Abstract
Fatigue is barrier of physical activity participation in adults with chronic conditions. However, physical activity alleviates fatigue symptoms. This systematic review and meta-analysis aimed to (1) synthesise evidence from randomised controlled trials (RCTs) exploring the effects of physical activity interventions on fatigue reduction and (2) evaluate their effectiveness. Medline/CINAHL/EMBASE/Web of Science and Scopus were searched up to June 24th, 2023. Two reviewers independently conducted study screening and selection (RCTs), extracted data and assessed risk of bias (RoB2). Outcome was the standardised mean difference (SMD) with 95% confidence intervals in fatigue between experimental and control groups. 38 articles met the inclusion criteria. Overall, physical activity interventions moderately reduced fatigue (SMD = 0.54, p < 0.0001). Interventions lasting 2–6 weeks demonstrated a larger effect on fatigue reduction (SMD = 0.86, p < 0.00001). Interventions with 18–24 sessions showed a large effect on fatigue reduction (SMD = 0.97, p < 0.00001). Aerobic cycling and combination training interventions had a large to moderate effect (SMD = 0.66, p = 0.0005; SMD = 0.60, p = 0.0010, respectively). No long-term effects were found during follow-up. Physical activity interventions moderately reduced fatigue among adults with chronic conditions. Duration, total sessions, and mode of physical activity were identified as key factors in intervention effectiveness. Further research is needed to explore the impact of physical activity interventions on fatigue.
Similar content being viewed by others
Introduction
The prevalence of chronic conditions rises with age, with approximately 62% of Americans aged 65 and above having at least one chronic condition1,2,3. Fatigue is a common and complex symptom reported by individuals with various chronic conditions (e.g., cancer, Parkinson’s Disease, inflammatory arthritis, and fibromyalgia)4,5,6,7. Despite its common occurrence, fatigue lacks a clear definition due to its multidimensionality, encompassing physical, mental, cognitive, emotional, and motivational fatigue8,9,10. Within the literature, fatigue is described as a disruptive, severe, and overwhelming symptom with cognitive elements among adults with chronic conditions8,11,12,13,14. The prevalence of fatigue in this population ranges from 39 to 80%6,15,16,17,18, making it a significant factor associated with limitations in functional independence and a barrier to engaging in physical activity (PA) for individuals with chronic conditions experiencing fatigue symptoms19,20,21.
Furthermore, fatigue often co-exists with other medical symptoms, such as pain, depression, and cognitive deficit22. Also, significant fatigue can have a detrimental effect on daily activities and health-related quality of life among individuals with chronic conditions19,23,24,25. In daily life context, fatigue exhibits multiple negative effects, which have been observed to progressively escalate from impaired attention and reduced PA levels to increased risk of falls, disabling conditions, and mortality9,26. In general, fatigue has a negative impact on health and functioning of individuals with chronic conditions, leading to a decrease in their health-related quality of life27. Yet, the experience of fatigue symptoms often correlates with lower exercise engagement and a lack of independence28,29.
Lack of PA engagement is prevalent among adults with chronic conditions across the lifespan19. Fatigue can act as a barrier to activity engagement, as intense exercise during periods of fatigue can cause a negative affective load, further discouraging future engagement in activities30,31. However, the relationship between PA and fatigue is complex, with conflicting outcomes suggesting both positive effects of PA on fatigue reduction and the negative impact of fatigue on PA participation9,20. While some recommendations suggest that PA might reduce fatigue symptoms32,33,34,35, other studies indicate limited or no effects of exercise therapy on fatigue among adults with chronic fatigue syndrome36 and Parkinson’s disease37. Nevertheless, PA offers benefits for overall functioning, health, well-being, and quality of life and is associated with a reduced risk of premature mortality13,38,39,40,41,42. Specifically, exercise and PA interventions in adults with mild cognitive impairments have shown positive effects on cognitive function40,43. Additionally, resistance training has also demonstrated favorable outcomes in measures of reasoning44 and exergaming has been found to improve cognitive function45. Furthermore, PA has proven mental health benefits, reducing morbidity and mortality and promoting improved sleep42,46.
PA is a critical element of fatigue management and has the potential to positively impact individuals’ well-being and quality of life47. While exercise-based interventions can lead to notable improvements in fitness within few weeks, the challenge lies in sustaining these outcomes over time. For this reason, the effectiveness of these interventions may rely on various intervention ingredients, including the duration, total sessions, and mode of PA. These ingredients are important considerations for both health-professionals and end-users, with implications for cost-effectiveness and sustained outcomes48. For instance, a study on adolescents found that an intervention of 7 weeks is adequate for improving physical fitness49. Therefore, further exploration of the importance of intervention duration and the role of different PA modes in addressing fatigue is crucial. Moreover, the mode of PA might play a key role on PA interventions and the long-term effects of it. It has been found that people are more likely to participate in PA if they are enjoying it50, which might be related to preferred exercise mode.
In the view of the above, it is important to explore how PA interventions reduce fatigue symptoms in adults with chronic conditions. PA interventions targeting fatigue often focus on one specific chronic condition rather than addressing the symptom itself, despite its prevalence in various chronic conditions. Interventions targeting fatigue are essential in the onset of symptoms and could align with individuals’ needs51. Therefore, gaining a better understanding of fatigue transdiagnostically, could provide valuable insights into the relationship between PA and fatigue. Taking a transdiagnostic approach in studying fatigue could offer a broader perspective and enhance our understanding of effective fatigue management strategies. The transdiagnostic approach involves examining fatigue across multiple chronic conditions, considering that experiencing a chronic condition is frequently associated with fatigue52,53. Targeting fatigue management early in its onset through PA, may yield beneficial outcomes, particularly in cases where the underlying causes remain undetermined.
In the current review, we adopt a transdiagnostic approach, focusing on diverse chronic conditions. This approach is valuable and innovative as it emphasizes the importance of symptom-dependency and disease-independency. Notably, the existing literature lacks comprehensive reviews encompassing a wide range of chronic conditions, including both oncological and non-oncological conditions, regarding the potential of PA in reducing fatigue symptoms. Furthermore, this review explores various ingredients of PA interventions (intervention length, total sessions, mode of PA) and their impact on perceived fatigue. To the best of our knowledge, there are currently no published systematic reviews/meta-analyses primarily investigating the effectiveness of PA intervention ingredients in reducing fatigue among adults with various chronic conditions. The outcomes of this review could offer novel insights into a transdiagnostic approach that targets fatigue symptoms and underscores the importance of PA for individuals with chronic conditions. Additionally, this systematic review and meta-analysis may inform researchers and health-professionals about the further development of PA interventions, while recognising the need for additional research in this area. The selection of randomised controlled trials (RCTs) for inclusion in this systematic review is based on their recognised status as the highest quality evidence in evaluating interventions54. Though, we also appreciate other research methodologies and designs, RCTs provide robust evidence regarding the effectiveness of interventions and their results can inform the design of future interventions55. Overall, the high-quality evidence derived from RCTs is valuable in guiding clinical practice and decision-making55.
