Abstract
Seaweed aquaculture has become a profitable and an attractive alternative of cultivation thanks to its quick biomass production for food, feed, and other non-food applications. In addition, the ecosystem services generated by seaweed cultivation towards carbon fixation represents a more sustainable solution to the ocean’s acidification. The growth of elkhorn sea moss (Kappaphycus alvarezii) was evaluated in three plots with 200 propagules during a period of 70 days in a floating raft system covered by a fishing net underneath. Initial weight of propagules was 159.3 ± 12.74 g in wet biomass and 15.3 ± 1.43 g in dry biomass and were sampled up to 19 days (in the lag growth phase; period I), up to 33 days (in the exponential growth phase; period II) and up to 70 days (in the stationarity growth phase; period III). The variations of sea surface water temperature, salinity, turbidity (Secchi depth), total ammonium, nitrites, nitrates, and phosphate were determined. The growth increase was more evident in the exponential phase II when a dry biomass of 28.0 ± 2.48 (1153.3 ± 6.25 g in wet mass) was reached, more than 7 times the biomass of propagules with an average daily growth rate of 15.2% g.day–1. The carrying capacity of the zone was estimated at 86.2% in the area where 53 cultivation units would be projected. The economic analysis presented a financial feasibility with a net profit of 19% over the projected income and an IRR of 16.5%, recovering the investment in an estimated period of 4.3 years. We recommend to continue with larger-scale studies to optimize the cultivation of K. alvarezii in the study area.
Similar content being viewed by others
Introduction
In 2022, more than 35 million tons of wet macroalgae were produced worldwide, generating a turnover of approximately 1.9 billion dollars1. In addition to their economic importance, algae production plays an important role in the ecological balance of aquatic ecosystems, contributing to the reduction of CO2 and eutrophication2. Therefore, they are an important tool in climate change mitigation, as algae culture can promote the elevation of the pH of water in their aquaculture areas, thus combating the acidification of the water3.
One of the species with the highest production in the tropics is the alga cottoni or elkhorn sea moss Kappaphycus alvarezii (Doty) Doty ex Silva, a species of red algae mainly exploited to produce carrageenan, commonly used as a food additive, but also in the pharmaceutical industry4. Moreover, recent studies propose the cultivation of K. alvarezii bioproducts for other uses in the nutraceutical field5.
The elkhorn sea moss K. alvarezii grows naturally in areas of Southeast Asia, mainly in Indonesia, Malaysia, and the Philippines at depths between 1-17 m6. It usually grows in warm waters (27–30 °C), with salinities between 30 and 35 ‰7,8,9, under high light levels10 and an intense degree of water movement11. In addition, the growth of K. alvarezii does not require water with a high nutrient content for its development11,12,13,14 and has a relatively faster growth rate than other macroalgae’s species15. Moreover, during the last decades the cultivation of the K. alvarezii has also been expanded to further circumtropical latitudes throughout the world, including Fiji, the Philippines, Malaysia, Tuvalu, the Maldives16, India, Tanzania17, Vietnam, Cambodia, and Myanmar16,18. In addition, cultivation of K. alvarezii has also successfully been implemented in Latin America in countries with tropical climates such as Brazil19, Cuba14, Venezuela20, Mexico4, Belize, Lesser Antilles21 and Colombia21,22, thanks to the inherent advantages previously mentioned of fast and easy production that this species has in comparison to other endemic.
In Ecuador, the cultivation of the elkhorn K. alvarezii began in 2014 with the government initiative through the Aquaculture Undersecretariat in association with artisanal fishermen (Santa Rosa de Salinas Artisanal Fishing Production Cooperative)23,24. Thus, K. alvarezii was included in the list of species suitable for mariculture as a “species under investigation” in 201725 and considered as one of the promising species for the diversification of aquaculture in the country. While there are no published records yet of its aquaculture and economic feasibility, this represents one of the main goals in our study.
Considering that one of the most used seaweed farming systems are horizontal floating rafts18,26, in this study we examined it, within the framework of best harvest yield over time (up 19, 33 and 70 days). This is the first time, K. alvarezii has been growing in a system of floating rafts in the Tropical Eastern Pacific, and thus determining environmental factors associated with the modulation of its growth. Finally, productive, and socioeconomic projections of its cultivation in Bahía Las Conchas, province of Santa Elena, Ecuador, are proposed.
Materials and methods
Location and culture system
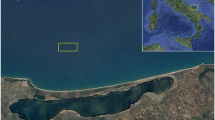
The study was carried out during a period of 3 months (July, August, and September in 2016) within the concession area of the Santa Rosa Artisanal Fishing Production Cooperative, located in Bahía Las Conchas, province of Santa Elena, Ecuador (Fig. 1). This area has a sandy substrate with depths of about 8–10 m23. The cultivation was carried out in a system of floating rafts using 110 mm diameter PVC tubes with a length of 3 m and 3 mm polypropylene ropes. These structures were fixed to the bottom by means of 250 kg cement weights located at an average depth of 5.4 ± 0.10 m. The cultivation unit was made up of 15 cells of 15 m2 (5 × 3 m) each for a total area of 225 m2. In each cell, 10 lines of 5 m length were placed, to which an average of 20 implants separated by 0.2 m each were attached. These cultivation structures were covered underneath with a fishing net to minimize the dispersion of detached seedlings and avoid possible herbivory by fish.
