for the different cultivars, with three cultivars showcasing the swiftest callus initiation at 15 days (Fig. 3A). Notably, Tables 2 and 3 indicate significant variations in callus induction rates among six cultivars and shoot formation across three cultivars, based on differing BA concentrations. After 30 days of incubation, six cultivars achieved callus induction rates exceeding 80%, amounting to an efficiency of 46% (Table 2 and Fig. 3B). By the 60-day mark, Rio and Blonde cultivars exhibited significant shoot formation, reaching 83% (Table 3 and Fig. 3B).
Seven cultivars with callus induction rates below 80% underwent dark treatment before a subsequent incubation attempt, including Blonde, Electra, Electronic Lime, Forever Red, Lime Ricky, Sweet Tart, and Sweet Tea. Callus induction rates for Blonde and Sweet Tea significantly increased after 5, 10, or 15 days of dark treatment (Fig. 4A). Electra and Sweet Tart displayed noteworthy improvements after 10 and 15 days of dark treatment, while Electronic Lime and Lime Ricky exhibited no significant changes (Fig. 4A). Notably, Forever Red experienced a decrease in callus induction rate (Fig. 4A). Moreover, these seven cultivars were subjected to a revised experiment involving diverse PGR combinations (with 0.5 mg/L BA + 0.5 mg/L NAA serving as the control). Compared to the control, treatment with 0.5 mg/L BA + 1.0 mg/L NAA did not substantially enhance induction rates across all cultivars (Fig. 4B). In the case of Blonde and Sweet Tea, induction rates significantly increased with the application of 0.5 mg/L BA + 0.5 mg/L 2,4-D, while other cultivars displayed negligible changes (Fig. 4B). Neither the dark pre-treatments nor the revised PGR treatments yielded improvements in shoot induction among the seven cultivars (Supplementary Fig. 1).
Effects of dark culture and modified PGR on callus induction. (A) Dark treatment with PGR combination 0.5 mg/L BA + 0.5 mg/L NAA. Explants without dark treatment were used as controls (0 day). Dark-treated explants with different durations (5 days, 10 days, 15 days) were analyzed for differences from controls and marked for significance. (B) Modified PGR combination. The 0.5 mg/L BA + 0.5 mg/L NAA combination was used as control, and the differences between other treatments and control were analyzed and marked as significant. ***, **, *, and ns indicate p value < 0.001, < 0.01, < 0.05, and > 0.05, respectively.
Correlation analysis of cutting and tissue culture initiation
Pearson correlation analysis was conducted between various measures and is presented in Fig. 5. There was a strong negative correlation (r = − 0.71) between callus induction rate and times of callus initiation. However, very weak or no correlations were found between shoot formation and callus induction or time of callus initiation. As expected, there were significant positive correlations among the cutting indexes (r ≥ 0.86).
The correlation between callus induction and cutting indexes is interesting because cutting indexes were negatively correlated with callus induction, positively correlated with the time of callus initiation, and only weakly or not correlated with shoot formation.
Discussion
Although studies have reported successful shoot regeneration from shoots, leaves, and petioles of Heuchera cultured in vitro3,5, petioles have advantages as explants over other plant parts. It has been established that using whole shoots is detrimental to mother plants, and petioles regenerate more shoots than leaves5. Additionally, leaves as explants are more susceptible to contamination due to their larger surface area, making disinfection more challenging. Furthermore, direct and indirect shoot organogenesis from petiole explants has been documented in various plants7,8. Multiple Heuchera cultivars in this study successfully regenerated shoots from petiole culture, confirming petioles as excellent explants for Heuchera in vitro culture.
There is an increasing consensus suggesting that high cytokinin-to-auxin ratio promotes shoot regeneration9,10,11, while a low ratio stimulates roots12, and specific ratios induce callus formation13,14. Previous Heuchera studies used BA and NAA combinations3,5, with BA acting as cytokinin and NAA acting as auxin. Since we foucsed on shoot formation, BA/NAA ratios favoring cytokinin were used. Treated cultivars showed varying shoot regeneration, though callus formation rates exceeded shoot rates in most cases. Some studies have reported that 2,4-D with cytokinin improves regeneration15, though 2,4-D suppressed shoot formation in the present study, increasing callus in some cultivars, which is in agreement with the suggestion that shoots form at undetectable 2,4-D levels16, indicating a minimal 2,4-D threshold for Heuchera shoot regeneration. Hence, we employed 2,4-D as an alternative PGR for certain cultivars. Surprisingly, BA plus 2,4-D hindered shoot regeneration across all treated cultivars while increasing callus formation frequencies in some cultivars. These results align with Sandal's suggestion from 2005, speculating that different species' tissue culture morphogenesis stages may require distinct intracellular 2,4-D concentration thresholds17. It is highly conceivable that Heuchera shoot formation might require a low or even zero threshold for intracellular 2,4-D concentration. Considering these outcomes, we excluded this PGR combination for shoot regeneration.