Based on the aforementioned rationale, the first aim of this review was to comprehensively synthesise evidence from RCTs on whether physical activity interventions significantly reduced fatigue symptoms among adults with chronic conditions. The second objective was to evaluate the effectiveness of PA interventions on perceived fatigue through a meta-analysis of the scientific literature.
Methods
Search strategy
The systematic review and meta-analysis were designed following the PRISMA statement for systematic review protocols and reporting guidelines56. The review was not registered. The search strategy was developed and tested in Medline using a combination of fatigue, PA, chronic disorder, and fatigue assessment terms. The search was modified for the following electronic databases: EMBASE; Web of Science; CINAHL; Scopus. No date limitations were applied. The search was performed on June 24th 2023. The full search strategy for all databases can be found in Table 1.
Eligibility criteria
Randomised controlled trials of PA interventions for adults with chronic diseases with fatigue reported as a primary or secondary outcome were included. Eligible RCTs included adult participants who were assigned randomly to a physical intervention or a control group. In addition, articles should have reported primary research studies in English. Excluded were protocol papers, editorials, discussion papers, and comments. Moreover, tailored interventions for the participants were excluded since this review aims to explore specific intervention programs that are based on objective and specific criteria for the PA intervention.
Data management and screening
The EndNote software version 20.4 was used to remove the duplicates and the remaining results were imported to Rayyan57, which is a web tool designed for systematic reviews. Titles and abstracts were screened by two independent reviewers (IB and KES) to determine whether they met the eligibility criteria. The non-eligible abstracts were rejected, and numbers were documented. The full texts of potentially eligible studies were retrieved and assessed independently by IB and KES. All decisions of inclusion or exclusion were automatically recorded in Rayyan, and reviewers were blinded to each other’s decisions. Any disagreements were discussed and resolved by consensus between the two reviewers or by consulting a third reviewer (FJH). The outcome data that were used for the meta-analysis were extracted by IB. Uncertainties about outcome data were discussed with KES and USA and the original paper was accessed to reach an agreement.
Risk of bias assessment
The risk of bias was assessed by two independent reviewers (IB, KES) using the Cochrane risk of bias tool consisting of five domains (version 2, ROB2)58. This approach addresses the following domains: the randomization process, the effect of assignment or adhering to intervention, missing outcome data, measurement of the outcome, and selection of the reported result. Each domain is scored as “low”, “some concerns”, or “high risk” of bias. Then, an overall risk of bias for each trial is provided through the tool’s algorithm. Disagreements about the risk of bias assessments were discussed and resolved by consensus by referring to the full text. ROB2 tool was used to create the risk of bias figures.
Statistical analysis
The primary outcomes in the meta-analysis included the effect of different PA interventions on perceived fatigue as a first step. Then, the effects of the length and total sessions of interventions and the mode of PA on perceived fatigue were investigated as a second step in which we aimed to explore different ingredients of the interventions. In the analyses, the studies were distributed in different subgroups based on:
-
the length of the interventions (2–6 weeks, 7–10 weeks, 11–15 weeks, 16 + weeks)
-
the total sessions of the interventions (8–16 sessions, 18–24 sessions, 30–36 sessions, 45–48 sessions, 54 + sessions)
-
the mode of PA (aerobic running, aerobic cycling, balance, resistance, combination, exergaming aerobic, horseback riding)
In addition, meta-analysis was conducted for the studies that included the post-trial follow ups, which assesses the effect retained at follow up, thus indicating the long-term effects. Random effects models were used for the inverse variance meta-analysis conducted in the Review Manager software (Review Manager 5.4; The Nordic Cochrane Centre, The Cochrane Collaboration). Because the interventions used different scales for the fatigue assessment, we performed standardised mean differences (SMD). SMD with 95% confidence intervals (CI) was used to describe the experimental and control group differences for post-intervention values in PA intervention subgroups as well as the overall effect. In addition, post-trial follow-ups were considered if appropriate. SMD values of 0.2, 0.5, and 0.8 represent a low effect, a moderate effect, and a large effect, respectively59. When the potentially eligible articles did not report mean (M) and standard deviation (SD), the corresponding authors were contacted by email to request the data. The articles were excluded from the meta-analysis if there was no reply within two weeks; however, they were included in the systematic review.
Statistical heterogeneity among studies was assessed by calculating the I2 index. Low heterogeneity was considered when I2 ≤ 25%, moderate when I2 ≤ 50% and > 25%, and high when ≤ 75% and > 50%60. Subgroup analysis was used to analyse the effectiveness of the PA interventions.
Moreover, among 31 studies, included in our analysis, five studies consisted of two experimental groups and one control group61,62,63,64,65. To ensure consistency, we examined whether the groups within each study belonged to the same PA mode. If both groups shared the same PA mode as well as intervention length, and total sessions, they were combined for the meta-analysis. Conversely, if different PA modes were used within the same study experimental groups, they were analysed separately.
Publication bias
Publication bias was conducted in RStudio using Egger’s test on the meta-analysis data66,67, which evaluates funnel plot asymmetry. The level of statistical significance was set to α < 0.05.
Results
Search result
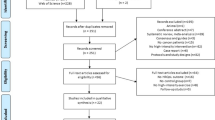
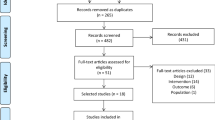
Figure 1 demonstrates the search process in a flow diagram. A total of 5004 unique articles were identified from our initial search of five databases, while 3288 titles and abstracts were screened after removing the duplicates. Following, 139 potentially eligible full text articles were assessed. After the screening process, 38 studies were included in this systematic review.
