Abstract
Traditional metabolic syndrome (MetS) criteria have several limitations, which hinder its use in clinical practice. To overcome the limitations, we investigated the association between age- and sex-specific continuous MetS severity score (cMetS-S) and cardiovascular disease (CVD) and mortality beyond MetS components in the framework of the Tehran Lipid and Glucose Study. Participants aged 20–60 years at baseline were included in the study. We excluded participants with CVD, cancer, use of corticosteroids, estimated glomerular filtration rate < 30 ml/min/1.73 m2, and those who were pregnant. We evaluated the association between cMetS-S with CVD and mortality over 18 years of follow-up among 8500 participants with continuous and quantile approaches using the Cox proportional hazard regression model. In addition, the model performance of cMetS-S for predicting CVD events was compared to the conventional MetS criteria. Participants with higher cMetS-S had a significantly increased risk for CVD, coronary (CHD) and non-coronary heart disease (non-CHD), and all-cause, cardiovascular, and sudden cardiac death. Independent of the confounders and MetS components, the cMetS-S had the HRs of 1.67 (95% CI 1.47–1.89), 1.60 (95% CI 1.37–1.86), and 1.88 (95% CI 1.50, 2.35) for CVD, CHD, and non-CHD events upon 1-SD increment, respectively. The risk of mortality was increased for 1-SD of cMetS-S (all-cause mortality, HR 1.24; 95% CI 1.09–1.41; CVD mortality, HR 1.72; 95% CI 1.20–2.45; sudden cardiac death, HR 1.60; 95% CI 1.03–2.49). The model fitness of cMetS-S was superior to the conventional MetS criteria in predicting CVD and mortality. The cMetS-S provided an additional risk for CVD and mortality beyond the individual MetS components. Standardized cMetS-S could be a potential universal measure to define MetS severity while considering the weighted contribution of MetS components and their variations by age, sex, and ethnicity.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a cluster of conditions defined by five cardiometabolic risk factors: waist circumference (WC), blood pressure, fasting plasma glucose (FPG), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C). In the Middle East, nearly one in four adults are living with MetS, and more people are affected by this increasing secular trend every day1,2. The latest study from Iran estimated an alarming prevalence of 47.6% for MetS in the general population3. Atherogenic dyslipidemia and endothelial dysfunction as central features of MetS contribute to the development of atherosclerosis and cardiovascular disease (CVD)4. Studies have shown that MetS increases the risk of CVD incidence and all-cause mortality by 2 and 1.5 times, respectively5. The association of MetS with CVD makes it an effective epidemiologic tool to prevent cardiovascular-related outcomes and mortality.
The dichotomous nature of the traditional definition of MetS (presence vs. absence) has limited its use in clinical settings; the binary definition results in a loss of data regarding the degree of abnormality of the MetS components. Moreover, a minimal change in the value of the MetS components could label the individuals as having MetS or not6. The term pre-metabolic syndrome has been described to address such individuals with one or two MetS components that do not fit the definition of MetS7. A measure of MetS severity enables clinicians to monitor changes in MetS and its related adverse health events over time within populations (i.e., individuals with and without MetS).
To prevail these limitations, a few researchers have formulated a MetS severity score using confirmatory factor analysis (CFA) in different ethnicities and demonstrated its predictability for CVD8,9. Recently, we developed a continuous Mets severity score (cMetS-S) using CFA in the Iranian population10. Briefly, we defined cMetS-S by the weighted contribution of MetS components to MetS and its variation by age and sex. However, whether this score predicts incident CVD and mortality is unnoted. In this article, we intend to verify our newly developed cMetS-S by investigating its association with CVD incidence and all-cause mortality and whether the association would be independent of individual MetS components.
Methods
Study population
Our study population includes participants from the Tehran Lipid and Glucose Study (TLGS), a 20-year large population-based cohort study. In brief, TLGS was first designed in 1999 to assess risk factors of non-communicable diseases and their prevalence in Iran. Detailed design of the TLGS has been published elsewhere11. For the current study, we included TLGS participants aged 20–60 years old at baseline. At baseline, individuals with cancer (n = 39), CVD (n = 300), use of systematic corticosteroids (n = 112), estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 (n = 2), missing data (missing covariates: n = 1714; missing follow-up: n = 46), and pregnant participants (n = 100) were excluded from the study. Hence, a total of 8500 participants were enrolled and followed for 18 years (Fig. 1).
