Abstract
This study assessed whether perioperative management is associated with postoperative acute exacerbations (AEs) in interstitial lung disease (ILD) patients. Using secondary data from the study “Postoperative acute exacerbation of interstitial lung disease: a case–control study,” we compared the perioperative clinical management of the AE and non-AE groups (1:4 case–control matching) selected by sex, year of surgery (2009–2011, 2012–2014, and 2015–2017), and multiple surgeries within 30 days. We compared 27 and 108 patients with and without AEs, respectively. Rates of one lung ventilation (OLV) cases (70 vs. 29%; OR, 5.9; 95%CI, 2.34–14.88; p < 0.001) and intraoperative steroid administration (48 vs. 26%; OR, 2.65; 95%CI, 1.11–6.33; p = 0.028), and average mean inspiratory pressure (9.2 [1.8] vs. 8.3 [1.7] cmH2O; OR, 1.36; 95%CI, 1.04–1.79; p = 0.026), were significantly higher in the AE group. There was a significant difference in OLV between the groups (OR, 4.99; 95%CI, 1.90–13.06; p = 0.001). However, the fraction of inspired oxygen > 0.8 lasting > 1 min (63 vs. 73%, p = 0.296) was not significantly different between the groups. OLV was significantly associated with postoperative AEs in patients with ILD undergoing both pulmonary and non-pulmonary surgeries. Thus, preoperative risk considerations are more important in patients who require OLV.
Similar content being viewed by others
Introduction
Acute exacerbation (AE) of interstitial lung disease (ILD) is one of the most common postoperative pulmonary complications1. The mortality rate due to AEs after pulmonary resection is 33.3–100%2,3, and fatal outcomes from AEs have been reported in patients with or without pulmonary resection4,5. Therefore, it is essential to identify high-risk patients preoperatively and to manage them without AEs in the perioperative period.
In previous studies, most risk factors for postoperative AEs were related to patient status and type of surgery. In 2014, Sato et al. identified the following predictors of AEs after pulmonary resection for lung cancer: surgical procedures, male sex, history of exacerbation, preoperative steroid use, serum sialylated carbohydrate antigen KL-6 levels, usual interstitial pneumonia appearance on computed tomography (CT), and reduced percentage predicted vital capacity6. Recently, Hosoki et al. demonstrated the following predictors of postoperative AEs in ILD after pulmonary and non-pulmonary surgeries: honeycombing on CT, percentage predicted forced vital capacity (FVC) < 80%, and Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score ≥ 457. These factors are preoperative risk factors for postoperative AEs in patients with ILD and are therefore used when considering indications for surgery. Furthermore, these predictors allow anesthesiologists and surgeons to evaluate the possibility of postoperative AEs in patients with ILD before surgery without an experienced respiratory physician. However, Hosoki et al. demonstrated risk factors for patient-related AEs and not the risk of perioperative clinical management. Up to now, perioperative management may affect postoperative AEs, there are few reports on perioperative management associated with AEs in patients with ILD despite it is highly anticipated.
A high fraction of inspired oxygen (FIO2) causes absorptive atelectasis and an increased generation of reactive oxygen species and proinflammatory cytokines, which can result in lung injury8,9,10. Therefore, empirically, a perioperative ventilatory strategy for patients with ILD is to set the FIO2 as low as possible. Furuya et al. reported that anesthetic management with propofol may be a risk factor for AEs after non-pulmonary resection surgery in patients with idiopathic interstitial pneumonia (IIP)5. Few other reports exist on the risk factors for anesthetic management that are associated with postoperative AEs. However, some reports suggest that the risk of AEs does not significantly differ between laparoscopic and open surgery, although hypercapnia and prolonged operative time are concerns11. Although perioperative pain may be a cause of prolonged postoperative recovery, few reports show an association between postoperative analgesia and postoperative AEs. Hickling et al. reported that low tidal volume management is recommended for patients with acute respiratory distress syndrome (ARDS), including hypercapnia12. Neto et al. reported that maintaining a driving pressure below a certain level and appropriate intraoperative positive end-expiratory pressure (PEEP) may reduce postoperative respiratory complications13. These methods may be adapted to intraoperative ventilation in patients with ILD, but there are few reports on ventilation management in patients with ILD.
