Abstract
Severe anemia is an important contributor to mortality in children with severe malaria. Anemia in malaria is a multi-factorial complication, since dyserythropoiesis, hemolysis and phagocytic clearance of uninfected red blood cells (RBCs) can contribute to this syndrome. High levels of oxidative stress and immune dysregulation have been proposed to contribute to severe malarial anemia, facilitating the clearance of uninfected RBCs. In a cohort of 552 Ugandan children with severe malaria, we measured the levels of xanthine oxidase (XO), an oxidative enzyme that is elevated in the plasma of malaria patients. The levels of XO in children with severe anemia were significantly higher compared to children with severe malaria not suffering from severe anemia. Levels of XO were inversely associated with RBC hemoglobin (ρ = − 0.25, p < 0.0001), indicating a relation between this enzyme and severe anemia. When compared with the levels of immune complexes and of autoimmune antibodies to phosphatidylserine, factors previously associated with severe anemia in malaria patients, we observed that XO is not associated with them, suggesting that XO is associated with severe anemia through an independent mechanism. XO was associated with prostration, acidosis, jaundice, respiratory distress, and kidney injury, which may reflect a broader relation of this enzyme with severe malaria pathology. Since inhibitors of XO are inexpensive and well-tolerated drugs already approved for use in humans, the validation of XO as a contributor to severe malarial anemia and other malaria complications may open new possibilities for much needed adjunctive therapy in malaria.
Similar content being viewed by others
Introduction
Malaria is a major cause of anemia in the areas of the world where this disease is endemic1. Severe anemia is a common complication of malaria, which occurs most frequently in children under the age of five and is a major contributor of death by malaria1. Although mortality associated with severe anemia in children with malaria is low with appropriate transfusion, unreliable blood availability in hospitals frequently results in avoidable deaths2. Additionally, children with severe malarial anemia (SMA) are at risk of repeated malarial episodes and increased post-discharge mortality3.
Malaria-induced anemia involves both dyserythropoiesis and increased loss of infected and uninfected red blood cells (RBCs)1,4. While Plasmodium infection of RBCs can lead to destruction and clearance of these cells, the majority of RBCs lost during Plasmodium infection are uninfected RBCs. For each RBC lysed directly due to Plasmodium infection, about eight uninfected erythrocytes are lost in P. falciparum5,6 infections. Although the mechanisms underlying the loss of uninfected RBCs during malaria are not completely understood, two mechanisms have been proposed: (1) increased RBCs rigidity from oxidative stress leads to subsequent splenic clearance of these cells7,8, and (2) immune dysregulation resulting in binding of antibodies and immune complexes (IC) to uninfected RBCs9,10 together with complement dysfunction11,12 would contribute to increased uptake by macrophages.
Oxidative stress is elevated during malaria and has been associated with different complications13, including anemia7,14, but also respiratory distress and acute kidney injury, as well as mortality after 6 months15,16. Here we have determined the levels of xanthine oxidase (XO), an oxidative enzyme not found in RBC17 that is upregulated during malaria18, in a cohort of Ugandan children with severe malaria. We have observed a highly significant correlation between the levels of this enzyme and severe anemia in these patients. We also measured the levels of other immune-related parameters previously described to correlate with SMA: anti-phosphatidylserine (anti-PS) antibodies19,20,21,22,23, anti-DNA antibodies21,23 and immune complexes (IC)10, finding that they are only weakly associated with the levels of XO. Taken together, these results indicate that XO is associated with SMA independently of autoimmune-related mechanisms. A significant association of XO levels and other common complications of severe malaria suggests a possible broad relation of XO with malaria-induced pathology.
Methods
Study population
Ethics and consent to participate: Written informed consent was obtained from parents or guardians of study participants. Ethical approval was granted by the Institutional Review Boards for human studies at Makerere University School of Medicine and Indiana University School of Medicine. All methods were performed in accordance with the relevant guidelines and regulations.