Measurements and definitions
Demographic data and medical and drug history were collected through a pretested questionnaire. Smoking status was defined as the occasional or daily consumption of tobacco products. Education level was divided into three categories based on years of education or the degree they hold as follows: (1) Illiterate/primary school (< 6 years). (2) High school (6–12 years). (3) Higher education (≥ 12 years). Diagnosis of CVD in at least one first-degree relative before 55 years in men and 65 years in women was considered to have a positive family history of CVD. The data on physical activity were collected using the Persian version of the Lipid Research Clinic (LRC) questionnaire. Physical activity was defined using the metabolic equivalent of task scale (METS), by which individuals with METS < 600 min-week −1 were categorized in the low physically active group. Weight and height were measured while patients were with minimal clothes and without shoes to the nearest 0.1 kg and 0.1 cm2 using standard protocols. Using a taped meter, WC was measured at the level of the umbilicus and recorded to the nearest 0.1 cm. Using a standardized mercury sphygmomanometer (calibrated by the Iranian Institute of Standards and Industrial Researches), systolic and diastolic blood pressure were measured twice after a 15-min rest, from the right arm, in the sitting position and the means were recorded as the participant's blood pressure.
After 12–14 h of fasting, blood samples were drawn from all the participants and were analyzed at the TLGS research laboratory on the day of blood collection. FPG, TG, HDL-C, and total cholesterol were measured using enzymatic colorimetric methods. Creatinine was assayed using the kinetic colorimetric Jaffe. All samples were analyzed when internal quality control met the acceptance criteria. Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg or the use of anti-hypertensive drugs. Dyslipidemia was defined as TG ≥ 150 mg/dL, TC ≥ 200 mg/dL, HDL-C < 40 mg/dL in men and HDL-C < 50 mg/dL in women, or the use of lowering lipid medication. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021 formula12.
According to the joint interim statement (JIS) definition, MetS is defined as having three or more of the five following components: (1) WC ≥ 90 cm in both sexes according to the population-specific cut-off presented by the national committee13, (2) serum HDL-C < 40 mg/dl for men and < 50 mg/dl for women. (3) TG ≥ 150 mg/dl or specific treatment, (4) FPG ≥ 100 mg/dl, and (5) SBP ≥ 130 mmHg, DBP ≥ 85 mmHg or use of anti-hypertensive medication. In the international diabetes federation (IDF) criteria, the definition is based on the presence of abdominal obesity and two of the four remaining MetS components14.
The cMetS-S definition
We have previously developed and validated age- and sex-specific cMetS-S score equations using CFA in each age and sex group separately (Supplementary Table S1)10. Briefly, this approach considers contributed weight of the traditional MetS components and their variations across age and sex subgroups. The cMetS-S can be calculated using the data of age, sex, and the MetS components’ values. Higher cMetS-S indicates higher MetS severity. In the current study, the cMetS-S was standardized at mean = 0 and SD = 1 based on the study population for straightforward interpretation and for generalizability of the results.
Study outcomes
Included participants were followed annually for any medical events over 18 years follow-up. A consulting committee consisting of specialists, an epidemiologist, and the physician that registered the data, reviewed and evaluated the collected data to assign each individual an outcome. CVD events, including coronary and non-coronary heart disease (CHD and non-CHD; ICD-10 rubric codes I20-I25, I46.1, and I60-I69), all-cause and CVD mortality, and sudden cardiac death (SCD; I46.1) were selected as desired outcomes of the study. According to ICD-10, CHD events (I20-I25) comprise, (1) cases of definite or probable myocardial infarction with positive electrocardiogram findings plus cardiac signs and/or biomarkers, (2) cases going through any cardiac procedures (angiography-proven CHD with ≥ 50% stenosis in at least one major coronary artery, history of angioplasty or bypass surgery), and (3) cases with unstable angina pectoris (participants with new cardiac symptoms or change of pattern in previous cardiac symptoms and positive ECG findings with normal biomarkers and admitted to cardiac care unit).