There is no clear association between anesthesia management and postoperative AEs in patients with ILD. In this report, we performed a secondary analysis of the retrospective observational study by Hosoki et al. titled “Predictors of postoperative acute exacerbation of interstitial lung disease: a case–control study” to elucidate whether perioperative management is associated with postoperative AEs in patients with ILD.
Methods
We performed a secondary analysis of the data presented in the publication of Hosoki et al. titled “Predictors of postoperative acute exacerbation of interstitial lung disease: a case–control study.” The study involved patients with ILD who underwent surgery under general anesthesia at the University of Tokyo Hospital between January 2009 and December 2017. Two or more respiratory physicians searched the electronic medical records of patients in the cohort to identify those with pre-existing ILD at the time of operations. A matched 1:4 case–control study was performed among 700 surgical patients with ILD matched by sex, year of surgery (2009–2011, 2012–2014, and 2015–2017), and multiple operations within 30 days. Patients under 18 years of age who had undergone solid organ or bone marrow transplantation were excluded. Furthermore, multiple operations within 30 days were considered as a single operation, and the first operation was adopted. Demographic and other baseline characteristics and the presence of blood transfusion were based on the original data from the previous study, and other data in our study were extracted from the anesthesia database. The anesthesia database contains minute-by-minute records of vital signs, ventilation settings, and medications of patients undergoing surgery. Patients who underwent surgery without mechanical ventilation and those with missing data were excluded. The definition of AE in our study was based on the ATS/ERS/JRS/ALAT statement14 similar to the previous study: (1) worsening of dyspnea within one month, (2) evidence of hypoxemia, (3) new radiographic alveolar infiltrates, and (4) the absence of alternative explanations such as infection, pulmonary embolism, pneumothorax, or heart failure.
We conducted a matched case–control study. The cases were patients with ILD who developed AE; matched controls were selected from the remaining cohort of ILD patients who did not develop AE. In our study, we compared anesthetic management factors in the postoperative AE and non-AE groups.
Intraoperative factors were collected for the following parameters: method of anesthesia (volatile or intravenous), presence of one lung ventilation (OLV), steroid use, type of surgery (laparoscopic or open), duration of anesthesia, blood loss, in–out balance, blood transfusion, postoperative analgesia methods (opioid and local anesthesia), and intraoperative desaturation events (pulse oximetry value [SpO2] ≤ 90% for > 1 min) or hypercapnia (end-tidal carbon dioxide [EtCO2] > 60 mmHg for > 1 min). Data on intraoperative respiratory parameters such as mean inspiratory pressure, mean PEEP, FIO2, and the duration of mechanical ventilation were also extracted. In this study, mean inspiratory pressure and PEEP were measured during mechanical ventilation. Patients with hyperoxia were defined as having an FIO2 > 0.8 for > 1 min during mechanical ventilation.
This study was approved by the Research Ethics Committee, Graduate School of Medicine and Faculty of Medicine, The University of Tokyo (IRB# 2203, October 2008 to June 2022). Due to the nature of the retrospective study, informed consent was waived by the Research Ethics Committee, Graduate School of Medicine and Faculty of Medicine, The University of Tokyo, provided that eligible patients were offered the opportunity to opt out. All study protocols were conducted in accordance with the amended Declaration of Helsinki.
Statistical analysis
Comparisons of demographic and other clinical characteristics between the two groups were made using Student’s t-test for continuous parametric variables and the chi-square test or Fisher’s exact test for categorical variables. Comparisons of intraoperative factors between the two groups were made using simple logistic regression analysis, and the odds ratio and 95%CIs were calculated for each risk factor. We considered p < 0.05 as a significant difference. Furthermore, factors that showed significant differences in the univariate analysis were used as independent factors, and multiple logistic regression analysis was performed for risk factors found to be significant. Goodness of fit was tested using the Hosmer–Lemeshow test. The discrimination power of significant factors was estimated using area under the receiver operating characteristic curve (AUC). All statistical analyses were performed using IBM Statistical Package for Social Sciences (SPSS) version 25 (IBM, Armonk, NY, USA).