Characteristics of included studies
The included studies involved 2091 participants from different medical condition populations (receiving a PA intervention: n = 1071, controls: n = 1020). The subjects in all studies were adults, aged 19–89 years. The gender of subjects in those 38 studies was: female = 75.37% and male = 24.63%. All the studies were conducted in adults with the following chronic disorders: multiple sclerosis (n = 17)61,62,63,64,68,69,70,71,72,73,74,75,76,77,78,79, cancer (n = 11)80,81,82,83,84,85,86,87,88,89,90,91, chronic obstructive pulmonary disease (n = 1)65, fibromyalgia syndrome (n = 1)92, Parkinson's disease (n = 3)37,93,94, axial spondylarthritis (n = 1)95, bowel syndrome (n = 1)96, stroke (n = 1)97, rheumatoid arthritis (n = 1)98 and kidney failure (n = 1)99.
Table 2 presents the characteristics of the included studies and PA interventions. The characteristics include the sample size and subjects’ age and body mass index (BMI), the fatigue assessment, the mode, description, and intensity of PA, the duration and frequency of the PA interventions as well as its adherence.
The fatigue severity scale (FSS) was used in twenty-three studies and was the most frequently used fatigue measure among the thirty-eight studies. The FSS is a validated and reliable 9-item questionnaire designed to assess the impact of perceived fatigue among adults diagnosed with chronic conditions100,101,102. The functional assessment of chronic illness therapy—fatigue scale (FACIT-F) was used in seven studies. FACIT-F (version 4) is a 40-item questionnaire evaluating self-reported fatigue and its influence on everyday activities and function among adults with cancer and older people who experience fatigue103. It is valid and reliable in several patients’ populations104,105,106. One study used the fatigue assessment questionnaire (FAQ), which is a validated 20-item questionnaire assessing fatigue among adults with cancer107,108. The modified fatigue impact scale (MFIS) was used by one study and contains 9 items109. MFIS provides an assessment of the effects of fatigue in physical and cognitive functioning and is reliable and valid in several clinical populations110. The multidimensional fatigue inventory (MFI) is 20-item questionnaire evaluating fatigue and was used by one study111. MFI is valid and reliable in several chronic conditions112,113. The visual analogue scale (VAS) is an 18-item questionnaire evaluating fatigue and is valid and reliable in stroke population114. The multidimensional fatigue symptom inventory (MFSI) is a 30-item questionnaire and was used by one study. It measures fatigue and it is valid and reliable in cancer populations115. Among the 38 PA interventions, eight used aerobic training, thirteen interventions used balance training, while sixteen interventions used a combination training of strength, balance, and/or aerobic. Strength training was used by six PA interventions. Two studies used dance (Tango), and horse-riding training.
Risk of bias
The Cochrane tool was used to assess the risk of bias in included studies (n = 38). Figure 2a and b show the risk of bias analysis. Overall, twenty-five studies showed a high risk of bias, and thirteen studies showed some concerns. The trials by Etnier et al.92, Rios Romenets et al.94 and Van Den Berg et al.75 presented a high risk of bias in the domain of randomization process. Whereas 18 trials showed a low risk of bias in this domain37,62,63,64,65,74,77,79,84,85,87,89,90,93,95,97,98,116. The trials conducted by Duruturk et al.65, Ozkul et al.72 and Petajan et al.73 showed high risk of bias in the deviations from intended intervention. While only three trials presented as low risk77,83,98. In the domain of missing outcome data, 22 trials presented a low risk of bias37,61,68,70,72,74,76,79,80,81,83,84,85,86,90,91,93,95,96,97,98,99 while only four presented a high risk of bias62,64,78,88. 15 trials showed a low risk of bias61,70,71,72,78,79,83,86,87,90,91,93,95,96,97 in the measurement of outcome domain while three showed a high risk of bias73,74,88. Ten trials presented a low risk of bias61,72,86,87,91,93,95,96,97,117 in the selection of reported results while no trials presented a high risk.
Effects of PA interventions on perceived fatigue
Initially, a meta-analysis was conducted to investigate the effects of all the included PA interventions on perceived fatigue. Eventually, 31 articles out of the 38 were included in the meta-analysis. The test for overall effect indicates that there is a moderate effect for reduction in perceived fatigue based on random effects model (SMD = 0.54; 95% CI = 0.79 to 0.29; p < 0.00001) with high heterogeneity results between studies (I2 = 79%). The outcomes are illustrated in Fig. 3.
The effects of the intervention length on perceived fatigue are presented in Fig. 4. The test for subgroup differences was not statistically significant with low heterogeneity (p = 0.38, I2 = 2.5%). Interventions that lasted for 2–6 weeks have a larger effect (SMD = 0.86; 95% CI = 1.24 to 0.48; p < 0.00001) compared to interventions that were 7–10 weeks (SMD = 0.76; 95% CI = 1.22 to 0.29; p = 0.001). The results from the interventions that were 2–6 weeks showed low heterogeneity (I2 = 10%) while the results from the interventions that were 7–10 weeks showed high heterogeneity (I2 = 79%). Moreover, the interventions that were 11–15 weeks showed a low effect for perceived fatigue (SMD = 0.48; 95% CI = 0.76–0.20; p = 0.0008) and results were heterogeneous between studies (I2 = 72%). No effect was found for the interventions that were 16 weeks + (SMD = 0.07; 95% CI = 1.86–1.73; p = 0.94) and results were heterogeneous between studies (I2 = 93%).
The effects of the intervention total sessions of PA interventions on perceived fatigue are illustrated in Fig. 5. The test for the subgroup differences was statistically significant with high heterogeneity (p = 0.01; I2 = 68%). Meta-analysis showed no effect in perceived fatigue in the 8–16 sessions (SMD = 0.23; 95% CI = 0.59–0.14; p = 0.23) and results were heterogeneous between studies (I2 = 51%). Interventions with 18–24 sessions had a large effect for perceived fatigue (SMD = − 0.97; 95% CI = − 1.29 to − 0.64; p < 0.00001) and results were found heterogenous (I2 = 70%). The interventions with 30–36 sessions showed no effect (SMD = 0.21; 95% CI = 1.16–0.75; p = 0.67) and heterogeneity was found between studies (I2 = 91%). Likewise, meta-analysis showed no effect for perceived fatigue in the interventions of 45–48 sessions (SMD = 0.22; 95% CI = 0.72–1.17; p = 0.64) and results between the studies were heterogeneous (I2 = 85%). Similarly, interventions with 54+ sessions were found to have no statistically significant results (SMD = 0.79; 95% CI = − 2.00 to 0.41; p = 0.20, I2 = 89%).