Panels (a) and (b) indicate the location of the cultivation site in the waters of Santa Elena province, Ecuador in South America. Panel (c) the propagules attached to the rope. Panel (d) a series of floating raft units, and finally panel (e) a branch of Kappaphycus alvarezii (Map source NASA: https://soto.podaac.earthdatacloud.nasa.gov/).
Culture experiments
Within the floating rafts system, 3 non-contiguous experimental culture units (5 × 3 m) were chosen, each unit being considered as an experimental replica. In each experimental unit, 200 propagules were sown (manually fixed to the culture unit with a piece of polyester rope) with an initial weight of 159.3 ± 12.74 g of wet biomass and 15.3 ± 1.43 g of dry biomass (95% confidence interval as dispersion index in all measurements).
Three sampling periods were conducted after sowing at up to 19 days (in the lag growth phase; period I), at up to 33 days (in the exponential growth phase; period II) and at up to 70 days (in the stationarity growth phase; period III). For the calculations of wet biomass growth, a sample of 15 tissue samples was taken at random in each period, which were weighed in situ with a digital portable scale (0.01 g precision). From this sample, 3 tissue samples were randomly taken for the evaluation of dry biomass, which were washed with fresh water and dried in an oven at 105 °C for 3 h following the recommendations of Ohno et al.7. The rest of the tissue samples were returned to the respective experimental replica.
For the growth curves we used the absolute dry weight end in each of the cultivation periods. For comparative use of other K. alvarezii cultures we use the equation of the daily growth rate in percentage (% DGR) in wet mass, proposed by Yong27 with the Eq. (1) as follows:
where Wo is the initial weight (g), Wt is the final weight (g), and t is the number of days of culture. The data is presented as mean daily growth rates for periods I, II and III, respectively.
Environmental factors
To estimate the possible influence of environmental factors on algal growth, sea surface water temperature and salinity were measured using a YSI Professional Plus (Pro Plus) multiparameter probe. Similarly, water samples were taken to determine the concentration of total ammonium (NH4+), nitrites (NO2–), nitrates (NO3–) and phosphate (PO43–) using a HI 83,200 Hanna instruments® equipment, previously calibrated by colorimetric analysis (0.01 ppm of precision).
Carrying capacity of culture area
As a feasibility factor in the study area, the carrying capacity was defined as the cultivation area that can be used for the mariculture activity of the macroalgae in a continuous way, considering that there are no social and ecological conflicts in the coastal system2. The calculations were based on the methodology applied by Azis28 taking into consideration the following Eqs. (2), (3) and (4). In this way, the system presented an area of 225 m2 (45 m length × 5 m width-crop unit) (L1P1). Additionally, a space between cultivation units of 10 m on all sides was considered, so the total projected area per cultivation unit was established at 1625 m2 (65 m length × 25 m width) (L2P2). The capacity of the water body was calculated according to the relationship:
where L1 = Width of a culture unit,
L2 = Appropriate width of a culture unit,
P1 = Length of a culture unit,
P2 = Appropriate length of a culture unit.
The method used to calculate the adequate area without exceeding the load capacity of a specific area was based on the following relationship formulas:
where Wa = area (ha),
Wc = capacity of water body (%).
The maximum number of culture units that the carrying capacity of the water body can support was calculated using the following formula:
where C = Carrying capacity of the water body (ha),
A = maximum area of use.
Economic viability
To determine the economic feasibility of cultivation of K. alvarezii, profitability was estimated with data on the maximum load capacity of the cultivation area, which was projected with a total of 53 floating rafts of 45 × 5 m of simple construction with floats of PVC pipes with a diameter of 110 mm and a length of 3 m. These rafts had a capacity of 150 lines or 2134 propagules. Seaweed production was estimated at the harvest time with the highest yield assuming 11 harvests/year (harvests of 30–35 days). The operational costs included an already operational infrastructure for maintaining the propagules as well as various activities (drying, packaging, salaries, mobility, etc.) necessary for the commercial production of macroalgae. Calc software functions were used to determine costs and financial indicators29,30,31. The financial analysis was projected over 10 years based on export prices for the weight of dehydrated seaweed as well as current local market prices for used inputs, expressed in US dollars. The projected revenue from the sale of the algae was based on a 4% annual increase in the sales price in the main international markets located in the Philippines, Indonesia, and Tanzania32.
Statistical analysis
The growth rates of the dry biomass of K. alvarezii during the initial, middle, and final periods were compared using a one-way ANOVA, after verification of the normal distribution and homogeneity of variances in the treatments (Shapiro–Wilk and Levene’s tests, respectively), followed by Tukey’s post hoc tests, according to recommendations of Zar33. Data of the environmental factors were analysed using the non-parametric Kruskal–Wallis test, establishing differences between periods using paired comparisons of Dwass-Steel-Critchlow-Fligner, and following the recommendations of Hsu34. The significance level for all tests was set at P = 0.05.