Statistical analysis
Baseline characteristics of the participants are reported as mean ± SD for continuous and frequency (percentage) for categorical variables. The comparison between the groups was made using an independent sample t-test for continuous and Chi-square test for categorical variables. The incident rate for the outcomes was calculated by dividing the total number of incident cases by the overall follow-up duration, measured in person-years. The association of cMetS-S with CVD and mortality was analyzed using continuous and tertile approaches with Cox proportional hazard regression models. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, smoking status, physical activity, CVD family history, and obesity (BMI ≥ 30 kg/m2). Model 3 was adjusted for model 2 and anti-hypertensive, anti-diabetic, and lipid-lowering medications. Model 4 was additionally adjusted for MetS components (high WC, high SBP, high TG, low HDL-C, and high FPG). We used restricted cubic spline regression analysis to demonstrate the relationship between cMetS-S and CVD and mortality. We also compared the model performance of JIS and IDF-based MetS and cMetS-S for predicting CVD and mortality using the Akaike information criterion (AIC) and log-likelihood ratio. The statistical analyses were performed using STATA 14 (StataCorp, college station, TX, USA) and R-3.0.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value < 0.05 was considered statistically significant.
Ethical approval and consent to participate
This study conformed to the ethical guidelines of the Helsinki Declaration and approved by the Human Research Review Committee of the Endocrine Research Center of Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.ENDOCRINE.REC.1401.085). All participants provided informed written consent.
Results
Baseline characteristics of the study population are presented according to cMetS-S tertiles in Table 1. Overall, we enrolled 8500 subjects (41.9% male) with a mean age of 37.4 ± 10.8 years. Approximately 32.2% and 27.9% of the participants were labeled as having MetS according to the JIS and IDF definitions; the MetS prevalence was higher in upper cMetS-S tertiles. Mean age, BMI, WC, lipid indices (except for HDL), SBP, and DBP increased toward upper cMetS-S tertiles (P < 0.001). Also, the prevalence of individuals who were smokers, low physically active, and used anti-hypertensive, anti-diabetic, and lipid-lowering drugs were higher in the higher cMetS-S tertiles (P < 0.001), while there was no difference in the prevalence of CVD family history across the tertiles.
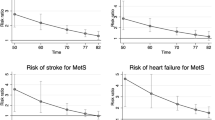
During 18 years of follow-up, 754 CVD incidents (524 CHD and 230 non-CHD events) and 292 all-cause mortality incidents (94 CVD and 198 non-CVD mortality) occurred. Incidence rates of CVD, mortality, and their subtypes increased incrementally across the cMetS-S tertiles (Tables 2, 3). The restricted cubic spline regression plots were generated according to the fully adjusted HRs obtained from the Cox proportional hazard model of participants for CVD and mortality. A non-linear positive association was found between cMetS-S and CVD and mortality outcomes (Fig. 2).
Restricted cubic spline regression analysis demonstrating the association of continuous metabolic syndrome severity score (cMetS-S) with all-cause, CVD, and sudden cardiac death (left panels), and CVD, CHD and non-CHD event (right panels). Heavy central lines represent the estimated fully adjusted hazard ratios in logarithmic scale, with shaded ribbons denoting 95% confidence intervals for each outcome. CVD Cardiovascular disease, CHD Coronary heart disease.
As shown in Table 2, cMetS-S was associated with future CVD events with an HR of 1.67 (95% CI 1.47–1.89) per 1-SD increase in cMetS-S. The results were congruent when calculating the risk of CVD event subtypes; the risk of CHD and non-CHD events increased with HRs of 1.60 (95% CI 1.37–1.86) and 1.88 (95% CI 1.50, 2.35) per 1-SD increase of cMetS-S, respectively. The risk of incident CVD (HR 3.39; 95% CI 2.31–4.96), CHD (HR 3.22; 95% CI 2.03–5.13), and non-CHD (HR 3.84; 95% CI 1.96–7.51) events increased in the highest tertile compared to the lowest tertile.
The cMetS-S had a significant association with all-cause mortality with an HR of 1.24 (95% CI 1.09–1.41) per 1-SD increase in cMetS-S after adjusting for confounding variables (Table 3); the result was consistent after further adjusting for individual MetS components, indicating that cMetS-S increased the risk of all-cause mortality independent of MetS components (HR 1.37; 95% CI 1.11–1.69). The risk of CVD mortality and SCD also increased upon a 1-SD increment of cMetS-S (CVD mortality, HR 1.72; 95% CI 1.20–2.45; SCD, HR 1.60; 95% CI 1.03–2.49) in the fully adjusted model. The risk of all-cause mortality (HR 1.65; 95% CI 0.93–2.90), CVD (HR 9.50; 2.41–37.9), and SCD (HR 8.68, 95% CI 1.62–47.2) increased upon higher cMetS-S tertile in the fully adjusted model (Table 3).