The effects of the mode of PA interventions on perceived fatigue are illustrated in Fig. 6. Overall, the test for subgroup differences suggests that there is a statistically significant subgroup effect based on a random model with high heterogeneity (p = 0.002; I2 = 71.2%). Meta-analysis showed no effect for perceived fatigue in the aerobic running training subgroup (SMD = 1.25; 95% CI = − 3.61 to 1.11; p = 0.30) and results between studies were heterogenous (I2 = 89%). The estimates showed a moderate effect for perceived fatigue in the aerobic cycling training interventions (SMD = − 0.66; 95% CI = − 1.03 to − 0.29; p = 0.0005) and the results between studies were not heterogeneous (I2 = 0%). Interventions with balance training showed a low effect for perceived fatigue (SMD = − 0.40; 95% CI = − 0.80 to − 0.01; p < 0.0001) and results between studies were moderately heterogeneous (I2 = 60%). Interventions with resistance training showed no statistically significant results for perceived fatigue (SMD = 0.54; 95% CI = 1.43–0.35; p = 0.24, I2 = 86%). Interventions with combination training showed a moderate effect for perceived fatigue (SMD = 0.60; 95% CI = 0.95–0.24; p = 0.0010) and results were heterogeneous between studies (I2 = 80%). Interventions with aerobic exergaming and horseback riding were not included in the subgroup meta-analysis since there was only one study in each group.
A meta-analysis was conducted for the post-trial follow ups. There were only eight studies that included post-trial follow up measurements. The test for the long-term effects showed no effect for perceived fatigue (SMD = 0.32; 95% CI = 0.60–0.04; p = 0.003) and results were moderately heterogenous between studies (I2 = 45%). The outcomes are illustrated in Fig. 7.
Publication bias
The publication bias assessment was estimated on the meta-analysis data. The analysis showed statistically significant evidence of publication bias (z = − 4.3163, p < 0.0001). The funnel plot demonstrated visible asymmetry, indicating the possibility of publication bias. The estimated intercept of the regression line was − 0.7805 (95% CI: − 1.0314 to − 0.5296) when the standard error of the effect sizes approached zero. The funnel plot is illustrated in Fig. 8.
Discussion
In this meta-analysis, data from 33 randomised control trials were synthesized to examine the effectiveness of PA interventions in reducing perceived fatigue among adults with chronic conditions. To the best of our knowledge, this is the first study to comprehensively evaluate the impact of PA interventions on perceived fatigue across a range of chronic conditions.
Firstly, this meta-analysis demonstrates that PA interventions have a moderate effect on perceived fatigue (SMD = 0.54) among adults with chronic conditions. Previous studies investigating the effects of PA on fatigue have yielded inconsistent findings. Some studies have demonstrated a positive association between exercise and fatigue in adults with chronic conditions118,119. Others, have reported that PA can reduce both fatigue and pain33,35,120, while contradictory results have indicated no effect of exercise on fatigue37,121. Our meta-analysis identifies a moderate effect of PA on fatigue reduction, providing new insights for the future design of targeted PA interventions to alleviate fatigue in individuals with various chronic conditions121,122.
Regarding the intervention length, this meta-analysis revealed that trials lasting from two to ten weeks2,3,4,5,6,7,8,9,10 demonstrated a large to moderate effect on perceived fatigue among adults with chronic conditions (SMD = 0.86, 0.76, respectively). Additionally, the subgroup of eleven to fifteen showed a low to moderate effect (SMD = 0.48) However, high heterogeneity was observed in the seven to ten weeks and eleven to fifteen weeks subgroups, potentially influenced by other intervention characteristics such as total sessions or mode of PA or even the disease diagnosis. In contrast, low heterogeneity was found in the 2–6 weeks intervention length, although the limited number of trials in this subgroup may be a limitation. Trials lasting from sixteen to 24 weeks were found to have no effect on perceived fatigue, with high heterogeneity and limited trials in this subgroup posing potential limitations. Additionally, adherence to the intervention was reported in two out of the four trials in this subgroup71,80,81,92, with adherence rates of 65%92 and 93.3%71. Due to lack of adherence reporting, we cannot definitively conclude that adherence might be a reason for the low effect on perceived fatigue. The duration of an intervention holds importance for health professionals and researchers, as clinical and health interventions often face budget constraints122. While this meta-analysis suggests a trend of shorter PA interventions being effective for alleviating fatigue, other factors may influence the results. Therefore, future research is necessary to determine the optimal and effective intervention duration, considering factors such as cost-effectiveness and time-efficiency in research and rehabilitation settings.
The exploration of total sessions in PA interventions yielded interesting findings. It was observed that interventions comprising 18–24 sessions had a substantial impact on perceived fatigue (SMD = 0.97). The considerable heterogeneity observed in the results could be partially attributed to other intervention characteristics, such as intervention duration and mode. On the other hand, subgroups with 8–16, 30–36, 45–48, and 54+ total sessions showed no effect on perceived fatigue. The uneven distribution of studies among these subgroups might have limited the analysis of their effects on the total sessions of the interventions. Thus, researchers should be careful when interpreting the pooled effect sizes and focus on the observed data patterns. Furthermore, the duration of PA sessions and adherence to weekly PA also play crucial roles in achieving desirable outcomes. However, investigating this element in the meta-analysis proved challenging due to the inclusion of various chronic conditions, each with their specific PA guidelines, although they share some similarities.
Moreover, this meta-analysis revealed that aerobic cycling and combination training have moderate effects on perceived fatigue in adults with chronic conditions (SMD = 0.66, and 0.60, respectively). However, there was variation in heterogeneity among these subgroups. The aerobic cycling training subgroup exhibited no heterogeneity. However, it is important to note that this subgroup had a limited number of studies, which could have influenced the results. In contrast, the combination training subgroup, incorporating different training components such as aerobic, resistance, and balance exercises, displayed high heterogeneity across the studies. The heterogeneity could be attributed by the variations in the combination training programs implemented. Existing literature suggests that aerobic and resistance training have been effective in alleviating fatigue symptoms among individuals with chronic conditions33,123,124,125. Conversely, treadmill running has shown no improvement in fatigue symptoms119. Therefore, the literature provides conflicting findings regarding the effects of different PA modes on fatigue, necessitating further exploratory studies in this area. Furthermore, the importance of PA enjoyment has been highlighted in the literature, as it has been observed that individuals who find an activity enjoyable are more likely to stay engaged and experience PA benefits while reducing their fatigue symptoms50. Additionally, individuals with chronic disorders may experience discomfort and other symptoms such as shortness of breath during aerobic training; underscoring the need for personalised options tailored to their specific needs. The inclusion of a range of PA modes seems promising, as it allows for choice, but further research is needed in this regard.