As described before24, between 2014 and 2017, 600 children with severe malaria were enrolled in the study designed to assess cognition in children < 4 years of age at 12 months’ follow-up25. Children were eligible if they: (1) were between 6 months and 4 years of age; (2) had diagnostic evidence of malaria with either a positive rapid diagnostic test for P. falciparum histidine-rich protein-2 (HRP2) or direct visualization of parasites by Giemsa microscopy; (3) required hospitalization; and (4) had 1 or more of the following severe malaria criteria: coma (Blantyre coma score < 3), respiratory distress (deep acidotic breathing or lower chest wall retractions), multiple seizures (≥ 2 generalized seizures in 24 h or a seizure > 30 min in duration), severe anemia (hemoglobin ≤ 5 g/dL), or prostration (≥ 1 year, unable to sit unsupported or stand; < 1 year, unable to breastfeed). Children were recruited at 2 referral hospitals in Central and Eastern Uganda: Mulago National Referral Hospital in Kampala and Jinja Regional Referral Hospital in Jinja. Exclusion criteria included the following: known chronic illness requiring medical care, history of coma, head trauma, known developmental delay, cerebral palsy, or prior hospitalization for malnutrition. Delayed exclusion criteria included an elevated cerebrospinal fluid white blood cell count in a child with coma. Children with severe malaria returned for an interim health assessment and blood draw at one-month follow-up.
For controls, 120 community children from the nuclear family, extended family, or household area of the children with severe malaria were enrolled. Additional exclusion criteria for the community children included an illness requiring medical care within the previous four weeks, a major medical or neurologic abnormality at screening physical examination, or active illness. Samples from 98 children in this group were used for this study. Samples from 11 children in this group presented positive levels of HRP2 (> 100 ng/mL), of which five were confirmed by Giemsa microscopy.
Other clinical complication definitions: Acute kidney injury (1.5-fold increase in creatinine over estimated baseline or a 0.3-mg/dL change in creatinine within 48 h)26; Acidosis: base deficit of > 8 mmol/L or, if unavailable, a plasma bicarbonate of < 15 mmol/L or venous lactate of > 5 mmol/L27.
At enrollment, study participants had serum collected for long-term storage at − 80 °C. All children had a complete blood count, blood smear to quantify malaria, stool testing for helminth infection, and were tested for HIV. At one month follow up the children with severe malaria returned for a health assessment and had serum collected and stored at − 80 °C.
Measurement of XO activity
XO activity in plasma samples from this cohort was measured using the Amplex Red Xanthine/Xanthine Oxidase Assay kit (Invitrogen A22182) according to the manufacturer’s instructions. Briefly, plasma samples (10 µl) taken from children upon hospital admission were incubated with hypoxanthine, the substrate of XO, Amplex Red reagent, and horseradish peroxidase (HRP). XO breaks down hypoxanthine into xanthine, which is then broken down into uric acid, and generates reactive oxygen species (ROS) along the way. In the presence of HRP, Amplex Red reacts with ROS to generate a fluorescent product (Excitation-530 nm/Emission-590 nm) that was quantified using a plate reader (Victor X3 from Perkin Elmer). ROS production was measured at different time points (1 h, 2 h, and for some samples also at 30 min) after the start of the reaction. ROS production in plasma from five healthy individuals were averaged and used as a reference to calculate relative units (RU) for each determination. RU at the different time points was averaged for each plasma sample and reported as the final XO activity. To determine XO levels at the one-month follow-up, we first selected the 100 patients with the highest levels of XO activity in plasma upon admission and determined XO levels from the 67 that had follow-up samples, all from children with severe malaria.
