Abstract
A morphology-based barcoding library of market teleost fishes (Teleostei) in Cebu is built based on cytochrome c oxidase subunit I (COI) sequences and voucher specimens which aimed to establish a reliable reference of frequently traded fishes in the province, a biodiversity hotspot at the center of the Philippine archipelago. A total of 1721 specimens were collected from 18 fish markets and landing sites around the province, in which 538 specimens were sequenced belonging to 393 species from 229 genera, 86 families, and 37 orders. Most speciose families are coral reef or reef-related shallow-water species. Twelve species from 11 families are newly recorded in the Philippine waters, among which 7 species are deep-sea inhabitants, while 3 species have expanded their distribution range. Only 20 taxa could not be identified to the species level due to the difficulty in morphological examinations, absence of matched reference sequences in online databases, and/or problematic species awaiting further studies. This first comprehensive DNA barcoding survey of Cebu fishes can facilitate further taxonomic research as well as the conservation and management of fisheries in the Philippines.
Similar content being viewed by others
Introduction
The DNA-based identification method has become increasingly popular in various taxa1. This can be attributed to the ease of using molecular markers (e.g., cytochrome oxidase subunit I, COI; cytochrome b; 12S ribosomal RNA) and the reduced reliance on taxonomic keys, which can be challenging for non-taxonomists2. DNA barcode reference libraries for bony fishes have been established in various geographic regions, aiding in the enrichment of local fish species lists. Starting from earlier Australian studies (e.g.3) and extending to more recent ones (e.g.4,5,6), the increasing utilization of DNA barcoding has expanded its scope from identifying species of conservation concern to revealing previously unrecognized cryptic species and accurately mapping the distribution ranges of known species. Accurate species identification is vital to ensure the successful management of fish stocks and will bring insight into processes maintaining marine biodiversity7.
In the Philippines, DNA barcoding has remained an underutilized tool in ichthyofaunal studies. This method has been increasingly used for identification and authentication of component species in commercial fishery products to ensure traceability and food safety (e.g.8,9); however, few studies have reported its use in the taxonomy, phylogeny, specimen-based identification, or to enrich species listing of marine fishes. To date, fish DNA barcoding studies in the country have primarily concentrated on major freshwater lakes10,11,12,13, while its application to marine ichthyofaunal diversity has been focused on specific groups of taxa with commercial importance, such as sardines (Dorosomatidae)14,15, groupers (Epinephelidae)16, trevallies (Carangidae) and snappers (Lutjanidae)17 which were collected from different sites in the country or from a specific area such as in the Cuyo Island, Palawan or in Northern Mindanao. Similarly, Willette & Padin18 and Torres & Santos19 used DNA barcodes of three marine Caranx species from the freshwater environment to resolve the identification and establish their phylogenetic relationship with extant marine species. Its recent use in specimen-based identification was reported by Bemis et al.5, who established the most comprehensive DNA barcode reference library of Philippine marine fishes based on 2,525 voucher specimens representing 984 species, significantly enhancing the coverage and utility of the method in the country.
The Cebu archipelago is located in the epicenter of marine biodiversity and its coasts harbor well-developed fringing reefs, strips of mangrove forests, and seagrass beds of varying patch sizes. Furthermore, the narrow and deep Tañon and Cebu Straits located in southern Cebu are the main channels for water exchanges in the Central Visayas, offering specific habitats for deep-sea fauna20. The variety of these habitats and ecosystems has made Cebu waters one of the seven most important marine biodiversity areas in the Coral Triangle21, and one of the most productive areas in the Philippines that have long served as a major fishing ground and source of fisheries revenues22. However, the presence of important coastal habitats and ecosystems amidst expanding human settlements has turned Central Visayas into a hotspot of overexploitation, thereby impacting species diversity in the region23,24.
Since the early 2000s, there has been a decrease in catch volume and changes in species composition (disappearance of some species and rise in number of invertebrates) landed in Cebu and surrounding waters due to changes in fishing gears and the increasing fishing pressure. This situation has had an impact on the livelihoods of artisanal fishermen20,23. Hence, the waters surrounding Cebu have become the priority areas for conservation, particularly for the reef fishes20. Nevertheless, previous fish species listings in Cebu waters were either site-specific (e.g.25,26) or focused on marine protected areas (e.g.27,28). While Bemis et al.5 have created the first DNA barcode reference library of marine market fishes in the Philippines, there is still a need to enhance comprehensive regional information on species composition through broader sampling coverage. As such, this study was conducted to identify the marine ichthyofauna of Cebu using DNA barcoding and voucher specimens. The aim is to expand the DNA barcode library of Philippine marine fishes and create a comprehensive fish species checklist for Cebu that encompasses additional economically important species, new species records, and the detection of cryptic species.