The effects of PA interventions on fatigue reduction on post-trial follow up were examined in eight studies included in this review, which revealed no significant effect of the PA intervention on fatigue reduction during the follow up. However, it is important to note that the level of PA participation between the end of the intervention and the follow up period was not clearly outlined in the included studies. Among the two studies that reported guidance during follow up, conflicting approaches were observed. One study motivated participants to maintain an active lifestyle and continue exercising, while another study encouraged them to resume their pre-intervention daily routine to assess the effects of the intervention after a period of inactivity. The contrasting guidance provided to participants could have influenced the effects of PA interventions on fatigue during follow up, as sustained PA during this period might suggest that the effects on fatigue could be diminished in the long term. Furthermore, the scarcity of studies and the high heterogeneity preclude definitive conclusions regarding the sustained effect of PA interventions on fatigue reduction. Nevertheless, it was observed that most PA interventions do not include post-trial follow up measurements, which are essential for identifying potential risks that may not be evident during the trial period126,127. In many cases, after the standardised intervention length, adults with chronic conditions struggle to remain physically active. Notably, a study aiming to promote long term PA adherence after rehabilitation discharge in individuals with chronic conditions demonstrated successful outcomes even one year after follow-up128,129.
Individuals with chronic conditions often experience fatigue, leading to activity avoidance or underactivity24. Therefore, it is important to distribute activities throughout the day more effectively24. Interestingly, none of the included interventions considered incorporating self-regulatory strategies such as activity pacing (regulation of activity levels) to participants130. Integrating activity pacing guidance, taught by healthcare professionals (e.g., occupational or physical therapists) can foster a balanced and active lifestyle, effectively managing fatigue symptoms and promoting physical activity engagement4,24,131,132,133. Moreover, self-regulatory skills have been found important for moderate to vigorous intensity PA and relevant for addressing fatigue complaints132,134,135. Thus, self-regulation should be considered in PA interventions, particularly when aiming for sustained physical activity engagement, facilitating the adaption of individuals with fatigue to the new PA lifestyle changes. Overall, the combination of activity pacing and self-regulation holds promise in achieving a physically active lifestyle while effectively managing in the long term135. Therefore, it is imperative to explore long term strategies and implement follow up measures that aim to reduce fatigue symptoms among adults with chronic conditions while maintaining PA engagement over time.
Furthermore, our transdiagnostic approach focusing specifically on fatigue symptoms, emphasizes the significance of addressing fatigue symptoms through PA, which can yield numerous benefits. Wilson and Cleary’s health-related quality of life model, highlights the interconnectedness of symptom status, which influences functional health, which influences the general health perception, which consequently influences the overall quality of life136. Applying this model to our findings may suggest that improving quality of life in individuals with chronic conditions can be achieved by reducing fatigue symptoms through PA51. This perspective could indicate the potential of PA to positively impact the overall well-being and functioning of individuals with chronic conditions. Further research is recommended to provide further evidence on this approach.
Strengths and limitations
This review possesses several key strengths. It is the first systematic review/meta-analysis to follow a transdiagnostic approach on this specific topic. This approach might enhance the generalizability of the findings and provide important insights into the effects of PA interventions on fatigue reduction across multiple chronic conditions. Additionally, this review synthesises data from thirty-three interventions, allowing for a comprehensive evaluation of the effects and providing a robust foundation for recommendations.
There are also some limitations of this review. Heterogeneity among the studies may limit the generalizability of our findings. Variations in the effects of PA interventions on fatigue may be attributed, in part, to intervention characteristics such as duration, sessions, and mode of PA. Furthermore, fatigue can vary across conditions with different severity levels137. Additionally, in the current review, over 65% of the studies were judged as high-risk based on the ROB2 assessment, primarily due to deviations from the intended interventions. Additionally, other elements of PA interventions posed challenges for inclusion in the meta-analysis. For instance, studies reported intensities differently or did not report them at all. Furthermore, the proportion of female participants was higher across the studies compared to males, which could have influenced the results given the higher prevalence of fatigue in females138,139,140,141,142,143.
Implications
In a transdiagnostic sample, this meta-analysis indicates that PA interventions have a moderate overall effect in reducing perceived fatigue among adults with chronic conditions. This finding indicates the potential integration of PA into fatigue management programs. However, the limited inclusion of follow up measures and long term effects emphasizes the need for further exploration of the sustained impact of PA on fatigue reduction. Additionally, the incorporation of activity pacing and self-regulation into long term interventions is crucial as these strategies have been identified as key factors in PA and fatigue management among adults with chronic conditions. Furthermore, it is important to critically assess and investigate the effectiveness of different ingredients of PA interventions while considering confounding factors, as these outcomes might have significant implications for both healthcare professionals and patients.
Conclusion
Fatigue poses a significant barrier to PA engagement among adults with chronic conditions but our findings provide robust evidence supporting the moderate effects of PA in reducing fatigue in this population. Our meta-analysis revealed that both aerobic cycling and combination training interventions demonstrated moderate effects on fatigue reduction. Furthermore, interventions lasting 2–10 weeks showed promising results in reducing fatigue in chronic conditions. However, the observed effects on fatigue during post-trial follow-ups were low, due to the lack of studies conducting follow up measurements underscoring the importance of further investigation into the long term effects of PA interventions. Additionally, further research is needed on the effects of the specific intervention ingredients as these findings hold valuable implications for health professionals and patients.
Data availability
All data generated or analysed during this meta-analysis review are included in this published article. There was no patient and public involvement in this study. Dissemination to study participants is not applicable.
Abbreviations
- BMI:
-
Body mass index
- M:
-
Mean
- SD:
-
Standard deviation
- SMD:
-
Standardised mean difference
- CI:
-
Confidence intervals
- RCTs:
-
Randomised controlled trials
- PA:
-
Physical activity
- MeSH:
-
Medical Subject Headings
- MS:
-
Multiple sclerosis
- BC:
-
Breast cancer
- COPD:
-
Chronic obstructive pulmonary disease
- FMS:
-
Fibromyalgia syndrome
- CC:
-
Colorectal cancer
- C:
-
Cancer
- KF:
-
Kidney failure
- PD:
-
Parkinson’s disease
- IBS:
-
Irritable bowel syndrome
- RA:
-
Rheumatoid arthritis
- AS:
-
Axial spondylarthritis
- FSS:
-
Fatigue severity scale
- FACIT-F:
-
Functional assessment of chronic illness therapy—fatigue
- FAQ:
-
Fatigue assessment questionnaire
- MFIS:
-
Modified fatigue impact scale
- MFI:
-
Multidimensional fatigue inventory
- VAS:
-
Visual analogue scale
- IG:
-
Intervention group
- EG:
-
Exercise group
- RC:
-
Relaxation group
- CG:
-
Control group
- MHR:
-
Maximum heart rate
- RPM:
-
Revolutions per minute
- APMHR:
-
Age predicted maximum heart rate
- mins:
-
Minutes
- MET:
-
Metabolic equivalent of task
- RM:
-
Repetition maximum
- RPM:
-
Revolutions per minute
- THR:
-
Target heart rate
- HRR:
-
Heart rate reserve
- ES:
-
Effect size
- TOCT:
-
Task-oriented circuit training
- AGU:
-
Augmented Reality Applications in Rehabilitation
- IB:
-
Ioulia Barakou
- KES:
-
Kandianos Emmanouil Sakalidis
- USA:
-
Ulric Sena Abonie
- FJH:
-
Florentina Johanna Hettinga
References
Ward, B. W. & Schiller, J. S. Prevalence of multiple chronic conditions among US adults: Estimates from the national health interview survey, 2010. Prev. Chron. Dis. 25(10), 120203 (2013).