Measurement of anti-PS and anti-DNA levels
Costar 3590 96-well ELISA plates were coated for16h at 4 °C with 100 µL of PS (Sigma) at 20 μg/ml in 200-proof Molecular Biology ethanol or calf thymus DNA (Sigma) at 10 μg/ml in PBS. DNA plates were allowed to evaporate at RT after coating. Plates were washed 5 times with 200 µL of PBS and then blocked for 1 h with 200 µL of PBS 3% BSA. Plasma from patients was diluted at 1:100 in PBS 1% BSA and incubated for 2 h at 37 °C. Plates were washed again five times and incubated with anti-human IgG-HRP (GE Healthcare) for 1 h at 37 °C. Plates were washed five more times and TMB substrate (BD Biosciences) was added until the desired color was obtained. The reaction was stopped by with Stop buffer (Biolegend) and absorbance was read at 450 nm. Positive and negative controls were the same used for XO determinations. The mean OD at 450 nm from triplicate wells was compared with the same dilution of the reference positive serum to calculate relative units (RU). Anti-PS and anti-DNA levels were determined in all available samples at one-month follow-up (n = 443).
Measurement of IC levels
Costar 3590 96-well ELISA plates were coated with 100 µL of C1q capture antibody (1 µg/mL) (Thermofisher PA5-29586) in sodium bicarbonate-sodium carbonate buffer (pH 9.6, 50 mM, Bioworld 40520017-1) overnight at 4 °C. Plates were washed 2 times with PBS 0.05% Tween 20 washing buffer and incubated with 100 µL 0.1% BSA in PBS blocking buffer for 1.5 h at RT. Plasma samples were diluted 1:80 in 0.1% blocking buffer. 100µL were added to the wells and incubated for 2 h at RT. Plates were washed 2 times and incubated with 100uL polyclonal goat anti-human IgG-HRP diluted 1:5000 (Invitrogen) for 1 h at RT. Plates were washed 3 times before developing with TMB substrate as described for anti-PS. Positive and negative controls were the same used for XO determinations. The mean OD at 450 nm from triplicate wells was compared with the same dilution of the reference positive serum to calculate relative units (RU).
Statistical analysis
Data were analyzed using Microsoft Excel, Graph Pad Prism and the StatsModels Python library. Differences in continuous variables between SMA group were analyzed using Wilcoxon rank sum analysis with Bonferonni correction to adjust for multiple testing. Differences in categorical variables were assessed using Fisher’s exact test or Pearson’s Chi squared test, as appropriate. Spearman’s rho was used to evaluate the correlation between the measured parameters and different clinical parameters. To evaluate differences in biomarker levels at admission and one-month follow-up, Wilcoxon signed rank test was used. To assess the interaction between XO levels and IC and anti-PS, we fitted two logistic regression models including the XO:IC and XO:anti-PS interaction terms, respectively. The p-value of the interaction term coefficient was considered as a measure of significant interaction. In addition, the log-likelihood of the fitted models was compared, correspondingly, to that of an additive model that did not include the interaction term. The log-likelihood ratio test was to evaluate model improvement upon inclusion of the interaction term.
Results
In this study, we analyzed 650 plasma samples from Ugandan children under 4 years old. This cohort included 552 children hospitalized with a clinical diagnosis of severe malaria24,28, of which 251 presented with severe anemia (hemoglobin ≤ 5 g/dL), and 98 community children (Fig. 1). The present evaluation for SMA includes all children with severe malaria in the study across any category who had a hemoglobin of ≤ 5 g/dL, so could, for example, include some children with coma or multiple seizures. The children classified as “severe malaria, non-SMA”, had a hemoglobin of > 5 g/dL and one or more other complications of severe malaria. The community children were comparable in age and sex to the children with severe malaria (Table 1). In addition, children with severe anemia had lower parasite density but comparable parasite biomass to children without severe anemia.
We conducted further analysis where we assessed clinical and laboratory indicators of hemolysis, and observed that children with SMA had lower RBC hemoglobin, lower levels of hemoglobin scavenger haptoglobin, and higher levels of lactate dehydrogenase (LDH), and heme-oxygenase 1 (HO-1), measures of RBC lysis and oxidative response, than community children or children with severe malaria but without SMA (Table 1).