Ethics declaration
The seedlings of K. alvarezii were imported from Punta Laurel sector, Bocas del Toro Archipelago, in the Republic of Panama, with its respective phytosanitary certificate of origin and invoice. The experimental farm in Panama has been certified by the Undersecretary of Aquaculture as an exporting establishment of Kappaphycus macroalgae, being certified free of any pathological agent under the National Government of the Republic of Panama through the National Directorate of Animal Health of the Ministry of Agricultural Development.
Results
Growth
No mortality was observed, nor was there any evidence of damage or grazing on the K. alvarezii cultivation during the study period. The final wet biomass average was 1620.7 g ± 12.74 g (95% CI, confidence interval), representing more than 10 times the initial one (159.3 ± 12.74 g). The increase was more evident in the period II when a wet biomass of 1153.3 ± 6.25 g was recorded. The growth shown in absolute values of dry biomass was similar, reaching an average initial dry biomass of the propagules (15.3 ± 1.43 g) to 28.0 ± 2.48, 108.3 ± 5.17 and 144.0 ± 8.61 g, for the I period (up to 19 days), II period (up to 33 days), and III period (up to 70 days), respectively, with the proportion daily growth rates of 3.0 ± 0.30, 15.2 ± 0.62 and 6.5 ± 0.25% g.day–1, respectively, being the values in the intermediate period significantly higher (Fig. 2).
Growth estimates presented in absolute values of dry biomass of Kappaphycus alvarezii under cultivation in a floating rafts system on the coast of the province of Santa Elena, Ecuador. Numbers presented above the figure indicate mean % daily growth rates for periods I (from 0 to 19 days), II (from 19 to up 33 days) and III (from 33 to up 70 days). Vertical lines indicate 95% CI, confidence intervals. The three colours highlight the corresponding three different sampled periods from July to September 2016.
Environmental parameters
The sea surface temperature presented an average of 28.9 ± 0.64 °C, with little difference among time periods (< 2 °C); however, the maximum temperatures recorded during the intermediate period (29.7 ± 0.06 °C) was significantly higher than the temperature in the final (28.7 ± 0.09 °C) and initial (28.3 ± 0.06 °C) periods (Fig. 3). The average turbidity was 1.3 ± 0.52 m, with a maximum of 2.0 ± 0.00 m in the initial period, significantly higher than that recorded in the intermediate (0.91 ± 0.025 m) and final period (1 ± 0.00 m). Regarding the dissolved inorganic nitrogenous compounds in the cultivation area, the values of NH4+, NO2− and NO3–, were generally 0.13, 0.13 and 1.92 ppm, respectively, without showing significant differences among the study periods (Fig. 4). Phosphate showed greater variability among periods, with values between 0.16 and 0.18, being the values in the initial period significantly higher.
Variation in the concentration of ammonium, nitrite, nitrate, and phosphate, measured in the sea surface water of the cultivation zone of Kappaphycus alvarezii on the coast of the province of Santa Elena, Ecuador. The three colours highlight the corresponding three different sampled period months from July to September 2016.
Carrying capacity
Since each cultivation unit requires an area of 0.16 ha, the concession area in Bahía Las Conchas has a carrying capacity of the water body equivalent to 86.2%, which represents a total of 53 cultivation units in the concession system with horizontal floating rafts (Table 1).
Economic viability
The 10-year financial study revealed a payback time from the fourth year of project start-up, with a return of 19% on the expected total income, with an expected annual production of 121.3 T in dry weight of K. alvarezii. The financial viability of the cultivation of the macroalga in floating farming systems was determined by the net present value (NPV) of $58,326.63, the internal rate of return (IRR) of 16.46% and the benefit–cost ratio (B/C) (1.67) (Table 2).
Discussion
Our trial study provides relevant new data on the biological feasibility of elkhorn sea moss (K. alvarezii) cultivation on the coast of the province of Santa Elena, Ecuador. During cultivation, we did not observe notable losses or damage to algae culture due to rupture, nor damage by grazers, which would attribute large losses in biomass production35. The recorded biomass growth after 70 days of cultivation, was more than 9 times (wet and dry) the original biomass sown, obtaining an average weight of 1600 g (144 g dry biomass) per propagule. In this period the % DGR was 6.5 ± 0.25% g.day–1.
The growth was more accelerated in the exponential phase (from day 20 to 33), reaching biomasses of about 1150 g (108 g dry biomass). During period II (initial to 33 days) the % DGR was 15.2 ± 0.62% g.day–1, which was more than twice if the algae were harvested after 70 days and 5 times more if the seaweed was harvested after 20 days. Slow growth in the first 20 days and after day 33 is characteristic in the culture of this species36. This suggests that the cultured biomass should be harvested after 30–35 days from out planting, when the highest growth rates are found37. Harvest time depends on the product yield and quality, and certain authors report that at a time close to a month after sowing, when the extraction of algae products (e.g. carrageenan) is feasible and recommended36,37. While, it has been documented that K. alvarezii has a lower concentration of carrageenan when harvested in a shorter period, other studies49,50,51 have recommended harvesting over longer periods of time, about 45 days after sowing.