While dark incubation has been shown to promote shoot regeneration in certain plants18,19,20,21, no in vitro studies on Heuchera explored the effects of dark treatment. Our study indicated that dark treatment favored callus formation but did not stimulate shoot regeneration in certain Heuchera cultivars. Dark culture has been linked to increased endogenous auxin accumulation22, potentially driving heightened callus formation in specific cultivars. Although enhanced endogenous auxin can reportedly promote shoot formation to some extent23, our findings contradict this prior assertion. This discrepancy may be attributed to the Heuchera genotype or the auxin overproduction induced by dark treatment, ultimately inhibiting shoot regeneration.
Clonal propagation through cutting or in vitro culture leverages the remarkable regenerative potential of plant cells and their capacity for cell division. Callus is commonly perceived as a mass of undifferentiated parenchyma cells24,25 that can form at the incision of cuttings and in vitro explants. It is now understood that plants recover via organogenesis, leading to the development of shoots or roots from callus26. Histological analysis of explants revealed that calli and organs originate from the incision point27. This observation led us to speculate about the relationship between cutting and in vitro propagation. Rooting results highlighted distinct recovery potentials among different cultivars, facilitating the ranking of the 13 cultivars. In tissue culture experiments, these cultivars produced both calli and shoots from petioles. The correlations between cutting and in vitro culture results yielded intriguing insights. Specifically, rooting from cuttings exhibited a negative correlation with callus induction in tissue culture, with no correlation evident regarding shoot formation—contrary to expectations. This divergence could stem from distinct endogenous mechanisms activated by varying cultural conditions. Our findings suggest that cultivars with lower difficulty in cutting propagation face greater challenges for tissue culture.
Efficient Heuchera seedling production through cutting and in vitro culture methods is crucial for large-scale propagation. Based on our findings, we propose a potential protocol for Heuchera propagation, as outlined in Fig. 6. Several key points are worth noting: petioles are recommended as explants; the BA concentration should not exceed 4.0 mg/L; and 2,4-D should be avoided. Regardless of whether primary shoots are directly formed or developed from callus, both approaches yield shoots. The next step involves shoot proliferation to expand production capacity when primary shoots are obtained, regardless of the number.
Conclusion
In the present study, plantlets were regenerated from petiole in vitro culture using either a two-step direct procedure—direct shoot formation and rooting—or a three-step indirect procedure involving callus induction, shoot development, and rooting. Petioles are recommended as explants, to be inoculated into MS medium with 0.5–2.0 mg/L BA and 0.5 mg/L NAA. We suggest utilizing comparatively lower BA concentrations due to the detrimental effect of 4.0 mg/L on induction. Moreover, 2,4-D should be excluded, given its inhibitory impact on shoot formation. Finally, we provide hitherto undocumented evidence of a negative correlation between cutting and tissue culture potential in Heuchera.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Felter, H. W. & Lloyd J. U. Heuchera americana. In King's American Dispensatory 988 (Ohio Valley Company, 1905).
Judd, W., Campbell, C., Kellogg, E., Stevens, P. & Donoghue, M. Structural and biochemical characters. In Plant Systematics: A Phylogenetic Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA (2002).
Hosoki, T. & Kajino, E. Shoot regeneration from petioles of coral bells (Heuchera sanguinea Engelm.) cultured in vitro, and subsequent planting and flowering ex vitro. In Vitro Cell. Dev. Biol. Plant. 39(2), 135–138 (2003).
Zhao, H. Q., He, Q. H., Song, L. L., Hou, M. F. & Zhang, Z. G. In vitro culture of Heuchera villosa ‘Caramel’. HortScience 52(4), 622–624 (2017).
Nowakowska, K., Bodych, A., Latkowska, M. J. & Pacholczak, A. The use of tissue cultures in the mass production of Heuchera ‘Silver Scrolls’. Ann. Warsaw Univ Life Sci. Hortic. Landsc. Architect. 41, 5–16 (2020).
Murashige, T. and Skoog, F.A. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497 (1962)
Anike, F. N., Konan, K., Olivier, K. & Dodo, H. Efficient shoot organogenesis in petioles of yam (Dioscorea spp.). Plant Cell Tissue Organ Culture 111(3), 303–313 (2012).
Mohammed, A., Chiruvella, K. K., Namsa, N. D. & Ghanta, R. G. An efficient in vitro shoot regeneration from leaf petiolar explants and ex vitro rooting of Bixa orellana L.—a dye yielding plant. Physiol. Mol. Biol. Plants 21(3), 417–424 (2015).
Jones, B. et al. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22(9), 2956–2969 (2010).