XO, anti-PS and IC are associated with SMA
A central goal of this study was to investigate whether the levels of XO are associated with severe anemia. We observed that children with SMA had significantly increased levels of XO compared to community controls and to children with severe malaria without SMA (Table 2). We also confirmed previous observations in other cohorts that both anti-PS antibodies and IC were associated with SMA10,19,20,21,22,23. However, we did not observe a significant association between anti-DNA antibodies and SMA and this parameter was excluded from further analysis related to SMA. When we performed these analysis using a more restrictive definition of SMA (which does not include children with P. falciparum densities lower than 10,000 parasites/µl; n = 96), we still observed increased levels of XO and anti-PS in this group compared to controls and children with severe malaria without SMA (Table S1).
To further investigate the relation of the measured parameters with severe anemia, a logit model based on XO levels, anti-PS levels, and an XO:anti-PS interaction term showed that the latter did not significantly contribute to the SMA outcome (XO:anti-PS: p = 0.14; XO: p < 10−3; anti-PS: p = 0.009). Moreover, this model was not significantly better than an additive model where the interaction was not present (log-likelihoods ratio test: chi-squared = 2.25, p = 0.13). Similar results were observed with a model based on XO and IC (XO:IC: p = 0.076; XO: p = 0.001; IC: p < 10−3). Overall, these findings indicate that XO, anti-PS and IC levels are independently associated with severe anemia during malaria.
Relationship between XO, anti-PS and IC
We observed that the levels of XO are only weakly associated with the levels of anti-PS or IC (Fig. 2), suggesting that XO relation to severe anemia is independent of IC and autoimmune antibodies. Conversely, we observed a strong correlation between the levels of anti-PS and anti-DNA, either at the time of admission or 1 month after discharge, indicating that individual patients tend to have similar levels of the two autoantibodies and suggesting that some patients are more prone to autoreactivity.
Correlation of XO, anti-PS, anti-DNA, and IC levels for all participants. Numbers indicate the Spearman’s ρ between the two indicated variables. Bonferroni-corrected α value is 0.001 (n = 49). The p values for each Spearman’s ρ are shown by yellow asterisks as follows: (*, p < 0.001; **, p < 0.0001).
XO, anti-PS and IC correlate with clinical parameters associated with hemolysis
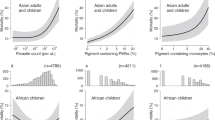
Correlation analysis between the levels of XO, anti-PS and IC, and clinical parameters related to anemia showed that all three are inversely correlated with RBCs hemoglobin, which is the clinical parameter that defines severe anemia (Fig. 3). They also correlate directly with three parameters that are associated with hemolysis: LDH, an enzyme that is released from lysed cells, including RBCs, and is often used as a marker of hemolysis29, HO-1, an enzyme that is upregulated in response to free heme30, and bilirubin, a product of heme degradation31. Haptoglobin binds to free hemoglobin in the circulation and is subsequently cleared. Therefore, decreased haptoglobin levels are associated with hemolysis29,32. An inverse correlation of XO, anti-PS and IC with haptoglobin was also observed (Fig. 3), indicating another association between each of the three parameters and hemolysis.
Correlation of levels of XO, anti-PS, and IC with RBC hemoglobin and parameters related to hemolysis, acidosis, and others. Numbers indicate the Spearman’s ρ between the two indicated variables. Bonferroni-corrected α value is 0.0013 (n = 39). The p values for each Spearman’s ρ are shown by yellow asterisks as follows: (*, p < 0.0013; **, p < 0.0001).
XO correlates with clinical parameters associated with acidosis
We also analyzed the relation of XO, anti-PS and IC with parameters related with acidosis. Multiple factors appear to contribute to acidosis in malaria, both in the buildup and impaired clearance of excess lactate33. To counteract this acidification of the blood, the body uses circulating bicarbonate ions to neutralize lactic acid, but in doing so, generates more carbon dioxide. Consequently, patients with acidosis normally have high levels of circulating lactate and low levels of bicarbonate33.
We observed a significant correlation between XO and acidosis-related parameters (Fig. 3), suggesting a potential relation of this oxidative enzyme to acidosis. An association between anti-PS and lactate, but not base or bicarbonate, was found. No associations were found between acidosis parameters and IC.