Results
Species identification and composition
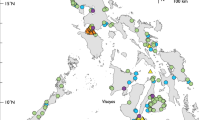
A total of 1721 specimens were collected from Cebu (Fig. 1, Supplementary Table S1), in which 538 specimens were sequenced, representing 393 species in 229 genera, 86 families, and 37 orders (Supplementary Fig. S1, Table S2). The length of obtained COI sequences ranged from 507 to 691 bp without any indel or stop codon. Twenty taxa could not be annotated at the species level because of any one or the combination of the following reasons: (1) species with difficulty in morphological identification; (2) species without corresponding blast result of genetic similarity higher than 98%; (3) species with blast results of genetic similarities higher than 98%, but the sequence from database was apparently misidentified; and (4) species may represent undescribed taxa awaiting further studies (Table 1). Twelve families contained ≥ 10 species and they accounted for 57.0% of the total species identified in this study, including Labridae (41 species, 10.4%), Pomacentridae (23 species, 5.9%), Lutjanidae (22 species, 5.6%), Carangidae (21 species, 5.3%), Muraenidae (19 species, 4.8%), Nemipteridae (17 species, 4.3%), Acanthuridae (16 species, 4.1%), Apogonidae (16 species, 4.1%), Epinephelidae (15 species, 3.8%), Chaetodontidae (12 species, 3.1%), Gobiidae (12 species, 3.1%), and Balistidae (10 species, 2.5%) (Fig. 2). Twelve species from 11 families were newly recorded in the Philippines, and three of these species exhibited expanded distribution ranges (Table 2). According to IUCN Red List status, none of the species involved in this study were categorized as Critically Endangered (CR) and Endangered (EN). Only Epinephelus fuscoguttatus (0.3% of all species) was considered Vulnerable (VU) species, while Choerodon schoenleinii and Sardinella lemuru (0.5%) were Near Threatened (NT) species. Two hundred and seventy-seven species (70.5%) were Least Concern (LC), 106 species (27.0%) were Not Evaluated (NE), and seven species (1.8%) were Data Deficient (DD) (Supplementary Table S2).
Map of 18 sampling localities in Cebu, the Philippines. Blue circles for sampling localities of the 23 deep-sea species in Table 5. Details of sampling localities in Supplementary Table S1. The map was generated by GMT version 5.4.5 (http://gmt.soest.hawaii.edu/doc/5.4.5/gmt.html) and modified by Photoshop CS5.
Genetic distance and species delimitation
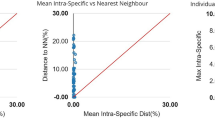
After trimming, 507 bp of all sequences were used in data analyses. The K2P genetic distances revealed an increased trend at higher taxonomies, with the mean values of 0.26 ± 0.03 (standard error) %, 15.30 ± 0.22%, 20.08 ± 0.12%, 23.42 ± 0.26%, and 24.50 ± 0.08% in intra-specific, -generic, -families, -orders, and -intraclass levels, respectively (Fig. 3, Table 3). At the intra-specific level, Plotosus lineatus showed large genetic distances between two genetic lineages that were far beyond 2%, but no obvious morphological difference was found between specimens (mean intra-specific K2P distance 15.00 ± 1.90%, n = 4; Fig. 4). Although this rare case may imply the exposure of divergent evolutionary lineages or cryptic species that have yet to be described, we temporarily annotated it under the same species and excluded it from the calculation of intra-specific distance. By contrast, two species pairs had clearly distinguishable morphology but revealed very low genetic divergences, viz., Abudefduf sexfasciatus vs. A. vaigiensis (inter-specific K2P distance 0.30 ± 0.17%, n = 3) and Arothron immaculatus vs. A. manilensis (0.79 ± 0.39%, n = 2), which were retained in the analysis. Except for the aforementioned three cases, the barcoding data showed a maximum intra-specific K2P distance of 1.30 ± 0.40% (Gymnothorax cf. chilospilus) and a minimum inter-specific K2P distance of 3.25 ± 0.80% (Satyrichthys welchi vs. S. laticeps), indicating a discernible barcoding gap spanning the range of 1.31–3.24% K2P distance.
Maximum likelihood tree of selected COI sequences (433 bp) from 10 BINs under the species names Plotosus lineatus + P. japonicus on BOLD Systems. Tree reconstructed based on GTR + Γ + I model. Numerals beside the internal branches for bootstrap values and those lower than 50 not shown. Plotosus canius, P. nkunga, and Paraplotosus albilabris are outgroups. Blue and red circles represent sequences of two lineages from Vietnam4 and sequences from this study, respectively. Insets show photos of Cebu specimens of the two lineages.
A total of 393 species were identified by the combination of morphology and blast results. Similarly, 379 existing BINs were recorded from BOLD Systems and 13 species represented unidentified BINs. Among all BINs, Abudefduf sexfasciatus vs. A. vaigiensis and Arothron immaculatus vs. A. manilensis were respectively designated in the same BINs, while Plotosus lineatus was represented by two BINs, resulting in a total of 392 BINs, which is one less than the total species number. The ABGD analysis revealed the same partition pattern of 392 OTUs with BINs. The ASAP showed 391 OTUs, which is very similar to BINs and ABGD, except for Satyrichthys welchi and S. laticeps, a species pair that had a K2P distance of 3.25 ± 0.80% but were deemed as the same OTU. The bPTP presented the most partitioned result that contained 420 OTUs.
The NJ tree based on 538 COI sequences revealed the monophyly in each species except for Abudefduf sexfasciatus vs. A. vaigiensis (Supplementary Fig. S2). However, 29 out of the 229 genera from 19 families were not monophyletic. We collected all sequences of these families from our COI data set and reconstructed an independent maximum likelihood (ML) tree for each family to test the monophyly of the aforementioned genera. Trees were constructed using the best-suggested models, along with 1000 bootstrap replications and outgroup sequences of the closest available taxa (according to the phylogenetic tree topology in Betancur-R et al.29) from our dataset. After checking, 19 genera in 12 families remained non-monophyletic (Supplementary Fig. S3, Table 4).