When comparing the % DGR of the present study harvesting K. alvarezii at 33 days, with previous studies in other tropical and subtropical regions (Table 3), the daily growth rate obtained for K. alvarezii growth (15.2% g.day–1) exceed all previous reports, either from studies carried out in the Atlantic (in Brazil, with the highest rate of 8.9% g.day–1) or in Asia with highest reported in India of 14% g.day–1, but in several other countries did not exceed 10.8% g.day–1. The high growth rate detected in the current study, in combination with environmental and littoral geography conditions of an extensive marine coastline (670 km) allow us to elucidate the positive biomass conditions for macroalgae production in Ecuador. Moreover, the possibility of culturing K. alvarezii in the upper estuary zone of Ecuador, where there is already a high level of aquaculture activity by coastal communities, reinforce the relevance of introducing the production of new species to diversify the aquaculture of the entire tropical Pacific region, in particular in Ecuador, where more than 95% is directly linked to shrimp farming1.
As such, to support the background idea of farming K. alvarezii and other macroalgae species in the region, more studies generating further evidence data should be promoted on the topic of assessing the production of macroalgae as one of the strategies to reduce the effects of climate change, counteract eutrophication and the crisis of biodiversity lost52. Given that macroalgae aquaculture is nowadays widely recognised as a strategic pathway to achieve a blue economy to meet more sustainability objectives53,54, the cultivation of macroalgae across the tropics, should be considered as one of main focus of public policies where the government, academic and private sector sectors must interact.
In our experimental design, although environmental factors related to the growth of macroalgae were recorded, there were no correlations detected with growth rates, except for the previously underlined intermediate II period, for which a positive correlation between increased temperature and higher biomass was observed. While temperature is a factor that modulates growth in aquatic organisms, including macroalgae and particularly photosynthesis55, the difference among the studied periods did not exceed 2 °C, which was possibly not decisive in causing notable physiological effects in the macroalgae. This suggests that differences in growth rate might be associated with endogenous factors of post-adaptability of the algae, after its initial growth phase56. However, an important feature of the higher % DGR found in K. alvarezii cultures in Vietnam, is that the temperature was notably higher (33 °C) than the one recorded in the present study, which reflects the higher metabolism at high temperatures of this species. Further studies are necessary to understand the effect of temperature in tropical ranges on this algae species. Though the species can grow at lower temperatures, such as those occurring in the subtropics (17–31 °C), its production is lower (e.g. South Japan41 and Bahía de Ubatuba in Brazil45,46) than those observed in tropical areas, where the temperature is much higher and less variable4,14,26,36,57,58.
Regarding salinity measurements in our study, the culture in the province of Santa Elena was developed in a range of 30–35 ‰, which is considered optimal for K. alvarezii8,9. The chemical nutrients dissolved in the water measured as NH4+ and PO43–, were higher than the minimum required values of 0.3 to 0.6 ppm, both in NH4+, NO2– and NO3–11,12,13,14, and (PO43–) 0.009–0.05 ppm, previously reported for a good development of the macroalgae59, which contributed to the good performance of the algae under cultivation. Both, the sowing density, and the depth of the cultivation set up, are also determining factors in productivity, and must be considered as one of the main factors affecting farm productivity49,50.
Quantification of the carrying capacity of an aquaculture system is important because its scale will determine the impact on the hydrodynamics of the area, the risk of spread of pests and diseases, as well as the probability of eutrophication due to the decomposition of the biomass60. However, eutrophication is an unlikely factor from the cultivation of this species as the biomass that breaks off and washes up on the shore is usually harvested due to its high commercial value. The calculations of the carrying capacity was based on multiple models and was adjusted to specific characteristics of the species and site where the project was performed61,62, the little information concerning to the cultivation of seaweeds is a limiting factor when comparing our results. For example, a recent work by Gomes Da Silva et al.63 showed that a plant cover area of 2 ha is considered by the Brazilian Government to have a low ecological impact due to the oceanographic characteristics in the Southeast region of that country. Being the area of this study less than the determined cultivation capacity (8.6 ha) for Santa Elena, and considering a maximum load capacity of 86.15%, would allow a production of 121.3 T in dry weight of algae for the total concession area of 10 ha.
Until now, the economic viability for this species has mainly been reported for “family scale” developments < 0.5 ha. For example, in Colombia where the internal rate of return (IRR) was 65%22, and in Brazil between 38.1 and 87.8%64. However, Nogueira and Henriques64, concluded that the financial unfeasibility for large-scale macroalgae production in Brazil, is because of the required plant cover area and current legislation. New economic models in multitrophic cultures of Kappaphycus algae with bivalves63 suggest features like those here reported, showing financial feasibility with an annual production of 121.3 T in dry weight of K. alvarezii, a period of recovery of 4.3 years of the investment and a rate of return of 16.46%. This activity generates an important socio-economic contribution to the sector (mainly constituted of associations of artisanal fishermen) since it guarantees the use of 259,200 man-hours for its development. Unlike Nogueira & Henriques64, this study demonstrates that in Ecuador the cultivation of macroalgae on a large scale is possible, based on financial viability, carrying capacity of the site and current legislation.