Relation to other clinical parameters and malaria complications
Comparing the levels of the measured parameters with clinical data revealed specific associations of each parameter. We observed that XO and anti-PS correlated significantly with parameters related with kidney function: blood urea nitrogen (BUN) and creatinine (Fig. 3), suggesting a possible relation of this oxidative enzyme with kidney damage. There were also a significant correlation between parasite biomass and anti-PS.
We also determined whether the measured parameters were related to specific malaria complications and clinical definitions of disease severity on admission (Table 3). In this table, if a child had the complication, they are included in the category, regardless of other complications they had. For example, most children with coma (cerebral malaria) also had multiple seizures. These children would be included in the coma and multiple seizures categories.
We observed that XO levels were significantly higher in severe malaria patients with acute kidney injury, prostration, acidosis or jaundice, indicating a relation of this enzyme to these severe manifestations of malaria. However, XO levels were not associated with multiple seizures or with the most severe neurological complication, coma (cerebral malaria). IC were also significantly elevated in children with jaundice, indicating a relation of IC to this complication.
Levels of XO and anti-PS after at 1-month follow-up
The levels of XO and anti-PS after 1 month showed a marked decrease compared to the samples collected at hospital admission (p < 0.0001 for both parameters), but still showed significant correlations with the levels at the time of admission (Fig. 2), indicating that levels of these parameters were still elevated for some patients. Median levels of XO in severe patients after 1 month were only slightly elevated when compared with community controls (Table 4), indicating that XO decreases almost to background levels in plasma after recovery. Levels of XO after 1 month still showed a significant difference compared to community controls (Table 4), but the difference was more significant for anti-PS, indicating persistence in plasma that is typical of these antibodies23.
Discussion
Since the oxidative enzyme XO is frequently elevated in malaria patients and correlates with disease severity18, we hypothesized that XO could contribute to severe anemia in malaria patients. The results presented here indicate that the levels of XO in plasma are associated with SMA, but also with hemolysis-related parameters (haptoglobin, LDH, HO-1 and bilirubin), as well as with related complications such as jaundice.
Oxidative stress has been proposed as a contributor to SMA, since it causes peroxidation of lipids on the surface of erythrocytes, reducing the deformability of these cells8, and ultimately leading to their clearance in the spleen7. This hypothesis is supported by previous patient-based studies showing that, in the context of P. falciparum infections, RBCs rigidity was mostly observed in uninfected RBCs and correlated strongly with severe anemia34 and death35. Some potential factors that contribute to the oxidative burden during malaria include elevated levels of oxidative enzymes, free heme released after the lysis of infected and uninfected RBCs, and the phagocytic oxidative burst that is induced after phagocytosis of infected RBCs by innate immune cells13. It is still unclear what is the relative contribution of these factors to the high levels of oxidative stress that are observed in malaria patients, but XO is probably an important contributor, since this enzyme degrades hypoxanthine and xanthine in plasma, releasing high levels of reactive oxygen species. XO is elevated in the blood of malaria patients, correlating with disease severity18 and with inflammatory cytokines36. Our results further support that XO may be an important source of oxidative stress in malaria given its association with severe anemia, hemolysis and acidosis parameters.
Since the relation between XO and anemia is associative, it is possible that XO directly contributes to the loss of RBC, but also that elevated levels of XO are a consequence of anemia or other associated factors. A direct relation involving the release of XO by lysed RBC is not likely since XO activity is not found in RBC17. It is also unlikely that the observed increase in XO levels in patients may be caused by high levels of its substrates hypoxanthine/xanthine in plasma, since the concentration of hypoxanthine in uninfected RBCs that would be released upon lysis is very low (9.3 nM)37 and there is a marked decrease in the levels of hypoxanthine in plasma during P. falciparum infections38, probably caused by the robust transport of hypoxanthine and its precursors into infected RBC39. However, mice studies showed that the enzyme precursor of XO, xanthine dehydrogenase, is upregulated early during Plasmodium infection by the activation of type I IFN receptor40, proposing an immune-related mechanism for the upregulation of XO during malaria. Since hemolysis can induce secretion of type I IFN in liver macrophages41, it is also possible that the correlation between hemolysis and XO levels may be driven by the levels of this cytokine. However, if this was the case, a strong correlation between XO levels and other parameters associated with hemolysis (anti-PS and IC) would have been expected.