Results
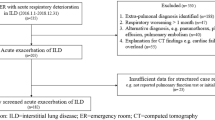
Among the 28 cases of AEs and 112 cases of non-AEs in our previous dataset, one case in the AE group and two cases in the non-AE group were excluded because these patients underwent surgery without mechanical ventilation. Two patients in the non-AE group lacked adequate intraoperative data and were therefore excluded (Fig. 1). Therefore, the total number of patients included differed from that in the study by Hosoki et al. However, the patient background was similar in both groups, even after the exclusion of five cases (Table 1). Moreover, 11 patients (41%) in the AE group died during hospitalization. Postoperatively, 22 (81%) and 102 (94%) patients in the AE and non-AE groups, respectively, were extubated in the operating room, whereas five (19%) patients in the AE group and six (5.6%) patients in the non-AE group were transferred to the intensive care unit (ICU) while. Pulmonary surgery performed 15 (56%) and 25 (23%) patients in the AE and non-AE groups, respectively. Five (19%) patients in the AE group and six (6%) patients underwent OLV in non-pulmonary surgery. The surgical site and type of surgery for each case in the AE and non-AE groups are shown in the supplementary Tables S1 and S2, and their percentages are shown in supplementary Figs. S1 and S2.
Characteristics of surgery and anesthesia management
There were nine (33%) total intravenous anesthesia (TIVA) cases out of 27 in the AE group and 27 (25%) out of 108 in the non-AE group, and this difference was not significant (p = 0.38). The rate of OLV cases was higher in the AE group (19, 70%) compared to that in the non-AE group (31, 29%) (OR, 5.9; 95%CI, 2.34–14.88; p < 0.001). There were 13 (48%) patients in the AE group and 28 (26%) patients in the non-AE group who received intraoperative steroid administration. The rate of intraoperative steroid administration was higher in the AE group (OR, 2.65; 95%CI, 1.11–6.33; p = 0.028). Two patients (7.4%) in the AE group and 14 (13%) in the non-AE group underwent laparoscopic surgery. The proportion of laparoscopic surgery and duration of anesthesia were not significantly different between the AE and non-AE groups (p = 0.43 and p = 0.36, respectively). Blood loss and in–out balance did not significantly differ between the groups (p = 0.069 and p = 0.44, respectively). Seven patients (26%) in the AE group and 18 (17%) in the non-AE group received blood transfusions (p = 0.26). There were 12 (44%) cases of perioperative opioid use in the AE group and 56 (52%) in the non-AE group (p = 0.49). The number of cases reporting the use of local anesthesia was 14 (52%) in the AE group and 61 (56%) in the non-AE group (p = 0.67) (Table 2). Regarding intraoperative events, there were 20 (74%) desaturation cases in the AE group and 60 (56%) in the non-AE group (p = 0.09). The number of hypercapnia cases was 5 (19%) in the AE group and 12 (11%) in the non-AE group. No significant differences were observed between the groups (p = 0.31).
Intraoperative ventilation management
The mean airway pressure during mechanical ventilation was 0.9 cmH2O higher in the AE group than in the non-AE group (9.2 ± 1.8 vs. 8.3 ± 1.7 cmH2O; OR, 1.36; 95%CI, 1.04–1.79; p = 0.026). No significant differences were observed in the mean PEEP between the groups (p = 0.47). Seventeen cases (63%) in the AE group and 79 cases (73%) in the non-AE group had intraoperative hyperoxic exposure (i.e., FIO2 > 0.8 for > 1 min) (p = 0.30). Mean inspiratory oxygen during mechanical ventilation did not significantly differ between the groups (p = 0.31). The duration of mechanical ventilation was not statistically different between the groups (p = 0.37) (Table 3).
Multiple logistic regression analysis of potential risk factors
We performed multiple logistic regression to identify potential risk factors, such as OLV, intraoperative steroid administration, and the average mean inspiratory pressure during intubation. The results revealed a significant difference in OLV (OR, 4.99; 95%CI, 1.91–13.06; p = 0.001) (Table 4).
Discussion
In this case-matched retrospective observational study, we found that OLV, intraoperative steroid administration, and high mean inspiratory pressure were potential predictors of postoperative AEs during general anesthesia in pulmonary and non-pulmonary surgeries. On the contrary, high intraoperative FIO2 levels were not revealed to be associated with postoperative AEs. Multiple logistic regression indicated a significant association between OLV and postoperative AEs.