Discussions
The majority of species examined in this study exhibit monophyly in the NJ tree (Supplementary Fig. S2), coupled with a barcoding gap of 1.31–3.24% K2P genetic distance, supporting the efficiency and reliability of using COI fragments to identify teleost fishes. Increasing genetic distances in higher taxonomic levels (Fig. 3, Table 3) conform with many previous barcoding studies (e.g.3,4,46,47,48). In the species delimitation, 392, 392, 391, and 420 OTUs are identified by BIN, ABGD, ASAP, and bPTP, respectively. The remarkable consistency in OTU numbers across BIN, ABGD, and ASAP implies the robustness of species delimitation through DNA barcoding. Combining various delimitation methods with the morphology of voucher specimens can effectively enhance the accuracy of identification. Nevertheless, 19 genera spanning 12 families do not exhibit monophyly (Supplementary Fig. S3, Table 4), suggesting either insufficient resolution of the used marker or the requirement for additional taxonomic revisions of these taxa. After careful comparison, all topologies of non-monophyletic genera based on COI sequences are similar to those in phylogenetic studies conducted with multi-loci or genome-wide data, in which their taxonomies are usually contentious (Table 4 and reference therein). This result supports that COI sequences may have certain resolutions at the generic level.
Only two species pairs cannot be delimitated from each other by COI sequences, viz., Abudefduf sexfasciatus vs. A. vaigiensis and Arothron immaculatus vs. A. manilensis. Both species in pairs possess specific coloration patterns that are easy to recognize and differentiate from one another (Supplementary Fig. S1). Similar results of these two species pairs have been observed in previous studies49,50. Incomplete lineage sorting of recent speciation or introgressive hybridization could result in two closely related species sharing haplotypes51, a common phenomenon found in marine fishes52,53,54. In contrast, two divergent genetic lineages of Plotosus lineatus are observed in Cebu specimens which is similar to the finding of Thu et al.4 in Vietnam (Fig. 4). Plotosus japonicus is a congener that resembles P. lineatus and could be misidentified. The two species can merely be separated by a few meristic characters but both are considered valid55. Nevertheless, COI sequences of P. lineatus + P. japonicus from BOLD Systems comprise 10 deeply evolved lineages (BINs) in Indo-West Pacific without a geographical distribution pattern (Fig. 4). Goren et al.56 have reviewed nine of the 10 BINs and designated them into four species, including P. lineatus (BOLD:AAB3017, BOLD:ACG5707, and BOLD:ACG8821), P. japonicus (BOLD:ABZ0283, BOLD:AAB3011, BOLD:AAB3013, and BOLD:ABY8174), Plotosus sp. 1 (BOLD:AAB3016), and Plotosus sp. 2 (BOLD:AAB3012). Although the two Cebu lineages of P. lineatus align with P. japonicus and Plotosus sp. 2 as defined by Goren et al.56, we take a more conservative decision that places both lineages under P. lineatus, since the morphology in each lineage has yet to be thoroughly examined for determining the molecular traits of true P. lineatus and P. japonicus.
Among the 12 Philippine new records, seven species are deep-sea fishes, suggesting that the deep-sea biodiversity in the Philippines may still be incompletely explored and underestimated (Tables 2, 5). Aside from deep-sea species, the other newly recorded species are either small-sized (e.g., Atherinidae and Gobiidae), morphologically similar to their congeners (e.g., Trichiuridae), or reclusive (e.g., Muraenidae). Three species have expanded distribution range, including Doboatherina duodecimalis (Atherinidae), Physiculus chigodarana (Moridae), and Parascolopsis rufomaculata (Nemipteridae). Doboatherina duodecimalis was widely distributed in Indo-West Pacific, ranging from Madagascar to Vanuatu, and north to the Gulf of Thailand and central Philippines. However, Sasaki & Kimura57 resurrected D. balabacensis, a Philippine endemic species previously considered a junior synonym of D. duodecimalis, and recognized all Philippine D. duodecimalis records as D. balabacensis. The authors also identified two genetic lineages (Madagascar & Andaman Sea vs. Ambon & Sulawesi) of D. duodecimalis, with no morphological differentiation observed among specimens. In the present study, a COI sequence from Cebu belongs to the Ambon & Sulawesi lineage of D. duodecimalis according to a GenBank sequence (AB849035) identified by Sasaki & Kimura57, representing a range expansion as well as a sympatric distribution with D. balabacensis in the Philippine waters. The identification of Physiculus chigodarana in this study is based on two diagnostic morphological characters (the anteriorly placed light organ and the extended first dorsal fin) that are unique in the genus58. There is no available COI sequence of P. chigodarana in databases; however, the BIN that matches this specimen comprises two monophyletic clades of COI sequences with a 1.8% inter-clade K2P distance, including (1) five sequences of P. natalensis from South Africa; and (2) five sequences of four species from various areas (our P. chigodarana from Cebu, two Physiculus sp. and one P. roseus from Western Australia, and one P. japonicus from Taiwan). This result suggests that the four sequences from Western Australia and Taiwan might have been misidentified and could potentially correspond to P. chigodarana. The record represents the range expansion of P. chigodarana from Japan and Taiwan to the central Philippines. Parascolopsis rufomaculata, on the other hand, is a rare deep-sea nemipterid that has only been documented in northwestern Australia and southern Java59. The Cebu record of P. rufomaculata expands its distribution range across the equatorial to the northern hemisphere, suggesting that this little-known nemipterid might have a broader distribution in northern Australia and the East Indies regions.