Oxidative stress has been proposed to induce SMA by causing increased rigidity of these cells that are subsequently recognized by phagocytes and cleared from the circulation7,8. It is possible that XO contributes to this mechanism, but in this study we did not quantify any parameter specifically associated to RBC phagocytosis in patients. However, we observed a significant association between XO levels and hemolysis parameters (haptoglobin, LDH, HO-1, bilirubin) that are compatible with the hypothesis that lysis of uninfected RBCs is directly induced by XO, as was observed in mice studies in the context of sickle cell disease42.
Other proposed causes of SMA include the binding of autoimmune antibodies to uninfected RBCs1, which increase their susceptibility to phagocytosis9 and their levels are associated with anemia in patients43. Mechanistic studies in a mouse model showed that binding of antibodies to the phospholipid PS, that is exposed in uninfected RBCs during malaria, increases RBCs phagocytosis and delays recovery from anemia20. In patients with P. falciparum and P. vivax infections, levels of anti-PS are associated with anemia19,20,21,22,23. However, we observed that they also correlate with parameters of hemolysis in patients, as described before22, suggesting they may contribute to SMA through both enhancing phagocytic clearance and hemolysis.
IC are also thought to contribute to phagocytosis of uninfected RBCs11 and correlate with SMA10. Here we observed that they correlate with parameters of hemolysis and also with anti-PS and anti-DNA, which probably indicates that part of the circulating IC are formed by these autoantibodies. It has been proposed that IC could contribute to anemia by increasing complement susceptibility of RBCs and increasing phagocytosis44. In this cohort, we also observed a correlation between IC and severe anemia, as well as hemolysis associated parameters.
The observed association of XO with different malaria complications points to a broader detrimental effect of this enzyme in severe malaria. It is possible that XO may contribute to complications such as jaundice and acute kidney injury as a consequence of increased hemolysis, since these complications are associated with it45,46. It is also possible that since XO is a highly oxidative and inflammatory enzyme36, the association with complications such as acute kidney injury may be driven by increased inflammation, which is thought to contribute to this complication47.
The novel finding of an association between XO and different severe malaria complications may open a possibility for adjunctive treatment, provided that a causative effect of XO in malarial anemia could be inequivocally demonstrated. Inexpensive and well-tolerated inhibitors of XO have been developed for the treatment of gout, since this condition is caused by accumulation of urate, the product of XO48. In mice studies, treatment with the XO inhibitor febuxostat reduced hemolysis induced in the context of sickle cell disease42. Given that elevated levels of XO may contribute to the development of severe anemia, treatment with the XO inhibitors allopurinol or febuxostat49, could contribute to alleviate this complication in malaria. Since febuxostat is not approved for use in children by the US Food and Drug Administration, and has a black box warning for adults, allopurinol is likely to be the preferred drug for studies in animals to evaluate prior to human use for malaria.
Data availability
Deidentified data are available from Dr. Chandy John on reasonable request.
References
White, N. J. Anaemia and malaria. Malar. J. 17, 371 (2018).
Bates, I., Hassall, O. & Mapako, T. Transfusion research priorities for blood services in sub-Saharan Africa. Br. J. Haematol. 177, 855–863 (2017).
Kwambai, T. K. et al. Post-discharge morbidity and mortality in children admitted with severe anaemia and other health conditions in malaria-endemic settings in Africa: A systematic review and meta-analysis. Lancet Child Adolesc. Health 6, 474–483 (2022).
Haldar, K. & Mohandas, N. Malaria, erythrocytic infection, and anemia. Hematol. Am. Soc. Hematol. Educ. Progr. https://doi.org/10.1182/asheducation-2009.1.87:87-93 (2009).