The AEs occurred in patients who underwent OLV, even in non-pulmonary surgery. It has been suggested that OLV itself is a risk factor for the development of AEs. In addition to pulmonary surgery (14 cases), this study included one case of distal arch descending aorta replacement, one case of thoracoscopic thymectomy, and three cases of esophagectomy among the OLV cases in the AE group. These surgeries were performed without direct lung invasion. Therefore, further investigation is necessary to assess whether OLV is an independent risk factor for postoperative AEs in non-pulmonary surgeries.
During OLV, the ventilated lung may be exposed to greater stress due to the higher pressure compared to that in two lung ventilation. In the present study, the mean airway pressure in the AE group was 0.9 cmH2O higher than that in the non-AE group, and the difference was statistically significant. Low tidal volume and high PEEP management are recommended treatment strategies for ARDS15,16. Moreover, high airway pressure can cause ventilator-induced lung injury (VILI)17. In 2015, Amato et al. reported that low driving pressure (single ventilation, volume/compliance) is associated with increased survival in patients with ARDS18. The probability of postoperative complications has also been reported to increase by 3.4% for every 1 cmH2O increase in driving pressure during OLV19,20. Considering that the mean airway pressure difference between the AE and non-AE groups in this study was 0.9 cmH2O, the effect of driving pressure could be related to AEs.
There was a significant difference in the average mean airway pressure in the AE group in the present study. Driving pressure is often considered an indicator of ventilation management13. However, we could not evaluate driving pressure because we could not extract data on tidal volume, respiratory system compliance, and plateau pressure from the anesthesia database. Therefore, it remains unclear whether high mean airway pressure affects the development of postoperative AEs.
Preoperative steroid use is reported to be a predictor of AE following pulmonary resection6. The results of the present study suggested that steroid use during general anesthesia may be a predictor of postoperative AE. In the present study, the purpose of steroid use was not limited to prophylaxis for AE (e.g., hydrocortisone for liver resection and methylprednisolone for cardiopulmonary bypass). Moreover, an association has been reported between high average daily steroid use for AE-IPF and in-hospital mortality21. Therefore, we should carefully assess perioperative steroid use in patients with ILD.
Several studies have suggested that high FIO2 levels may damage lung tissue8,9,10. However, in the present study, there was no significant difference in FIO2 between the AE and non-AE groups. One possible reason for this is that the anesthesiologists in charge who are aware of the risk are expected to maintain inspiratory oxygen levels at the minimum necessary. It is unclear whether AEs occur more frequently when higher oxygen concentrations are administered.
There were no differences between the two groups in the use of volatile or intravenous anesthesia in the present study. We could not determine the anesthesia method that was more favorable in preventing respiratory complications in patients with ILD. More studies are needed to determine whether the choice of anesthesia for patients with ILD is associated with postoperative AEs.
The factors evaluated in the present study are those that anesthesiologists can observe or intervene to treat during the perioperative period. Higher peak pressure might be needed because of a "difficult to ventilate" lung, so that this would be an unmodifiable factor. However, since high airway pressure itself may be the cause of AEs, it is worthwhile to consider lung protective ventilation during surgery. In addition, since OLV may be avoided in pulmonary surgery22, it is worthwhile to consider the indication for OLV in patients who are at high risk. Furthermore, the findings of this study can be useful for anesthesiologists in determining perioperative strategies for ILD patients.
Limitations
Our study has some limitations. First, causality and the effect of unmeasured confounding factors could not be determined, as this was a retrospective study. Second, we were unable to extract the data of some parameters from the anesthesia database. Comparison of the tidal volume, respiratory system compliance, and plateau pressure between patients in the AE and non-AE groups would have facilitated clarification of whether AEs are due to OLV itself or ventilator management. Third, this was a single-center study. Therefore, a study on the management of general anesthesia in patients with ILD at multiple centers is needed.
Although case matching of pulmonary and non-pulmonary surgery requires a large number of cases, we did not perform this study because it was a secondary analysis, and the number of cases was limited. However, we thought it would be worthwhile to examine whether OLV itself contributes to the occurrence of AEs, regardless of whether pulmonary surgery is performed.
We recommend that clinical studies be carried out to determine clinical strategies for better AE management. In addition, a prospective study with a larger number of AEs should be conducted to compare the results.
Conclusions
Perioperative clinical management was compared between ILD patients with and without postoperative AE when surgery was performed under general anesthesia. OLV was significantly associated with postoperative acute exacerbation of ILD. OLV should be carefully considered in general anesthesia for ILD patients.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Choi, S. M. et al. Postoperative pulmonary complications after surgery in patients with interstitial lung disease. Respiration 87, 287–293 (2014).