Song, J. Y., Mattson, N. S. & Jeong, B. R. Efficiency of shoot regeneration from leaf, stem, petiole and petal explants of six cultivars of Chrysanthemum morifolium. Plant Cell Tissue Organ Culture. 107(2), 295–304 (2011).
Hesami, M., Daneshvar, M. H., Yoosefzadeh-Najafabadi, M. & Alizadeh, M. Effect of plant growth regulators on indirect shoot organogenesis of Ficus religiosa through seedling derived petiole segments. J. Genet. Eng. Biotechnol. 16(1), 175–180 (2018).
Che, P., Lall, S. & Howell, S. H. Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta 226(5), 1183–1194 (2007).
Feher, A., Pasternak, T. P. & Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 74(3), 201–228 (2003).
Birnbaum, K. D. & Alvarado, A. S. Slicing across kingdoms: Regeneration in plants and animals. Cell 132(4), 697–710 (2008).
Chauhan, H., Desai, S. A. & Khurana, P. Comparative analysis of the differential regeneration response of various genotypes of Triticum aestivum, Triticum durum and Triticum dicoccum. Plant Cell Tissue Organ Cult. 91(3), 191–199 (2007).
Sandal, I. et al. Gradual depletion of 2, 4-D in the culture medium for indirect shoot regeneration from leaf explants of Camellia sinensis (L.) O. Kuntze. Plant Growth Regul. 47(2), 121–127 (2005).
Ivarson, E., Ahlman, A., Li, X. & Zhu, L.-H. Development of an efficient regeneration and transformation method for the new potential oilseed crop Lepidium campestre. BMC Plant Biol. 13(1), 1–9 (2013).
Marutani-Hert, M. et al. A dark incubation period is important for Agrobacterium-mediated transformation of mature internode explants of sweet orange, grapefruit, citron, and a citrange rootstock. PLoS ONE 7(10), e47426 (2012).
Jin, W., Wang, Y. & Wang, H. Adventitious shoot regeneration from leaves of apple rootstock ‘Pingyitiancha’(Malus hupehensis var. pinyiensis) and genetic fidelity of regenerated plantlets using SSR markers. Can. J. Plant Sci. 94(8), 1345–1354 (2014).
Wang, H., Yang, Y., Li, M., Liu, J. & Jin, W. Reinvigoration of diploid strawberry (Fragaria vesca) during adventitious shoot regeneration. Sci. Rep. 9(1), 1–9 (2019).
Zhang, Y. et al. An efficient in vitro regeneration system from different wild apple (Malus sieversii) explants. Plant Methods 16(1), 1–10 (2020).
Fattorini, L. et al. Jasmonate promotes auxin-induced adventitious rooting in dark-grown Arabidopsis thaliana seedlings and stem thin cell layers by a cross-talk with ethylene signalling and a modulation of xylogenesis. BMC Plant Biol. 18(1), 1–18 (2018).
Liu, Y. et al. Transcriptomic and hormonal analyses reveal that YUC-mediated auxin biogenesis is involved in shoot regeneration from rhizome in Cymbidium. Front. Plant Sci. 8, 1866 (2017).
Wang, X., Nolan, K. E., Irwanto, R. R., Sheahan, M. B. & Rose, R. J. Ontogeny of embryogenic callus in Medicago truncatula: The fate of the pluripotent and totipotent stem cells. Ann. Bot. 107(4), 599–609 (2011).
Ali, D., Alarifi, S. & Pandian, A. Somatic embryogenesis and in vitro plant regeneration of Bacopa monnieri (Linn.) Wettst., a potential medicinal water hyssop plant. Saudi J. Biol. Sci. 28(1), 353–359 (2021).
Ji, L. et al. Genome-wide reinforcement of DNA methylation occurs during somatic embryogenesis in soybean. Plant Cell 31(10), 2315–2331 (2019).
Huang, H. et al. High frequency regeneration of plants via callus-mediated organogenesis from cotyledon and hypocotyl cultures in a multipurpose tropical tree (Neolamarkia Cadamba). Sci. Rep. 10(1), 1–10 (2020).
Acknowledgements
This study was supported by the Chongqing Bishan National Agricultural Science and Technology Park Management Committee.
Funding
This work was funded by Chongqing Bishan National Agricultural Science and Technology Park Management Committee (Grant no. 202109).
Author information
Authors and Affiliations
Contributions
C.X.: conceptualization, supervision, writing, reviewed the manuscript. H.G.: investigation, resources, formal analysis, reviewed the manuscript. Z.W.: investigation, data curation, reviewed the manuscript. Y.C.: funding acquisition and project administration, reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, C., Guo, H., Wang, Z. et al. Development and comparative analysis of initiation ability in large-scale Heuchera propagation using tissue culture versus cuttings. Sci Rep 13, 14785 (2023). https://doi.org/10.1038/s41598-023-42001-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42001-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.