Jakeman, G. N., Saul, A., Hogarth, W. L. & Collins, W. E. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 119(Pt 2), 127–133 (1999).
Price, R. N. et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65, 614–622 (2001).
Griffiths, M. J. et al. Oxidative stress and erythrocyte damage in Kenyan children with severe Plasmodium falciparum malaria. Br. J. Haematol. 113, 486–491 (2001).
Becker, K. et al. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 34, 163–189 (2004).
Waitumbi, J. N., Opollo, M. O., Muga, R. O., Misore, A. O. & Stoute, J. A. Red cell surface changes and erythrophagocytosis in children with severe Plasmodium falciparum anemia. Blood 95, 1481–1486 (2000).
Mibei, E. K., Orago, A. S. & Stoute, J. A. Immune complex levels in children with severe Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 72, 593–599 (2005).
Stoute, J. A. et al. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J. Infect. Dis. 187, 522–525 (2003).
Fernandez-Arias, C. et al. Malaria inhibits surface expression of complement receptor 1 in monocytes/macrophages, causing decreased immune complex internalization. J. Immunol. 190, 3363–3372 (2013).
Vasquez, M., Zuniga, M. & Rodriguez, A. Oxidative stress and pathogenesis in malaria. Front. Cell. Infect. Microbiol. 11, 768182 (2021).
Greve, B., Kremsner, P. G., Lell, B., Luckner, D. & Schmid, D. Malarial anaemia in African children associated with high oxygen-radical production. Lancet 355, 40–41 (2000).
Elphinstone, R. E. et al. Alterations in systemic extracellular heme and hemopexin are associated with adverse clinical outcomes in Ugandan children with severe malaria. J Infect Dis 214, 1268–1275 (2016).
Plewes, K. et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: An observational study. BMC Infect. Dis. 17, 313 (2017).
Lewis, S. E. et al. Human and rodent red blood cells do not demonstrate xanthine oxidase activity or XO-catalyzed nitrite reduction to NO. Free Radic. Biol. Med. 174, 84–88 (2021).
Iwalokun, B. A., Bamiro, S. B. & Ogunledun, A. Levels and interactions of plasma xanthine oxidase, catalase and liver function parameters in Nigerian children with Plasmodium falciparum infection. APMIS 114, 842–850 (2006).
Barber, B. E. et al. Antiphosphatidylserine immunoglobulin M and immunoglobulin G antibodies are higher in vivax than falciparum malaria, and associated with early anemia in both species. J. Infect. Dis. 220, 1435–1443 (2019).
Fernandez-Arias, C. et al. Anti-self phosphatidylserine antibodies recognize uninfected erythrocytes promoting malarial anemia. Cell Host Microbe 19, 194–203 (2016).
Rivera-Correa, J. et al. Autoantibody levels are associated with acute kidney injury, anemia and post-discharge morbidity and mortality in Ugandan children with severe malaria. Sci. Rep. 9, 14940 (2019).
Rivera-Correa, J. et al. Atypical memory B-cells are associated with Plasmodium falciparum anemia through anti-phosphatidylserine antibodies. Elife 8, e48309 (2019).
Rivera-Correa, J. et al. Atypical memory B-cells and autoantibodies correlate with anemia during Plasmodium vivax complicated infections. PLoS Negl. Trop. Dis. 14, e0008466 (2020).
Namazzi, R. et al. Acute kidney injury interacts with coma, acidosis, and impaired perfusion to significantly increase risk of death in children with severe malaria. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciac229 (2022).
Namazzi, R. et al. Acute kidney injury, persistent kidney disease, and post-discharge morbidity and mortality in severe malaria in children: A prospective cohort study. EClinicalMedicine 44, 101292 (2022).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179–c184 (2012).
WHO. Severe malaria. Trop. Med. Int. Health 19(Suppl 1), 7–131 (2014).
Conroy, A. L. et al. Blackwater fever and acute kidney injury in children hospitalized with an acute febrile illness: Pathophysiology and prognostic significance. BMC Med. 20, 221 (2022).