To our knowledge, this is the first report on the cultivation of K. alvarezii and its feasibility in the Tropical Eastern Pacific waters. It was observed that the algae increased its biomass by more than 7 times after 33 days of cultivation, with an average daily growth rate of 15.2% g.day–1. These values are almost three times higher than those proposed as suitable for commercial cultivation of eucheumatoides seaweeds worldwide16. The productivity and growth rates show the biological feasibility of K. alvarezii cultivation in the province of Santa Elena under the previously described conditions of temperature, salinity, and nutrients.
The load capacity established in the study area was 53 floating rafts in total, with a profitability of 67%. Although this profitability seems to be low, it is higher than that established by Gomes Da Silva et al.63 in multitrophic culture (K. alvarezii with Perna perna, and Nodipecten nodosus) carried out in the state of Santa Catarina, Brazil. The data supplied for the economic feasibility exercise is real and comes from government support for the “Mariculture Macroproject on the Ecuadorian Coast”, which is subject to various administrative control procedures that normally slow down and increase the cost of the initial investment, so the projection of costs in the present study could be overestimated.
We recommend continuing with the evaluation and refinement of K. alvarezii cultivation practices in the same study region and similar areas along the coast of Ecuador. Studies should include optimization of mass, number and seeding distance of propagules, control of biofouling, improvement of the product, etc. together with more detailed studies on phytopathology, product quality and commercialization. Special emphasis should be given to social inclusion, particularly in the active participation of women in the cultivation. The effect of different environmental factors on the culture should be evaluated before managing a large-scale commercialization phase, based on a K. alvarezii mariculture establishment with an adequate social and environmental impact within the framework of productive sustainability. Finally, given the systemic services that macroalgae can generate, we encourage and recommend to focus efforts on carbon sink studies for K. alvarezii cultures, as well as evaluating the effect on eutrophication reduction from discharge water systems from the shrimp industry and other similar discharge systems.
Data availability
Data supporting the conclusions of this study are available from the corresponding author upon request.
References
FAO The state of world fisheries and aquaculture 2022. In Towards Blue Transformation (FAO, 2022). https://doi.org/10.4060/cc0461en.
Campbell, I. et al. The environmental risks associated with the development of seaweed farming in Europe—Prioritizing key knowledge gaps. Front. Mar. Sci. 6, 107. https://doi.org/10.3389/fmars.2019.00107 (2019).
Langton, R., Augyte, S., Price, N., Forster, J., Noji, T., Grebe, G., Gelais A. S. & Byron CJ. An ecosystem approach to the culture of seaweed. NOAA technical memoirs. NMFS-F/SPO-195, pp 24 https://repository.library.noaa.gov/view/noaa/35734 (2019).
Muñoz, J., Freile-Pelegrín, Y. & Robledo, D. Mariculture of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) color strains in tropical waters of Yucatán México. Aquaculture 239, 161–177. https://doi.org/10.1016/j.aquaculture.2004.05.043 (2004).
Shah, M. D. et al. Therapeutic potential and nutraceutical profiling of north bornean seaweeds: A review. Mar. Drugs 20, 101. https://doi.org/10.3390/md20020101 (2022).
Rodgers, S. K. & Cox, E. F. Rate of spread of introduced Rhodophytes Kappaphycus alvarezii, Kappaphycus striatum, and Gracilaria salicornia and their current distributions in Kane ‘ohe Bay, O‘ahu, Hawai‘i. Pac. Sci. 53, 232–241 (1999).
Ohno, M., Nang, H. Q. & Hirase, S. Cultivation and carrageenan yield and quality of Kappaphycus alvarezii in the waters of Vietnam. J. Appl. Phycol. 8, 431–437. https://doi.org/10.1007/bf02178588 (1996).
FAO. Cultured aquatic species information programme Eucheuma spp. cultured aquatic species fact sheets. FAO Fish. Aquac. Dep. 1, 1–13 (2005).
Reis, R. P., Loureiro, R. R. & Mesquita, F. S. Does salinity affect growth and carrageenan yield of Kappaphycus alvarezii (Gigartinales/Rhodophyta)?. Aquac. Res. 42, 1231–1234. https://doi.org/10.1111/j.1365-2109.2010.02699.x (2010).
Dawes, C. J., Lluisma, A. O. & Trono, G. C. Laboratory and field growth studies of commercial strains of Eucheuma denticulatum and Kappaphycus alvarezii in the Philippines. J. Appl. Phycol. 6, 21–24. https://doi.org/10.1007/BF02185899 (1994).
Glenn, E. P. & Doty, M. S. Growth of the seaweeds Kappaphycus alvarezii, K. striatum and Eucheuma denticulatum, as affected by environment in Hawaii. Aquaculture 84, 245–255. https://doi.org/10.1016/0044-8486(90)90090-A (1990).