The species composition of collected specimens in this study may reflect the environmental condition of fishery grounds, fishing gears and methods used by local fishers, or the diet habits of the local people since most materials are obtained from local markets and fish landing sites. In the species list, the majority are coral reef or reef-related shallow-water species, suggesting that coastal communities in Cebu have heavily relied on the coral reef resource60,61 (Fig. 2). Furthermore, 23 deep-sea species in 17 families that can inhabit a depth range greater than 200 m are discovered (Table 5). All the deep-sea species are collected from either the southwestern (Badian and Santander) or northeastern (Bogo) coasts of the province connecting to the Tañon Strait and Camotes Sea fishery ecosystems, respectively, the two deepest marine areas in Central Visayas20.
A notable difference is observed in species composition compared to another market fish barcoding survey in Vietnam4. The most speciose 10 families in Thu et al.4 are the Gobiidae (8.2%), Carangidae (6.1%), Lutjanidae (3.8%), Nemipteridae (3.6%), Epinephelidae (3.3%), Apogonidae (2.9%), Callionymidae (2.1%), Cynoglossidae (2.1%), Leiognathidae (2.1%), and Platycephalidae (2.1%). Most of these species are associated with sandy or muddy substrates, particularly the latter four families, which are relatively uncommon in Cebu. The discrepancy can largely be attributed to variations in habitats, as two of the three sampling areas in Thu et al.4, the Halong Bay and Gulf of Thailand, are characterized by soft-bottomed terrains62,63. In contrast, our Cebu checklist and that of Bemis et al.5 share the identical composition of the top 11 speciose families, differing only slightly in their rankings. Nevertheless, this study identified 120 species that were not included in Bemis et al.5, in which the top five speciose families are Pomacentridae (nine species), Labridae (eight species), Muraenidae (eight species), Gobiidae (seven species), and Apogonidae (six species) (Fig. 2). Moreover, six families (Atherionidae, Bembropidae, Cynoglossidae, Myctophidae, Sillaginidae, and Zenarchopteridae) were absent from Bemis et al.5, demonstrating the significant contribution of this study in expanding the barcode reference library for Philippine marine fishes. Likewise, the Cebu market survey and a recent fish checklist in Palawan, based on underwater visual census (UVC) in coral reefs64, show a very similar composition of speciose families. The 10 most prevalent families in the latter (Pomacentridae, Labridae, Lutjanidae, Chaetodontidae, Epinephelidae, Carangidae, Acanthuridae, Gobiidae, Scaridae, and Apogonidae) closely align with those in this study, with the exception of Muraenidae and Nemipteridae, which hold higher proportions in our findings. Balisco et al.64 reported a higher number of nemipterids (24 vs. 16 in this study), while the count of muraenid species is lower (11 vs. 19). The predictable underestimation of reclusive taxa like moray eels is a recognized limitation of using UVC to assess fish diversity64. On the other hand, moray eels are an important component of popular local dishes and have been regarded as one of the main targets of commercial fisheries in the Central Visayas region65, resulting in more species obtainable from fish markets.
In the present study, 12 (3%) of species documented in Cebu are new records in the Philippines, indicating a rich reserve of biodiversity yet to be fully explored. While no species classified as critically endangered or endangered are identified, it is important to highlight that nearly a third of the species remain unevaluated or lack adequate information (IUCN catalogs NE and DD), hindering comprehensive assessments. The Central Visayas, formerly renowned for hosting one of the world’s most prolific coral reef fish populations, now has faced a contrasting reality23,24. Recent investigations revealed that both this area and the southern Philippine Seas exhibit the lowest species richness. This significant decline in biomass and diversity has largely been attributed to prolonged overexploitation and habitat degradation23,24,66. Apparently, there have been no published references comprehensively documenting the marine fishes of Cebu waters prior to this study. With the reported decline in species diversity and biomass in the area, some species could have become locally extinct before being properly documented. Therefore, the establishment of a reliable morphology-based DNA barcoding library for commonly caught fishes in Cebu is an urgent necessity. This resource can assist researchers and fishery authorities in accurately identifying fish species present in the province, as well as in detecting threatened and cryptic species. It can serve as a foundation for fishery managers to implement biodiversity conservation and formulate suitable management policies, particularly for species that are already at risk.
Material and methods
Sample collection
Two fish collection activities were conducted in February 2010 and December 2018–January 2019, spanning a total of 21 days. Marine fish specimens were collected from 18 fish markets and landing sites across Cebu province, including Mactan and Bantayan islands (Fig. 1, Supplementary Table S1). Each fish was photographed, and either their fins or muscle tissue were clipped, stored in 95% ethanol, and frozen at − 20 °C before DNA extraction. Small specimens were taken as voucher specimens, while only the fin clips or tissue samples were preserved for large and/or expensive specimens (Supplementary Table S2). Voucher specimens were fixed using 10% formalin and gradually transferred to 70% ethanol for further preservation. All fin clip or tissue samples and voucher specimens were deposited in the ichthyological collection of the Department of Oceanography, National Sun Yat-sen University, Kaohsiung (DOS), Marine Ecology and Conservation Research Center, National Academy of Marine Research, Kaohsiung (NAMR), and National Museum of the Philippines, Manila (PNM). Necessary permits were obtained from the Bureau of Fisheries and Aquatic Resources Region 7 before the specimen transport. An initial checklist was created by assessing the morphology of the species using photographs and voucher specimens.