Jaul, E. & Barron, J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front. Public Health 11, 5 (2017).
Hayek, S., Ifrah, A., Enav, T. & Shohat, T. Prevalence, correlates, and time trends of multiple chronic conditions among israeli adults: Estimates from the israeli national health interview survey, 2014–2015. Prev. Chron. Dis. 10(14), 170038 (2017).
Abonie, U. S., Sandercock, G. R. H., Heesterbeek, M. & Hettinga, F. J. Effects of activity pacing in patients with chronic conditions associated with fatigue complaints: A meta-analysis. Disabil. Rehabil. 1, 1–10. https://doi.org/10.1080/09638288.2018.1504994 (2018).
Swain, M. G. Fatigue in chronic disease. Clin. Sci. 99(1), 1 (2000).
Enoka, R. M. & Duchateau, J. Translating fatigue to human performance. Med. Sci. Sports Exerc. 48(11), 2228–2238 (2017).
Kluger, B. M., Krupp, L. B. & Enoka, R. M. Fatigue and fatigability in neurologic inllnesses. Neurology 80, 409–416 (2013).
Billones, R., Liwang, J. K., Butler, K., Graves, L. & Saligan, L. N. Dissecting the fatigue experience: A scoping review of fatigue definitions, dimensions, and measures in non-oncologic medical conditions. Brain Behav. Immun. Health. 15, 100266 (2021).
Knoop, V. et al. Fatigue and the prediction of negative health outcomes: A systematic review with meta-analysis. Ageing Res. Rev. 67, 101261 (2021).
Ericsson, A., Bremell, T. & Mannerkorpi, K. Usefulness of multiple dimensions of fatigue in fibromyalgia. J. Rehabil. Med. 45(7), 685–693 (2013).
Li, Y. et al. Multidimensional daily diary of fatigue-fibromyalgia-17 items (MDF-fibro-17): Part 2 psychometric evaluation in fibromyalgia patients. BMC Musculoskelet. Disord. 18(1), 198 (2017).
Kratz, A. L., Schilling, S., Goesling, J. & Williams, D. A. The PROMIS FatigueFM Profile: Q self-report measure of fatigue for use in fibromyalgia. Qual. Life Res. 25(7), 1803–1813 (2016).
van Hoogmoed, D., Fransen, J., Bleijenberg, G. & van Riel, P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology 49(7), 1294–1302 (2010).
Falup-Pecurariu, C. Fatigue assessment of Parkinson’s disease patient in clinic: Specific versus holistic. J. Neural Transm. 120(4), 577–581 (2013).
de Groot, M. H., Phillips, S. J. & Eskes, G. A. fatigue associated with stroke and other neurologic conditions: Implications for stroke rehabilitation. Arch. Phys. Med. Rehabil. 84(11), 1714–1720 (2003).
Kluger, B. M. et al. Parkinson’s disease-related fatigue: A case definition and recommendations for clinical research. Mov. Disord. 31(5), 625–631 (2016).
Aldughmi, M., Bruce, J. & Siengsukon, C. F. Relationship between fatigability and perceived fatigue measured using the neurological fatigue index in people with multiple sclerosis. Int. J. MS Care 19(5), 232–239 (2017).
Al-Maqbali, M., Al-Sinani, M., Al-Naamani, Z., Al-Badi, K. & Tanash, M. I. Prevalence of fatigue in patients with cancer: A systematic review and meta-analysis. J. Pain Symptom. Manage. 61(1), 167–189 (2021).
Ginis, K., Ma, J., Latimer-Cheung, A. & Rimmer, J. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol. Rev. 2016, 1–31 (2016).
Elshahat, S., Treanor, C. & Donnelly, M. Factors influencing physical activity participation among people living with or beyond cancer: A systematic scoping review. Int. J. Behav. Nutr. Phys. Act. 18(1), 50 (2021).
Fritschi, C. & Quinn, L. Fatigue in patients with diabetes: A review. J. Psychosom. Res. 69(1), 33–41 (2010).
Kinsinger, S. W., Lattie, E. & Mohr, D. C. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology 24(5), 573–580 (2010).
Mead, G. et al. Evaluation of fatigue scales in stroke patients. Stroke 38(7), 2090–2095 (2007).
Abonie, U. S., Edwards, A. M. & Hettinga, F. J. Optimising activity pacing to promote a physically active lifestyle in medical settings: A narrative review informed by clinical and sports pacing research. J. Sports Sci. 38(5), 590–596 (2020).
Abonie, U. & Hettinga, F. Effect of a tailored activity pacing intervention on fatigue and physical activity behaviours in adults with multiple sclerosis. J. Phys. Act Health. 15(10), S76 (2020).
Kapur, N. & Webb, R. Suicide risk in people with chronic fatigue syndrome. The Lancet. 387(10028), 1596–1597 (2016).
Fisk, J. D., Pontefract, A., Ritvo, P. G., Archibald, C. J. & Murray, T. J. The impact of fatigue on patients with multiple sclerosis. Can. J. Neurol. Sci. 21(1), 9–14 (1994).
Puetz, T. W. Physical activity and feelings of energy and fatigue. Sports Med. 36(9), 767–780 (2006).
McIlvenny, S., DeGlume, A., Elewa, M., Fernandez, O. & Dormer, P. Factors associated with fatigue in a Family Medicine clinic in the United Arab Emirates. Fam. Pract. 17(5), 408–413 (2000).