Doty, M. S. & Norris, J. N. Eucheuma species (Solieriaceae, Rhodophyta) that are major sources of carrageenan. In Taxonomy of Economic Seaweeds with Reference to Some Pacific and Caribbean Species (eds Abbott, I. A. & Norris, J. N.) (California Sea Grant College Program, 1985).
Luxton, D. M. Aspects of the farming and processing of Kappaphycus and Eucheuma in Indonesia. Hydrobiologia 260, 365–371. https://doi.org/10.1007/BF00049042 (1993).
Areces, A. J. Cultivo comercial de carragenófitas del género Kappaphycus Doty, 529–550. In: Alveal, K., M. E. Ferrario, E. C. Oliveira y E. Sar (Eds). Phycological Methods Manual. Univ. Concepción, 863 (1995).
Setthamongkol, P., Tunkijjanukij, S., Satapornvanit, K. & Salaenoi, J. Growth and nutrients analysis in marine macroalgae. Kasetsart J. Nat. Sci. 49, 211–218 (2015).
Ask, E. & Azanza, V. Advances in the cultivation of commercial eucheumatoide species: A review with suggestions for future research. Aquaculture 206, 257–277. https://doi.org/10.1016/S0044-8486(01)00724-4 (2002).
Tanaka, H. Foreword. In: Adams, T., Foscarini, R. (Eds) Proceeding of Regional Workshop on Seaweeds Culture and Marketing. South Pacific Aquaculture Development Project, Food and Agriculture Organisation of The United Nations, pp. piii–iv (1990).
Hurtado, A. Q., Neish, I. C. & Critchley, A. T. Phyconomy: The extensive cultivation of seaweeds, their sustainability and economic value, with particular reference to important lessons to be learned and transferred from the practice of eucheumatoid farming. Phycologia 58, 472–483. https://doi.org/10.1080/00318884.2019.1625632 (2019).
de Paula, E. J., Pereira, R. T. L. & Ohno, M. Strain selection in Kappaphycus alvarezii var. alvarezii (Solieriaceae, Rhodophyta) using tetraspore progeny. J. Appl. Phycol. 11, 111–121. https://doi.org/10.1023/A:1008085614360 (1999).
Rincones, R. E. & Rubio, J. N. Introduction and commercial cultivation of the red alga Eucheuma in Venezuela for the production of phycocolloids. World Aquacult. Mag. 30, 57–61 (1999).
Smith, A. H. & Rincones, R. E. Seaweed Resources of the Caribbean. In World Seaweed Resources: An authoritative reference system [DVD-ROM] (eds Critchley, A. T. et al.) (University of the Netherlands, 2006).
Rincones, R. E. & Moreno, D. A. Technical and economical aspects for the commercial establishment of seaweed mariculture in Colombia: Experiences in the Guajira Peninsula. Ambiente y Desarollo 28, 123–144 (2011).
Sepúlveda, M. Environmental File “Experimental Cultivation of Seaweed Macro as a productive and sustainable alternative for Artisanal Fishermen of Ecuador” (2014).
Montúfar, M. & Avendaño, U. Technical report for recategorization of the Macroalga Kappaphycus alvarezii in Ecuador. Instituto Nacional de Pesca (2017).
Instituto Publico de Investigación de Acuicultura y Pesca (IPIAP), Mariculture of macroalgae (Kappaphycus alvarezii) in the first nautical mile of the coastal zone of the province of Santa Elena, Ecuador. Acta de reunión acuicultura-01–2017 (2017).
Kasim, M. & Mustafa, A. Comparison growth of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) cultivation in floating cage and longline in Indonesia. Aquac. Rep. 6, 49–55. https://doi.org/10.1016/j.aqrep.2017.03.004 (2017).
Yong, Y. S., Yong, W. T. L. & Anton, A. Analysis of formulae for determination of seaweed growth rate. J. Appl. Phycol. 25, 1831–1834. https://doi.org/10.1007/s10811-013-0022-7 (2013).
Azis, H. Y. [Optimizing seaweed processing in the coastal areas of Bantaeng in South Sulawesi]. PhD Dissertation, Postgraduate School, Bogor Agricultural University. Bogor [In Indonesian], 163. https://docplayer.info/40296530-Optimasi-pengelolaan-sumberdaya-rumput-laut-di-wilayah-pesisir-kabupaten-bantaeng-provinsi-sulawesi-selatan-hasni-yulianti-azis.html (2011).
Shang, Y. C. Aquaculture Economic Analysis: An Introduction (The World Aquaculture Society, 1990).
Engle, C. R. Aquaculture Economics and Financing Management and Analysis 1st edn. (Wiley Blackwell, 2010).
Johnston, B., Kishore, P., Vuibeqa, G. B., Hine, D. & Southgate, P. C. Economic assessment of community-based pearl oyster spat collection and mabé pearl production in the western Pacific. Aquaculture 514, 734505. https://doi.org/10.1016/j.aquaculture.2019.734505 (2020).