DNA sequencing
At least one specimen per species (according to the initial morphology-based checklist) was chosen for DNA extraction and molecular analysis to confirm the identification. A GeneMark DNA Purification Kit (GMbiolab, Taichung, Taiwan) was used to extract DNA from tissue samples. Partial fragments of the mitochondrial COI gene were amplified by polymerase chain reaction (PCR) using different combinations of fish-specific primers designed by Ward et al.3: FishF1 (5′-TCA ACC AAC CAC AAA GAC ATT GGC AC-3′), FishF2 (5′-TCG ACT AAT CAT AAA GAT ATC GGC AC-3′), FishR1 (5′-TAG ACT TCT GGG TGG CCA AAG AAT CA-3′), and FishR2 (5′-ACT TCA GGG TGA CCG AAG AAT CAG AA-3′). The PCR material, thermal cycling profile of PCR, quality check and purification of the PCR products, and DNA sequencing followed Thu et al.4. Sequences were trimmed and edited manually in MEGA version 1167. The edited sequences were then translated into amino acid sequences to identify the possible occurrence of insertion-deletion mutations (indels) or stop codons, which may represent a sign of nuclear mitochondrial pseudogenes68. All COI sequences have been submitted to GenBank, and their accession numbers are provided in Supplementary Table S2.
Data analyses
Obtained COI sequences were blasted in GenBank and BOLD Systems online public databases to create another species list. Species identification was accepted only when the similarity value exceeded 98% with the nearest DNA barcode in the database69. The barcode index number (BIN) of each sequence was also recorded. Each BIN corresponds to an operational taxonomical unit (OTU) designated via a sequence-based clustering method from BOLD Systems70, which is helpful for filtering possible misidentifications of sequences or revealing cryptic species. In cases where a specimen displayed conflicting morphological and molecular identifications, or online databases proposed two or more species for a specimen with high similarities, we further examined the photo and voucher specimen, if any, using taxonomic keys and published literature to confirm their identification (e.g.58,71). We followed the taxonomic classification by Betancur-R et al.29 for the order level, and by Eschmeyer's Catalog of Fishes72 for the family, genus, and species levels. The final species list was cross-referenced with records from FishBase73, Eschmeyer's Catalog of Fishes72, Balisco et al.64, and Bemis et al.5 to ascertain the number of newly recorded species in the Philippines. Additionally, we determined the conservation status of each species by referencing the International Union for Conservation of Nature Red List of Threatened Species74.
After species identification, all sequences were aligned and trimmed to the same length using MEGA version 11 for further analyses. The Kimura-2-Parameter (K2P)75 genetic distances at different taxonomic levels were calculated, including intra-specific distance (excluding species with only one sequence), inter-specific distance within the same genus (excluding genera with only one species), inter-genus distance within the same family (excluding families with only one genus), inter-family distance within the same order (excluding orders with only one family), and inter-order distance within the infraclass Teleostei. All COI sequences were used to construct a neighbor-joining (NJ) tree using Tamura-Nei + Γ model with 1000 bootstrap replications76,77,78. Both the model testing and tree construction were conducted using MEGA version 11.
Lastly, we used the barcoding data set from Cebu to test the species delimitation using three clustering models, including the Automatic Barcode Gap Discovery (ABGD79), the Assemble Species by Automatic Partitioning (ASAP80), and the Bayesian implementation of the Poisson Tree Processes (bPTP81). These results were compared with the final species list (based on morphology and blast results) and the number of BINs.
Data availability
Catalog numbers and GenBank accession numbers for all sequenced specimens are provided in Supplementary Table S2, and their corresponding photos can be found in Supplementary Fig. S1. All sequences are available on GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with accession numbers OM037461, OM037474, OM037539, ON351491, OQ508847, and OR113751–OR114283.
References
Hebert, P. D. N., Cywinska, A., Ball, S. L. & DeWaard, J. R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 270, 313–321 (2003).
Hajibabaei, M., Singer, G. A. C., Hebert, P. D. N. & Hickey, D. A. DNA barcoding: How it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 23, 167–172 (2007).
Ward, R. D., Zemlak, T. S., Innes, B. H., Last, P. R. & Hebert, P. D. N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 1847–1857 (2005).
Thu, P. T. et al. DNA barcoding of coastal ray-finned fishes in Vietnam. PLoS ONE 14, e0222631 (2019).
Bemis, K. E. et al. Biodiversity of Philippine marine fishes: A DNA barcode reference library based on voucher specimens. Sci. Data 10, 411 (2023).
Guimarães-Costa, A. J. et al. Fish diversity of the largest deltaic formation in the Americas—A description of the fish fauna of the Parnaíba delta using DNA barcoding. Sci. Rep. 9, 7530 (2019).
Marko, P. B. & Moran, A. L. Out of sight, out of mind: High cryptic diversity obscures the identities and histories of geminate species in the marine bivalve subgenus. Acar. J. Biogeogr. 36, 1861–1880 (2009).
Maralit, B. A. et al. Detection of mislabeled commercial fishery by-products in the Philippines using DNA barcodes and its implications to food traceability and safety. Food Control 33, 119–125 (2013).