Ekkekakis, P. & Dafermos, M. Exercise Is a Many-Splendored Thing, but for Some It Does Not Feel So Splendid: Staging a Resurgence of Hedonistic Ideas in the Quest to Understand Exercise Behavior (Oxford University Press, 2012).
Brand, R. & Ekkekakis, P. Affective-reflective theory of physical inactivity and exercise. German J. Exerc. Sport Res. 48(1), 48–58 (2018).
Belloni, S., Arrigoni, C. & Caruso, R. Effects from physical exercise on reduced cancer-related fatigue: A systematic review of systematic reviews and meta-analysis. Acta Oncol. (Madr.) 60(12), 1678–1687 (2021).
Cramp, F. & Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2021, 9 (2012).
Wender, C. L. A., Manninen, M. & O’Connor, P. J. The effect of chronic exercise on energy and fatigue states: A systematic review and meta-analysis of randomized trials. Front. Psychol. 3, 13 (2022).
Oberoi, S. et al. Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: A systematic review and meta-analysis of randomized trials. Crit. Rev. Oncol. Hematol. 122, 52–59 (2018).
Larun, L., Brurberg, K. G., Odgaard-Jensen, J. & Price, J. R. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst. Rev. 2021, 3 (2019).
Winward, C. et al. Weekly exercise does not improve fatigue levels in Parkinson’s disease. Mov. Disord. 27(1), 143–146 (2012).
Warburton, D. E. R. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 174(6), 801–809 (2006).
Ferrante, M. et al. Benefits of physical activity during and after thyroid cancer treatment on fatigue and quality of life: A systematic review. Cancers (Basel) 14(15), 3657 (2022).
Erickson, K. I. et al. Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 51(6), 1242–1251 (2019).
Caru, M. et al. A scoping review to map the evidence of physical activity interventions in post-treatment adolescent and young adult cancer survivors. Crit. Rev. Oncol. Hematol. 171, 103620 (2022).
Onyeaka, H. et al. Is engagement in physical activity related to its perceived mental health benefits among people with depression and anxiety? A population-scale survey study. Am. J. Lifestyle Med. 19, 155982762211160 (2022).
Barha, C. K., Davis, J. C., Falck, R. S., Nagamatsu, L. S. & Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 46, 71–85 (2017).
Kelly, M. E. et al. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res. Rev. 16, 12–31 (2014).
Ogawa, E. F., You, T. & Leveille, S. G. Potential benefits of exergaming for cognition and dual-task function in older adults: A systematic review. J. Aging Phys. Act. 24(2), 332–336 (2016).
Banno, M. et al. Exercise can improve sleep quality: A systematic review and meta-analysis. PeerJ 6, e5172 (2018).
Ma, J. K. & Martin Ginis, K. A. A meta-analysis of physical activity interventions in people with physical disabilities: Content, characteristics, and effects on behaviour. Psychol. Sport Exerc. 37, 262–273 (2018).
Paton, F. et al. Initiatives to reduce length of stay in acute hospital settings: A rapid synthesis of evidence relating to enhanced recovery programmes. In Southampton (UK): NIHR Journals Library (2014).
Buchan, D. S. et al. Physical activity interventions: Effects of duration and intensity. Scand. J. Med. Sci. Sports 21(6), e341–e350 (2011).
Salmon, J., Owen, N., Crawford, D., Bauman, A. & Sallis, J. F. Physical activity and sedentary behavior: A population-based study of barriers, enjoyment, and preference. Health Psychol. 22(2), 178–188 (2003).
Bickel, E. A. et al. Looking at individual symptoms: The dynamic network structure of depressive symptoms in cancer survivors and their preferences for psychological care. J. Cancer Survivorship https://doi.org/10.1007/s11764-022-01246-4 (2022).
Menting, J. et al. Is fatigue a disease-specific or generic symptom in chronic medical conditions?. Health Psychol. 37(6), 530–543 (2018).
Goërtz, Y. M. J. et al. Fatigue in patients with chronic disease: Results from the population-based Lifelines Cohort Study. Sci. Rep. 11(1), 20977 (2021).
Young, A. E., Davies, A., Bland, S., Brookes, S. & Blazeby, J. M. Systematic review of clinical outcome reporting in randomised controlled trials of burn care. BMJ Open 9(2), e025135 (2019).
Athens, B. A. et al. A systematic review of randomized controlled trials to assess outcomes of genetic counseling. J. Genet. Couns. 26(5), 902–933 (2017).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 29, n71 (2021).
Ouzzani, M., Hammady, H., Fedorowicz, Z. & Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5(1), 210 (2016).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 28, l4898 (2019).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences 2nd edn. (Springer, 1988).
Huedo-Medina, T. B., Sánchez-Meca, J., Marín-Martínez, F. & Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index?. Psychol. Methods 11(2), 193–206 (2006).
Yazgan, Y. Z., Tarakci, E., Tarakci, D., Ozdincler, A. R. & Kurtuncu, M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 39, 101902 (2020).
Ozkul, C., Guclu-Gunduz, A., Yazici, G., Atalay Guzel, N. & Irkec, C. Effect of immersive virtual reality on balance, mobility, and fatigue in patients with multiple sclerosis: A single-blinded randomized controlled trial. Eur. J. Integr. Med. 35, 101092 (2020).
Escudero-Uribe, S., Hochsprung, A., Heredia-Camacho, B. & Izquierdo-Ayuso, G. Effect of training exercises incorporating mechanical devices on fatigue and gait pattern in persons with relapsing-remitting multiple sclerosis. Physiother. Can. 69(4), 292–302 (2017).
Cakit, B. D. et al. Cycling progressive resistance training for people with multiple sclerosis. Am. J. Phys. Med. Rehabil. 89(6), 446–457 (2010).
Duruturk, N., Arıkan, H., Ulubay, G. & Tekindal, M. A. A comparison of calisthenic and cycle exercise training in chronic obstructive pulmonary disease patients: A randomized controlled trial. Expert. Rev. Respir. Med. 10(1), 99–108 (2016).
RStudio Team (2020). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/.
Sterne, J. A. C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343(1), d4002–d4002 (2011).
Ahmadi, A., Nikbakh, M., Arastoo, A. & Habibi, A. H. The effects of a yoga intervention on balance, speed and endurance of walking, fatigue and quality of life in people with multiple sclerosis. J. Hum. Kinet. 2010(23), 71–78 (2010).
Ahmadi, A., Arastoo, A. A., Nikbakht, M., Zahednejad, S. & Rajabpour, M. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iran. Red. Crescent Med. J. 15(6), 449–454 (2013).