Watanabe, A., Kawaharada, N. & Higami, T. Postoperative acute exacerbation of IPF after lung resection for primary lung cancer. Pulm. Med. 2011, 960316 (2011).
Chida, M. et al. Incidence of acute exacerbation of interstitial pneumonia in operated lung cancer: Institutional report and review. Ann. Thorac. Cardiovasc. Surg. 18, 314–317 (2012).
Ghatol, A., Ruhl, A. P. & Danoff, S. K. Exacerbations in idiopathic pulmonary fibrosis triggered by pulmonary and nonpulmonary surgery: A case series and comprehensive review of the literature. Lung 190, 373–380 (2012).
Furuya, K. et al. Acute exacerbation of idiopathic interstitial pneumonia after nonpulmonary surgery under general anesthesia: A retrospective study. Sarcoidosis Vasc. Diffuse Lung Dis. 34, 156–164 (2017).
Sato, T. et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J. Thorac. Cardiovasc. Surg. 147, 1604-1611.e3 (2014).
Hosoki, K. et al. Predictors of postoperative acute exacerbation of interstitial lung disease: A case-control study. BMJ Open Respir. Res. 7, e000634 (2020).
Chow, C. W., Herrera Abreu, M. T., Suzuki, T. & Downey, G. P. Oxidative stress and acute lung injury. Am. J. Respir. Cell Mol. Biol. 29, 427–431 (2003).
Duggan, M. & Kavanagh, B. P. Pulmonary atelectasis: A pathogenic perioperative entity. Anesthesiology 102, 838–854 (2005).
Kotani, N. et al. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology 93, 15–25 (2000).
Hayashi, K., Nakashima, K., Noma, S., Aoshima, M. & Kusanagi, H. Laparoscopic surgery in patients with interstitial lung disease: A single-center retrospective observational cohort study. Asian J. Endosc. Surg. 13, 279–286 (2020).
Hickling, K. G., Henderson, S. J. & Jackson, R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 16, 372–377 (1990).
Neto, A. S. et al. Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: A meta-analysis of individual patient data. Lancet Respir. Med. 4, 272–280 (2016).
Raghu, G. et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 183, 788–824 (2011).
Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 342, 1301–1308 (2000).
Villar, J., Kacmarek, R. M., Pérez-Méndez, L. & Aguirre-Jaime, A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: A randomized, controlled trial. Crit. Care Med. 34, 1311–1318 (2006).
Ricard, J. D., Dreyfuss, D. & Saumon, G. Ventilator-induced lung injury. Eur. Respir. J. Suppl. 42(Suppl 42), 2s–9s (2003).
Amato, M. B. et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 372, 747–755 (2015).
Park, M. et al. Driving pressure during thoracic surgery: A randomized clinical trial. Anesthesiology 130, 385–393 (2019).
Blank, R. S. et al. Management of one-lung ventilation: Impact of tidal volume on complications after thoracic surgery. Anesthesiology 124, 1286–1295 (2016).
Cuerpo, S. et al. Acute exacerbations of idiopathic pulmonary fibrosis: Does clinical stratification or steroid treatment matter?. Chron. Respir. Dis. 16, 1479973119869334 (2019).
Pompeo, E. et al. Nonintubated surgical biopsy of undetermined interstitial lung disease: A multicentre outcome analysis. Interact. Cardiovasc. Thorac. Surg. 28, 744–750 (2019).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
F.S. and G.K. had full access to all the data used in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. K.H., T.J., M.U., and K.U. substantially contributed to the study design, data analysis and interpretation, and the writing of this manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
K. Uchida is involved in two reports receiving collaborative research contracts between the University of Tokyo and grants from (1) Nihon Kohden Corporation and (2) Nipro Medical Corporation. T. Jo is a member of the Department of Health Services Research, which is a cooperative program between The University of Tokyo and Tsumura & Co. The other authors have no conflict of interest with any companies and/or organizations whose products or services may be discussed in this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seto, F., Kawamura, G., Hosoki, K. et al. Secondary analysis of preoperative predictors for acute postoperative exacerbation in interstitial lung disease. Sci Rep 13, 13955 (2023). https://doi.org/10.1038/s41598-023-41152-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41152-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.