Sarmiento, K. P. et al. Not fish in fish balls: Fraud in some processed seafood products detected by using DNA barcoding. Philipp. Sci. Lett. 11, 30–36 (2018).
Abdulmalik-Labe, O. P. & Quilang, J. P. DNA barcoding of fishes from Lake Lanao, Philippines. Mitochondrial DNA B Resour. 4, 1890–1894 (2019).
Abdulmalik-Labe, O. P., Picart, N. M. R., Francisco, M. E. M., Castillo, R. M. G. & Quilang, J. P. DNA barcoding of Glossogobius species (Teleostei: Gobiidae) from major lakes in the Philippines reveals the presence of cryptic species and species complexes. Conserv. Genet. Resour. 14, 309–320 (2022).
Aquilino, S. V. L. et al. DNA barcoding of the ichthyofauna of Taal Lake, Philippines. Mol. Ecol. Resour. 11, 612–619 (2011).
Aquino, L. M. G. et al. DNA barcoding of fishes of Laguna de Bay, Philippines. Mitochondrial DNA 22, 143–153 (2011).
Quilang, J. P., Ong, P. S., Kendrich, I. & Fontanilla, C. DNA barcoding of the Philippine endemic freshwater sardine Sardinella tawilis (Clupeiformes: Clupeidae) and its marine relatives. Philipp. Agric. Sci. 94, 248–257 (2011).
Labrador, K. et al. DNA barcoding of dominant species in the sardine fishery of northern Mindanao. Philipp. J. Sci. 150, 433–437 (2021).
Alcantara, S. G. & Yambot, A. V. DNA barcoding of commercially important grouper species (Perciformes, Serranidae) in the Philippines. Mitochondrial DNA A 27, 3837–3845 (2016).
Templonuevo, R. M., Alcantara, S., Juanico, C. S. & Yambot, A. DNA barcoding of two commercially important fish families (Carangidae and Lutjanidae) collected from Cuyon, Palawan, Philippines. Int. J. Agric. Technol. 14, 2051–2066 (2018).
Willette, D. A. & Padin, J. I. M. Identifying the biodiversity of marine jacks (Carangidae) in the freshwater Taal Lake, Philippines using phenotypic features and mitochondrial DNA. J. Appl. Ichthyol. 30, 490–495 (2014).
Torres, S. K. M. & Santos, B. S. Species identification among morphologically similar Caranx species. Turk. J. Fish. Aquat. Sci. 20, 159–169 (2020).
Green, S. J. et al. The fisheries of Central Visayas, Philippines: status and trends. Coastal Resource Management Project of the Department of Environment and Natural Resources and the Bureau of Fisheries and Aquatic Resources of the Department of Agriculture (2004).
Asaad, I., Lundquist, C. J., Erdmann, M. V. & Costello, M. J. Delineating priority areas for marine biodiversity conservation in the Coral Triangle. Biol. Conserv. 222, 198–211 (2018).
Tamayo, N. C. A., Anticamara, J. A. & Acosta-Michlik, L. National estimates of values of Philippine reefs’ ecosystem services. Ecol. Econ. 146, 633–644 (2018).
Nañola, C. L., Aliño, P. M. & Carpenter, K. E. Exploitation-related reef fish species richness depletion in the epicenter of marine biodiversity. Environ. Biol. Fishes 90, 405–420 (2011).
Guanco, M. R., Mesa, S. V., Belga, P. B. & Nuñal, D. R. M. Assessment of the commercial fisheries of western and central Visayas. BFAR NFRDI Tech. Paper Ser. 12, 1–44 (2009).
Edullantes, B. et al. A benchmark survey of plankton, fish and benthic composition in Poblacion and Kadurong Reefs in Liloan, Cebu, Philippines. Biodivers. Data J. 9, 1–20 (2021).
Caballero, J. E. et al. Coral recruitment rate and fish aggregation in Pandong Bato Puente, Carmen, Cebu, Philippines. J. Wetl. Biodivers. 11, 27–47 (2021).
Corrales, C. M. et al. A baseline study on coral reef fishes in the marine protected areas in southern Cebu, Philippines. Trop. Technol. J. 19, 1–8 (2015).
White, A. T., Apistar, D., Tesch, S. C., White, E. & Martinez, R. Summary field report: Coral reef monitoring expedition to Southeastern Cebu, Philippines, March 22–29, 2010. www.coast.ph (2010).
Betancur, R. R. et al. Phylogenetic classification of bony fishes. BMC Evol. Biol. 17, 162 (2017).
Thacker, C. E. & Roje, D. M. Phylogeny of cardinalfishes (Teleostei: Gobiiformes: Apogonidae) and the evolution of visceral bioluminescence. Mol. Phylogenet. Evol. 52, 735–745 (2009).
McCord, C. L. & Westneat, M. W. Phylogenetic relationships and the evolution of BMP4 in triggerfishes and filefishes (Balistoidea). Mol. Phylogenet. Evol. 94, 397–409 (2016).
Hundt, P. J., Iglésias, S. P., Hoey, A. S. & Simons, A. M. A multilocus molecular phylogeny of combtooth blennies (Percomorpha: Blennioidei: Blenniidae): Multiple invasions of intertidal habitats. Mol. Phylogenet. Evol. 70, 47–56 (2014).