We also examined whether cMetS-S has added value over MetS definitions and its components in predicting CVD and mortality independent of its components using model fit analysis (Tables 4, 5). The initial model was built based on MetS components (high TG, high WC, high FPG, high SBP, and low HDL-C) and the risk factors as covariates. We then separately added cMetS-S, MetS defined by JIS, and MetS defined by IDF to the initial model. Unlike JIS and IDF-based MetS definitions, cMetS-S performed better than the initial model by decreasing the AIC value by 24.1 and 59 on df = 1 for mortality and CVD prediction, respectively (P < 0.001). The association of JIS- and IDF-based definition with CVD and mortality are presented in Supplementary Tables S2 and S3.
The Kaplan–Meier curves demonstrated poorer event-free probability for individuals in upper tertiles of cMetS-S for CVD event and mortality outcomes (log P rank < 0.0001) (Fig. 3).
Kaplan–Meier survival curves showing event-free probability according to cMetS-S tertile for all-cause, CVD, sudden cardiac death (left panels), and CVD, CHD, and non-CHD event (right panels). P values from the log-rank test are displayed within the plots. cMetS-S Continuous metabolic syndrome severity score, CVD Cardiovascular disease, CHD Coronary heart disease.
Discussion
In the current study, we assessed the potential applicability of cMetS-S, an age- and sex-specific measure of MetS severity, to predict incident CVD and mortality during 18 years in a population-based cohort study. We found that cMetS-S was independently associated with CVD (CHD and non-CHD) and all-cause and CVD mortality among young and middle-aged populations using continuous and quantile approaches. The aforementioned association persisted even after controlling for MetS components. Additionally, our findings indicated that cMetS-S demonstrates a superior predictive ability for future CVD events and mortality compared to the conventional definitions of MetS based on JIS and IDF criteria.
The clinical utility of MetS as a predictive tool to assess the future risk of diabetes and CVD is limited due to its binary nature. No universal measure has been presented to define the severity of cardiometabolic abnormality based on MetS. Some researchers employ the number of MetS components as a metric for measuring MetS severity, thereby ignoring the extent of abnormality of the individual MetS components. A recent international multicenter study reported that the prevalence and the risk of future CVD were higher in individuals with MetS compared to those with a single MetS component; however, this risk was not elevated when compared to individuals with two MetS components15. This indicates the continuum nature of MetS and its attributed risk for CVD. The cMetS-S is a newly developed definition for MetS severity based on the values of traditional MetS components and their contribution to MetS on a continuous scale. In this newly developed definition for severity, the limitations in the traditional MetS criteria are overcome.
Recently, several useful phenotypes of preMetS and their corresponding indices have been defined. Studies demonstrated that hypertriglyceridemic-waist (HTGW) phenotype and triglyceride-glucose (TyG) index (representative of high FPG and TG phenotype) increased the risk of CVD events even after controlling for established risk factors16,17,18. The joint effect of high TG and FPG also provided an additional predictive ability for CVD incidence19. Although these phenotypes and indices can be helpful indicators of cardiometabolic health, their multiplicity creates confusion in health assessment at individual and public levels. The cMetS-S is a tangible quantitative measure that considers all these phenotypes and the degree of abnormality in MetS components. The cMetS-S provides a comprehensive health risk assessment for the whole population regardless of whether or not individuals meet the traditional definition of MetS.
In this study, we demonstrated that cMetS-S could well predict CVD incidence over long-term follow-up. Individuals with higher cMetS-S had a three times higher risk for CVD, CHD, and non-CHD events after adjusting for age, sex, education, physical activity, CVD family history, smoking, obesity, medications, and MetS components. This aligns with the findings of previous studies despite differences in confounding adjustments, methods, and study populations. In the study on the American population8, in contrast to the current and the Korean study9, the developed MetS severity was not formulated based on age categories; in addition, the adjustments were limited to age, sex, and individual MetS components. We also demonstrated a non-linear positive association between cMetS-S and CVD using continuous and tertile approaches. Previous studies only investigated this association using quantile classification and analysis8,9. We found that each SD increase in MetS severity leads to more than 60% increased risk of future CVD. Using a standardized continuous approach will allow for comparison and generalization of the results among different studies and populations and also prevent the loss of data due to quantile classification.
To the best of our knowledge, this is the first study to show the association between MetS severity derived from CFA and mortality. We found that cMetS-S is associated with an increased risk of all-cause, cardiovascular, and sudden cardiac death even after adjusting for MetS components. MetS is a complex and heterogeneous entity with variations in the pathophysiology, prevalence, and associated risk of individual MetS components for CVD and mortality across age and sex groups20,21,22. Using cMetS-S, we could overcome this limitation by defining MetS severity based on the contribution of MetS components to MetS in each sex and age subgroup. However, further research is needed to assess how defining MetS severity in different age and sex categories could improve the prediction of future adverse health outcomes.