Fendel, R. et al. Hemolysis is associated with low reticulocyte production index and predicts blood transfusion in severe malarial anemia. PLoS ONE 5, e10038 (2010).
Ryter, S. W., Alam, J. & Choi, A. M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 86, 583–650 (2006).
Tenhunen, R., Marver, H. S. & Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. U. S. A. 61, 748–755 (1968).
di Masi, A. et al. Haptoglobin: From hemoglobin scavenging to human health. Mol. Aspects Med. 73, 100851 (2020).
Possemiers, H., Vandermosten, L. & Van den Steen, P. E. Etiology of lactic acidosis in malaria. PLoS Pathog. 17, e1009122 (2021).
Dondorp, A. M. et al. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am. J. Trop. Med. Hyg. 60, 733–737 (1999).
Dondorp, A. M. et al. Prognostic significance of reduced red blood cell deformability in severe falciparum malaria. Am. J. Trop. Med. Hyg. 57, 507–511 (1997).
Ty, M. C. et al. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol. Med. 11, e9903 (2019).
Casali, E., Berni, P., Spisni, A., Baricchi, R. & Pertinhez, T. A. Hypoxanthine: A new paradigm to interpret the origin of transfusion toxicity. Blood Transfus. 14, 555–556 (2016).
Cassera, M. B. et al. Plasmodium falciparum parasites are killed by a transition state analogue of purine nucleoside phosphorylase in a primate animal model. PLoS ONE 6, e26916 (2011).
Downie, M. J., Kirk, K. & Mamoun, C. B. Purine salvage pathways in the intraerythrocytic malaria parasite Plasmodium falciparum. Eukaryot. Cell 7, 1231–1237 (2008).
Guermonprez, P. et al. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat. Med. 19, 730–738 (2013).
Liu, Y. et al. Type I interferon is induced by hemolysis and drives antibody-mediated erythrophagocytosis in sickle cell disease. Blood 138, 1162–1171 (2021).
Schmidt, H. M. et al. Xanthine oxidase drives hemolysis and vascular malfunction in sickle cell disease. Arterioscler. Thromb. Vasc. Biol. 41, 769–782 (2021).
Mourao, L. C. et al. Anti-band 3 and anti-spectrin antibodies are increased in Plasmodium vivax infection and are associated with anemia. Sci. Rep. 8, 8762 (2018).
Owuor, B. O. et al. Reduced immune complex binding capacity and increased complement susceptibility of red cells from children with severe malaria-associated anemia. Mol. Med. 14, 89–97 (2008).
Anand, A. C. & Puri, P. Jaundice in malaria. J. Gastroenterol. Hepatol. 20, 1322–1332 (2005).
Batte, A. et al. Malaria-associated acute kidney injury in African children: Prevalence, pathophysiology, impact, and management challenges. Int. J. Nephrol. Renovasc. Dis. 14, 235–253 (2021).
Katsoulis, O., Georgiadou, A. & Cunnington, A. J. Immunopathology of acute kidney injury in severe malaria. Front. Immunol. 12, 651739 (2021).
Abhishek, A., Roddy, E. & Doherty, M. Gout—A guide for the general and acute physicians. Clin. Med. (Lond.) 17, 54–59 (2017).
Sivera, F., Andres, M. & Dalbeth, N. A glance into the future of gout. Ther. Adv. Musculoskelet. Dis. 14, 1759720X221114098 (2022).
Author information
Authors and Affiliations
Contributions
A.R. and A.L.C. wrote the main manuscript text, M.V., M.S. and J.S. performed the experiments and prepared figures; M.V., M.S. and M.D.F. analyzed the data, R.N., R.O.O., D.D., J.M.S., C.C.J, and A.L.C. collected samples and associated data, and validated data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasquez, M., Sica, M., Namazzi, R. et al. Xanthine oxidase levels and immune dysregulation are independently associated with anemia in Plasmodium falciparum malaria. Sci Rep 13, 14720 (2023). https://doi.org/10.1038/s41598-023-41764-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41764-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.