Konda, N. V. S. N. M., Singh, S., Simmons, B. A. & Klein-Marcuschamer, D. An investigation on the economic feasibility of macroalgae as a potential feedstock for biorefineries. BioEnergy Res. 8, 1046–1056. https://doi.org/10.1007/s12155-015-9594-1 (2015).
Zar, J. H. Biostatistical Analysis 5th edn, 944 (Prentice Hall, 2010).
Hsu, J. C. Multiple Comparisons: Theory and Methods (CRC Press, 1996).
Russell, D. J. Ecology of the imported red seaweed Eucheuma striatum Schmitz on Coconut Island, Oahu, Hawaii. Pac. Sci. 37, 87–108 (1983).
Budiyanto, K. F. & Abadi, S. Y. Growth and carrageenan content of local and tissue culture seed of Kappaphycus alvarezii cultivated in floating cage. AACL Bioflux 12, 167–178 (2019).
Sudjiharno Teknologi Budidaya Rumput Laut (Lampung: Balai Budidaya Laut), 91 (2001).
Glenn, E. P. & Doty, M. S. Water motion affects the growth rates of Kappaphycus alvarezii and related red seaweeds. Aquaculture 108, 233–246 (1992).
Li, R., Li, J. & Wu, C. Effect of ammonium on growth and carrageenan content in Kappapycus alvarezii (Gigartinales, Rhodophyta). Hydrobiologia 204, 499–503 (1990).
Hurtado-Ponce, A. Q. Cage culture of Kappaphycus alvarezii var. Tambalang (Gigartinales, Rhodophyceae). J. Appl. Phycol. 4, 311–313 (1992).
Ohno, M., Largo, D. B. & Ikomoto, T. Growth rate, carrageenan yield and gel properties of culture kappa-carrageenan producing red alga Kappaphycus alvarezii (Doty) Doty in the subtropical water oh Shikoku, Japan. J. Appl. Phycol. 6, 1–5. https://doi.org/10.1007/BF02185896 (1994).
Hurtado-Ponce, A. Q. Carrageenan properties and proximatencomposition of three morphotypes of Kappaphycus alvarezii Doty (Gigartinales Rhodophyta) grown at two depths. Bot. Mar. 38, 215–219 (1995).
Hurtado, A. Q., Agbayani, R. F., Sanares, R. & Castro-Mallare, M. T. R. The seasonality and economic feasibility of cultivating Kappaphycus alvarezii in Panagatan Cays, Caluya, Antique Philippines. Aquaculture 199, 295–310 (2001).
Eswaran, K., Ghosh, P. K. & Mairh, O. P. Experimental celd cultivation of Kappaphycus alvarezii (Doty) Doty.ex.P. Silva at Mandapam region. Seaweed Res. Util. 24, 67–72 (2002).
de Paula, E. J., Pereira, R. T. L. & Ohno, M. Growth rate of the carrageenophyte Kappaphycus alvarezii (Rhodophyta, Gigartinales) introduced in subtropical waters of São Paulo State, Brazil. Phycol. Res. 50, 1–9. https://doi.org/10.1046/j.1440-1835.2002.00248.x (2002).
Hayashi, L., de Paula, E. J. D. & Chow, F. Growth rate and carrageenan analyses in four strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales) farmed in the subtropical waters of Sao Paulo State, Brazil. J. Appl. Phycol. 19, 393–399 (2007).
Rao, P. V., Kumar, K. S., Ganesan, K. & Thakur, M. C. Feasibility of cultivation of Kappaphycus alvarezii (Doty) Doty at different localities on the Northwest coast of India. Aquac. Res. 39, 1107–1114. https://doi.org/10.1111/j.1365-2109.2008.01976.x (2008).
Kasin, M. & Mustafa, A. Comparison growth of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) cultivation in floating cage and longline in Indonesia. Aquac. Rep. 6, 49–55. https://doi.org/10.1016/j.aqrep.2017.03.004 (2017).
Neish, I. C. The ABC of Eucheuma Seaplant Production Agronomy, Biology and Crop-handling of Betaphycus, Eucheuma and Kappaphycus the Gelatinae, Spinosum and Cottonii of Commerce. Monograph #1-0703 Surialink (2003).
Neish, I. C. Good agronomic practices for Eucheuma and Kappaphycus seaplants: Including an overview of basic biology. SEAPlant.net Monograph no. HB2F 1008 V3 GAP. (2008).
Rincones, R. E. Manual para el cultivo experimental de la macroalga Kappaphycus alvarezii como alternativa productiva y sostenible para los pescadores artesanales del Ecuador proyecto “maricultura y piscicultura para el fomento acuícola en el Ecuador” Subsecretaría de Acuicultura, MAGAP, 127 (2016).
Arzeno-Soltero, I. B. et al. Large global variations in the carbon dioxide removal potential of seaweed farming due to biophysical constraints. Commun. Earth Environ. 4, 185. https://doi.org/10.1038/s43247-023-00833-2 (2023).