MetS is a well-known risk factor for CVD, CHD, stroke, and mortality23,24,25; however, whether the traditional-based MetS definitions as a whole convey the same risk independent of its components is controversial26,27,28. Our findings suggest that unlike JIS and IDF-based definitions, cMetS-S has significant additional predictive value for CVD and mortality beyond MetS components. Our study is in line with the studies in the literature on American and Korean individuals showing cMetS-S to have an increased risk for CVD independent of the MetS components8,9,29. These findings indicate that MetS defined in continuous scale by CFA may better incorporate the complex interactions between metabolic processes involved in MetS by considering the weighted contribution of the MetS components.
The present study has several notable strengths. We validated the newly developed cMetS-S in a large, representative sample of Iranian adults with nearly two decades of follow-up. Additionally, this is the first study to evaluate cMetS-S utility for predicting all-cause, CVD, and sudden cardiac death. Furthermore, unlike the previous studies in the literature, we demonstrated the association of standardized MetS severity with CVD and mortality on a continuous scale (for each SD increase), allowing for comparison and generalization of the results from different studies. Moreover, age, sex, education, smoking, physical activity, CVD family history, obesity, medications, and individual MetS components were adjusted to reflect the independent association of MetS severity with CVD and mortality. Regarding our limitations, we restricted our participants to individuals aged between 20 and 60 years to investigate this association in the non-elderly population.
In conclusion, the newly developed age- and sex-specific MetS severity score is a reliable predictive tool for CVD and mortality among the Iranian population independent of MetS components. Unlike JIS and IDF-based MetS criteria, the cMetS-S provides additional value beyond MetS components in predicting CVD and mortality. The standardized cMetS-S has the potential to be presented as a new universal scoring system for MetS with respect to the variations in age, sex, and ethnicity.
Data availability
Datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- MetS:
-
Metabolic syndrome
- WC:
-
Waist circumference
- FPG:
-
Fasting plasma glucose
- TG:
-
Triglyceride
- HDL-C:
-
High density lipoprotein cholesterol
- CVD:
-
Cardiovascular disease
- CFA:
-
Confirmatory factor analysis
- cMetS-S:
-
Continuous Mets severity score
- TLGS:
-
Tehran Lipid and Glucose Study
- eGFR:
-
Estimated glomerular filtration rate
- METS:
-
Metabolic equivalent of task scale
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CKD-EPI:
-
Chronic Kidney Disease Epidemiology Collaboration
- JIS:
-
Joint interim statement
- IDF:
-
International Diabetes Federation
- CHD:
-
Coronary heart disease
- AIC:
-
Akaike information criterion
- HTGW:
-
Hypertriglyceridemic-waist
- TyG:
-
Triglyceride-glucose
References
Ansarimoghaddam, A. et al. Prevalence of metabolic syndrome in Middle-East countries: Meta-analysis of cross-sectional studies. Diabetes Metab. Syndr. 12(2), 195–201 (2018).
Sliem, H. A., Ahmed, S., Nemr, N. & El-Sherif, I. Metabolic syndrome in the Middle East. Indian J. Endocrinol. Metab. 16(1), 67–71 (2012).
Tabatabaei-Malazy, O. et al. A nationwide study of metabolic syndrome prevalence in Iran; A comparative analysis of six definitions. PLoS ONE 16(3), e0241926 (2021).
Huang, P. L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2(5–6), 231–237 (2009).
Mottillo, S. et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 56(14), 1113–1132 (2010).
DeBoer, M. D. & Gurka, M. J. Clinical utility of metabolic syndrome severity scores: Considerations for practitioners. Diabetes Metab. Syndr. Obes. 10, 65–72 (2017).
Cho, A. R., Kwon, Y. J. & Kim, J. K. Pre-metabolic syndrome and incidence of type 2 diabetes and hypertension: From the Korean genome and epidemiology study. J. Pers. Med. 11(8), 700 (2021).
DeBoer, M. D. et al. Independent associations between metabolic syndrome severity and future coronary heart disease by sex and race. J. Am. Coll. Cardiol. 69(9), 1204–1205 (2017).
Jang, Y. N. et al. Metabolic syndrome severity score for predicting cardiovascular events: A nationwide population-based study from Korea. Diabetes Metab. J. 45(4), 569–577 (2021).