Lin, H.-C. & Hastings, P. A. Phylogeny and biogeography of a shallow water fish clade (Teleostei: Blenniiformes). BMC Evol. Biol. 13, 210 (2013).
Bellwood, D. R. et al. Evolutionary history of the butterflyfishes (f: Chaetodontidae) and the rise of coral feeding fishes. J. Evol. Biol. 23, 335–349 (2010).
Zhuang, X., Qu, M., Zhang, X. & Ding, S. A comprehensive description and evolutionary analysis of 22 grouper (Perciformes, Epinephelidae) mitochondrial genomes with emphasis on two novel genome organizations. PLoS ONE 8, e73561 (2013).
Ma, K. Y. & Craig, M. T. An inconvenient monophyly: an update on the taxonomy of the groupers (Epinephelidae). Copeia 106, 443–456 (2018).
Fadli, N., Muchlisin, Z. A. & Siti-Azizah, M. N. DNA barcoding of commercially important groupers (Epinephelidae) in Aceh, Indonesia. Fish. Res. 234, 105796 (2021).
Wang, D. Taxonomy, phylogeny and evolutionary correlation between body elongation and paired fin reduction in Trichiuroidea (Teleostei: Scombriformes). (National Taiwan University, 2022).
Tavera, J., Balart, E. F. & Bernardi, G. Molecular phylogeny of grunts (Teleostei, Haemulidae), with an emphasis on the ecology, evolution, and speciation history of New World species. BMC Evol. Biol. 12, 57 (2012).
Westneat, M. W. & Alfaro, M. E. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 36, 370–390 (2005).
Guo, Y., Bai, Q., Yan, T., Wang, Z. & Liu, C. Mitogenomes of genus Pristipomoides, Lutjanus and Pterocaesio confirm Caesionidae nests in Lutjanidae. Mitochondrial DNA A 27, 2198–2199 (2016).
Veneza, I. et al. Multiloci analyses suggest synonymy among Rhomboplites, Ocyurus and Lutjanus and reveal the phylogenetic position of Lutjanus alexandrei (Lutjanidae: Perciformes). Neotrop. Ichthyol. 17, e180109 (2019).
Reece, J. S., Bowen, B. W., Smith, D. G. & Larson, A. Molecular phylogenetics of moray eels (Muraenidae) demonstrates multiple origins of a shell-crushing jaw (Gymnomuraena, Echidna) and multiple colonizations of the Atlantic Ocean. Mol. Phylogenet. Evol. 57, 829–835 (2010).
Friedman, M. et al. A phylogenomic framework for pelagiarian fishes (Acanthomorpha: Percomorpha) highlights mosaic radiation in the open ocean. Proc. R. Soc. B Biol. Sci. 286, 20191502 (2019).
McCord, C. L., Nash, C. M., Cooper, W. J. & Westneat, M. W. Phylogeny of the damselfishes (Pomacentridae) and patterns of asymmetrical diversification in body size and feeding ecology. PLoS ONE 16, e0258889 (2021).
Zhang, J. & Hanner, R. Molecular approach to the identification of fish in the South China Sea. PLoS One 7, e30621 (2012).
Chang, C. H. et al. DNA barcodes of the native ray-finned fishes in Taiwan. Mol. Ecol. Resour. 17, 796–805 (2017).
Lakra, W. S. et al. DNA barcoding Indian marine fishes. Mol. Ecol. Resour. 11, 60–71 (2011).
Bertrand, J. A. M., Borsa, P. & Chen, W.-J. Phylogeography of the sergeants Abudefduf sexfasciatus and A. vaigiensis reveals complex introgression patterns between two widespread and sympatric Indo-West Pacific reef fishes. Mol. Ecol. 26, 2527–2542 (2017).
Marie, Z. A. & Allam, M. Molecular phylogenetic linkage for Nile and marine puffer fishes using mitochondrial DNA sequences of cytochrome b and 16S rRNA. Egypt. J. Aquat. Biol. Fish. 23, 67–80 (2019).
Victor, B. C. How many coral reef fish species are there? Cryptic diversity and the new molecular taxonomy. in Ecology of Fishes on Coral Reefs, pp. 76–87 (ed. Mora, C.) (Cambridge University Press, 2015).
Allen, G. R., Erdmann, M. V. & Dailami, M. Cirrhilabrus marinda, a new species of wrasse (Pisces: Labridae) from eastern Indonesia, Papua New Guinea, and Vanuatu. J. Ocean Sci. Found. 15, 1–15 (2015).
Huang, W.-C., Chen, H. M., Tseng, Y. C. & Liao, T.-Y. Assessing phenovariant species pairs of moray eels: hybridization, introgression, and morphology. Zool. Scr. 51, 676–694 (2022).
Victor, B. C. Hypoplectrus floridae n. sp. and Hypoplectrus ecosur n. sp., two new barred hamlets from the Gulf of Mexico (Pisces: Serranidae): more than 3% different in COI mtDNA sequence from the Caribbean Hypoplectrus species flock. J. Ocean Sci. Found. 5, 2–19 (2012).
Yoshino, T. & Kishimoto, H. Plotosus japonicus, a new eeltail catfish (Siluriformes-Plotosidae) from Japan. Bull. Natl. Mus. Nat. Sci. Ser. A 2, 1–11 (2008).