Duarte, C. M., Bruhn, A. & Krause-Jensen, D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 5, 185–193. https://doi.org/10.1038/s41893-021-00773-9 (2022).
Bhushan, S., Veeragurunathan, V., Kavale, M. G., Jaiswar, S. & Mantri, V. A. Regulatory ecosystem services through large-scale commercial farming of Kappaphycus alvarezii: Pan-India potential estimates. J. Appl. Phycol. 35, 1945–1956. https://doi.org/10.1007/s10811-023-03001-3 (2023).
Davison, I. R. Environmental effects on algal photosynthesis: Temperature. J. Phycol. 27, 2–8. https://doi.org/10.1111/j.0022-3646.1991.00002.x (1991).
Terada, R. et al. The effect of irradiance and temperature on the photosynthesis and growth of a cultivated red alga Kappaphycus alvarezii (Solieriaceae) from Vietnam, based on in situ and in vitro measurements. J. Appl. Phycol. 28, 457–467. https://doi.org/10.1007/s10811-015-0557-x (2015).
Villanueva, R. D., Romero, J. B., Montano, M. N. E. & de la Pena, P. O. Harvest optimization of four Kappaphycus species from the Philippines. Biomass Bioenergy 35, 1311–1316. https://doi.org/10.1016/j.biombioe.2010.12.044 (2011).
Abdullah, N., Wibowo, E. S., Irfan, M., Muchdar, F. & Malan, S. Seaweed Kappaphycus alvarezii cultivation using longline method in Kastela waters, Ternate Island, Indonesia. AACL Bioflux 13, 2336–2342 (2020).
Parakkasi, P., Rani, C., Syam, R. & Zainuddin & Achmad, M. Growth response and quality of seaweed Kappaphycus alvarezii cultivated in various coastal ecosystems in the waters of West Sulawesi, Indonesia. AACL Bioflux 13, 627–639 (2020).
Roberts, T. & Upham, P. Prospects for the use of macro-algae for fuel in Ireland and the UK: An overview of marine management issues. Mar. Policy 36, 1047–1053. https://doi.org/10.1016/j.marpol.2012.03.001 (2012).
Ferreira, J. G., Hawkins, A. J. S. & Bricker, S. B. Management of productivity, environmental effects and profitability of shellfish aquaculture—The Farm Aquaculture Resource Management (FARM) model. Aquaculture 264, 160–174. https://doi.org/10.1016/j.aquaculture.2006.12.017 (2007).
Cubillo, A. M. et al. Role of deposit feeders in integrated multi-trophic aquaculture—A model analysis. Aquaculture 453, 54–66. https://doi.org/10.1016/j.aquaculture.2015.11.031 (2016).
Gomes, E., Castillo-Barros, L. & Henriques, M. B. Economic feasibility of integrated multi-trophic aquaculture (mussel Perna perna, scallop Nodipecten nodosus and seaweed Kappaphycus alvarezii) in Southeast Brazil: A small-scale aquaculture farm model. Aquaculture 552, 738031. https://doi.org/10.1016/j.aquaculture.2022.738031 (2022).
Nogueira, M. C. F. & Henriques, M. B. Large-scale versus family-sized system production: Economic feasibility of cultivating Kappaphycus alvarezii along the southeastern coast of Brazil. J. Appl. Phycol. 32, 1893–1905. https://doi.org/10.1007/s10811-020-02107-2 (2020).
Acknowledgements
To Instituto Público de Investigación de Acuicultura y Pesca; Cooperativa de Pesca Artesanal “Santa Rosa de Salinas”; Viceministerio de Acuicultura y Pesca; Ministerio de Ambiente, Agua y Transición Ecológica; Ministerio de Obras Públicas y Transporte Marítimo; Secretaría de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT) (Agreement No. CZ05-000735-2018); Agencia Nacional de Investigación y Desarrollo (ANID)—Subdirección de Capital Humano/Doctorado Nacional (Agreement No. 2021-21210630) and AquaCEAL Corporation (Agreement No. CEAL-2022-001); or funding the study. To Jose Alió for reviewing the manuscript.
Author information
Authors and Affiliations
Contributions
M.M.R. conceived the study design, carried out fieldwork and sample collection, data analysis and drafting; R.R. participated in its design, carried out field work and sample collection and proofreading of the final manuscript; L.B.C.F. and M.M.E.V. data analysis, performed economic analysis and draft of the manuscript; U.A. carried out field work and sample collection; LC. participated in its design; J.T.C. methodology design, carried out field work and sample collection; W.R. participated in its design; W.R. participated in its design; U.A. carried out field work and sample collection; C.L., A.A.N. and L.C.F. visualization, data analysis, draft of the manuscript and proofreading of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montúfar-Romero, M., Rincones-León, R.E., Cáceres-Farias, L.B. et al. Feasibility of aquaculture cultivation of elkhorn sea moss (Kappaphycus alvarezii) in a horizontal long line in the Tropical Eastern Pacific. Sci Rep 13, 14751 (2023). https://doi.org/10.1038/s41598-023-41795-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41795-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.