Honarvar, M., Masoumi, S., Mehran, L., Khalili, D., Amouzegar, A. & Azizi, F. Development and validation of a continuous metabolic syndrome severity score in the Tehran lipid and glucose study. Article In Press Sci. Rep. (2023).
Azizi, F. et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials 10, 5 (2009).
Inker, L. A. et al. New creatinine- and cystatin c-based equations to estimate GFR without race. N. Engl. J. Med. 385(19), 1737–1749 (2021).
Azizi, F. et al. Appropriate waist circumference cut-off points among Iranian adults: The first report of the Iranian National Committee of Obesity. Arch. Iran. Med. 13(3), 243–244 (2010).
Alexander, C. M., Landsman, P. B., Teutsch, S. M. & Haffner, S. M. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 52(5), 1210–1214 (2003).
Ahmadi, A. et al. Is metabolic syndrome predictive of prevalence, extent, and risk of coronary artery disease beyond its components? Results from the multinational coronary CT angiography evaluation for clinical outcome: An International Multicenter Registry (CONFIRM). PLoS ONE 10(3), e0118998 (2015).
da Silva, A., Caldas, A. P. S., Rocha, D. & Bressan, J. Triglyceride-glucose index predicts independently type 2 diabetes mellitus risk: A systematic review and meta-analysis of cohort studies. Prim. Care Diabetes 14(6), 584–593 (2020).
Ding, X., Wang, X., Wu, J., Zhang, M. & Cui, M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: A meta-analysis of cohort studies. Cardiovasc. Diabetol. 20(1), 76 (2021).
Zheng, X., Ren, X., Jiang, M. & Han, L. Association between hypertriglyceridemic-waist phenotype and cardiovascular disease: A cohort study and meta-analysis. Front. Cardiovasc. Med. 9, 940168 (2022).
Si, S. et al. Causal effect of the triglyceride-glucose index and the joint exposure of higher glucose and triglyceride with extensive cardio-cerebrovascular metabolic outcomes in the UK Biobank: A Mendelian Randomization Study. Front. Cardiovasc. Med. 7, 583473 (2020).
Ju, S. Y., Lee, J. Y. & Kim, D. H. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: A meta-analysis of prospective cohort studies. Medicine (Baltimore) 96(45), e8491 (2017).
Meloni, A. et al. Gender differences and cardiometabolic risk: The importance of the risk factors. Int. J. Mol. Sci. 24(2), 1588 (2023).
Kuk, J. L. & Ardern, C. I. Age and sex differences in the clustering of metabolic syndrome factors: Association with mortality risk. Diabetes Care 33(11), 2457–2461 (2010).
Galassi, A., Reynolds, K. & He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 119(10), 812–819 (2006).
Li, X. et al. Metabolic syndrome and stroke: A meta-analysis of prospective cohort studies. J. Clin. Neurosci. 40, 34–38 (2017).
Wu, S. H., Liu, Z. & Ho, S. C. Metabolic syndrome and all-cause mortality: A meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 25(6), 375–384 (2010).
Guembe, M. J. et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 19(1), 195 (2020).
Georgiopoulos, G. et al. Metabolic syndrome, independent of its components, affects adversely cardiovascular morbidity in essential hypertensives. Atherosclerosis 244, 66–72 (2016).
Sundström, J. et al. Risk associated with the metabolic syndrome versus the sum of its individual components. Diabetes Care 29(7), 1673–1674 (2006).
Guo, Y. et al. Assessing the added predictive ability of a metabolic syndrome severity score in predicting incident cardiovascular disease and type 2 diabetes: The Atherosclerosis Risk in Communities Study and Jackson Heart Study. Diabetol. Metab. Syndr. 10, 42 (2018).
Acknowledgements
We express our appreciation to TLGS participants and the research team members for their contribution to the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing the manuscript. M.H.: designing, methodology, analysis, preparation and editing the manuscript; L.M.: designing, methodology, and editing the manuscript; S.M.: data analysis; S.A.: writing the original draft; S.K.: reviewing and editing the manuscript; F.A.: project supervision and editing the final draft; AA: project administration and editing the final draft. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Honarvar, M., Mehran, L., Masoumi, S. et al. Independent association between age- and sex-specific metabolic syndrome severity score and cardiovascular disease and mortality. Sci Rep 13, 14621 (2023). https://doi.org/10.1038/s41598-023-41546-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41546-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.