Goren, M., Stern, N. & Diamant, A. New records of Plotosus japonicus in the Red Sea and genetic indications for its presence throughout the Indo-Pacific (Osteichthyes: Plotosidae). Zool. Middle East 66, 124–131 (2020).
Sasaki, D. & Kimura, S. A new atherinomorine genus Doboatherina (Atheriniformes: Atherinidae) with a review of included species. Ichthyol. Res. 67, 225–261 (2020).
Nakabo, T. Fishes of Japan with pictorial keys to the species. (Tokai University Press, 2013).
Oktaviyani, S., Peristiwady, T., Tan, H. H. & Ho, H. C. New record of Parascolopsis rufomaculata Russell, 1986 (Perciformes: Nemipteridae) from Indonesian waters, Eastern Indian Ocean. Raffles Bull. Zool. 36, 491–495 (2021).
Muallil, R. N. et al. Effectiveness of small locally-managed marine protected areas for coral reef fisheries management in the Philippines. Ocean Coast. Manag. 179, 104831 (2019).
Ochavillo, D., Hodgson, G., Shuman, C. & Ruz, R. Status of the Philippine marine aquarium fish trade. in Turbulent seas: The status of Philippine marine fisheries, pp. 60–64 (eds. Silvestre, G. et al.) (Department of Agriculture-Bureau of Fisheries and Aquatic Resources, 2004).
Di Geronimo, I., Sanfilippo, R., Chaimanee, N., Robba, E. & Negri, M. P. An actuopalaeontological approach to the assessment of recent changes in benthic molluscan biodiversity: Preliminary results in the Northern Gulf of Thailand. Rend. Soc. Paleont. Ital. 2, 37–68 (2005).
Mekhova, E. S. & Britayev, T. A. Soft substrate crinoids (Crinoidea: Comatulida) and their macrosymbionts in Halong Bay (North Vietnam). Raffles Bull. Zool. 63, 438–445 (2015).
Balisco, R. A. T. et al. Marine fishes of Palawan, Philippines: Species diversity, new records, and conservation status. Reg. Stud. Mar. Sci. 60, 102825 (2023).
Tsuji, T. The technique and ecology surrounding moray fishing: A case study of moray trap fishing on Mactan Island, Philippines. in Prehistoric marine resource use in the Indo-Pacific Region, pp. 167–181 (eds. Ono, R., Addison, D. & Morrison, A.) (Australian National University Press, 2013).
Parras, D. A. ‘Toni’. Coastal resource management in the Philippines: A case study in the Central Visayas region. J. Environ. Dev. 10, 80–103 (2001).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Song, H., Buhay, J. E., Whiting, M. F. & Crandall, K. A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Natl. Acad. Sci. U.S.A. 105, 13486–13491 (2008).
Ward, R. D. DNA barcode divergence among species and genera of birds and fishes. Mol. Ecol. Resour. 9, 1077–1085 (2009).
Ratnasingham, S. & Hebert, P. D. N. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 8, e66213 (2013).
Heemstra, P. C., Heemstra, E., Ebert, D. A., Holleman, W. & Randall, J. E. Coastal fishes of the Western Indian Ocean. (South African Institute for Aquatic Biodiversity, 2022).
Fricke, R., Eschmeyer, W. N. & van der Laan, R. Eschmeyer’s catalog of fisheries: Genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (2023).
Froese, R. & Pauly, D. FishBase, version 07/2023. www.fishbase.org (2023).
IUCN. The IUCN Red List of Threatened Species, version 07/2023. https://www.iucnredlist.org (2023).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980).
Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39, 783–791 (1985).
Saitou, N. & Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 (1987).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877 (2012).
Puillandre, N., Brouillet, S. & Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 21, 609–620 (2021).
Zhang, J., Kapli, P., Pavlidis, P. & Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869–2876 (2013).
Acknowledgements
We appreciate the logistical support during sampling provided by Tzu-Yu Chen, Tsu-Jung Jhang (NSYSU), and the staff from the University of the Philippines Cebu and University of San Carlos. We also thank Po-Hao Chiu, You-Chen Liu, Pin Man Tseng, and Ching-Hsun Lin (NSYSU) for helping with sequencing. Special thanks to Nok Sum Leung (NSYSU), Shing-Lai Ng, Jian-Fu Huang (NTOU), and Chi-Ngai Tang (NTU) for their assistance in fish identification. Finally, we extend our gratitude to Sih-Yu Chen (NSYSU) for assistance in the map drawing. This study is supported by the Grants to TYL (106-2923-B-110-002-MY3 and 111-2611-M-110-026) and CWC (100-2923-B-291-001-MY2) from the National Science and Technology Council.
Author information
Authors and Affiliations
Contributions
T.Y.L., F.C.E., C.L.N., C.W.C., K.N.S., and K.T.S. conceived and designed the research. W.C.H., F.C.E., T.K.C., C.W.C., and K.N.S. conducted field work and collected specimens. W.C.H., R.A.B., T.K.C., and W.C.J. identified and sequenced the specimens. W.C.H., F.C.E., R.A.B., and T.Y.L. analyzed the data and prepared the manuscript. All authors contributed to the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, WC., Evacitas, F.C., Balisco, R.A. et al. DNA barcoding of marine teleost fishes (Teleostei) in Cebu, the Philippines, a biodiversity hotspot of the coral triangle. Sci Rep 13, 14867 (2023). https://doi.org/10.1038/s41598-023-41832-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41832-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.