Gomez-Illan, R. et al. Effects of maximal strength training on perceived-fatigue and functional mobility in persons with relapsing-remitting multiple sclerosis. Med. (B. Aires) 56(12), 718 (2020).
Langeskov-Christensen, M. et al. Efficacy of high-intensity aerobic exercise on common multiple sclerosis symptoms. Acta Neurol. Scand. 145(2), 229–238 (2021).
Ozkul, C. et al. Effect of combined exercise training on serum brain-derived neurotrophic factor, suppressors of cytokine signaling 1 and 3 in patients with multiple sclerosis. J. Neuroimmunol. 316, 121–129 (2018).
Petajan, J. H. et al. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann. Neurol. 39(4), 432–441 (1996).
Straudi, S. et al. A task-oriented circuit training in multiple sclerosis: A feasibility study. BMC Neurol. 14(1), 124 (2014).
van den Berg, M. et al. Treadmill training for individuals with multiple sclerosis: A pilot randomised trial. J. Neurol. Neurosurg. Psychiatry 77(4), 531–533 (2006).
Tarakci, E., Yeldan, I., Huseyinsinoglu, B. E., Zenginler, Y. & Eraksoy, M. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: A randomized controlled trial. Clin. Rehabil. 27(9), 813–822 (2013).
Surakka, J. et al. Effects of aerobic and strength exercise on motor fatigue in men and women with multiple sclerosis: A randomized controlled trial. Clin. Rehabil. 18(7), 737–746 (2004).
Pan, Y., Huang, Y., Zhang, H., Tang, Y. & Wang, C. The effects of Baduanjin and yoga exercise programs on physical and mental health in patients with Multiple Sclerosis: A randomized controlled trial. Complement. Ther. Med. 70, 102862 (2022).
Andreu-Caravaca, L. et al. Effects of fast-velocity concentric resistance training in people with multiple sclerosis: A randomized controlled trial. Acta Neurol. Scand. 146(5), 652–661 (2022).
Cerulli, C. et al. Therapeutic horseback riding in breast cancer survivors: A pilot study. J. Altern. Complement. Med. 20(8), 623–629 (2014).
de Luca, V. et al. Effects of concurrent aerobic and strength training on breast cancer survivors: A pilot study. Public Health 136, 126–132 (2016).
Oliveira, P. F. et al. Effects of exergaming in cancer related fatigue in the quality of life and electromyography of the middle deltoid of people with cancer in treatment: A controlled trial. Asian Pac. J. Cancer Prev. 19(9), 2591–2597 (2018).
Kim, J. Y. et al. Effects of a 12-week home-based exercise program on quality of life, psychological health, and the level of physical activity in colorectal cancer survivors: A randomized controlled trial. Support. Care Cancer 27(8), 2933–2940 (2019).
Steindorf, K. et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: Results on cancer-related fatigue and quality of life. Ann. Oncol. 25(11), 2237–2243 (2014).
Yee, J. et al. Physical activity for symptom management in women with metastatic breast cancer: A randomized feasibility trial on physical activity and breast metastases. J. Pain Symptom Manage. 58(6), 929–939 (2019).
Mostafaei, F., Azizi, M., Jalali, A., Salari, N. & Abbasi, P. Effect of exercise on depression and fatigue in breast cancer women undergoing chemotherapy: A randomized controlled trial. Heliyon 7(7), e07657 (2021).
Koevoets, E. W. et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 24(1), 36 (2022).
Headley, J. A., Ownby, K. K. & John, L. D. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol. Nurs. Forum 31(5), 977–983 (2004).
Parent-Roberge, H. et al. Effects of combined exercise training on the inflammatory profile of older cancer patients treated with systemic therapy. Brain Behav. Immun. Health. 2, 100016 (2020).
Castro-Sánchez, A. M. et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: A randomized controlled trial. Evid.-Based Complement. Altern. Med. 2012, 1–8 (2012).
Wei, X. et al. Effects of Baduanjin exercise on cognitive function and cancer-related symptoms in women with breast cancer receiving chemotherapy: A randomized controlled trial. Support. Care Cancer 30(7), 6079–6091 (2022).
Etnier, J. L. et al. Exercise, fibromyalgia, and fibrofog: A pilot study. J. Phys. Act Health 6(2), 239–246 (2009).
Ribas, C. G., Alves-da-Silva, L., Corrêa, M. R., Teive, H. G. & Valderramas, S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: A pilot randomized controlled trial. Parkinson. Relat. Disord. 38, 13–18 (2017).
Rios Romenets, S., Anang, J., Fereshtehnejad, S. M., Pelletier, A. & Postuma, R. Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: A randomized control study. Complement. Ther. Med. 23(2), 175–184 (2015).
Sveaas, S. H. et al. High-intensity exercise improves fatigue, sleep, and mood in patients with axial spondyloarthritis: Secondary analysis of a randomized controlled trial. Phys. Ther. 100(8), 1323–1332 (2020).
D’Silva, A. et al. Meditation and yoga for irritable bowel syndrome: A randomized clinical trial. Am. J. Gastroenterol. 118(2), 329–337 (2022).
Deijle, I. A. et al. Effect of an exercise intervention on global cognition after transient ischemic attack or minor stroke: The MoveIT randomized controlled trial. BMC Neurol. 22(1), 289 (2022).
Loeppenthin, K. et al. Efficacy and acceptability of intermittent aerobic exercise on polysomnography-measured sleep in people with rheumatoid arthritis with self-reported sleep disturbance: A randomized controlled trial. ACR Open Rheumatol. 4(5), 395–405 (2022).
Parvan, K., Jabar-Zadeh, F., Sarbakhsh, P., Akhtari-Shojai, E. & Zarei, T. The effect of exercise during hemodialysis on fatigue and self-efficacy in patients: A blind randomized clinical trial. Ann. Clin. Anal. Med. 08, 5 (2017).
Batur, E. B., Ozyemisçi-Taskiran, O., Yuksel, S., Cengiz, M. & Karatas, G. K. Validity and reliability of the fatigue impact scale in stroke. Top. Stroke Rehabil. [Internet] 29 (7), 526–537 (2022). https://search.ebscohost.com/login.aspx?direct=true&db=c8h&AN=159297615&site=ehost-live&scope=site.
Ghajarzadeh, M., Jalilian, R., Eskandari, G., Ali Sahraian, M. & Reza, A. A. Validity and reliability of Persian version of Modified Fatigue Impact Scale (MFIS) questionnaire in Iranian patients with multiple sclerosis. Disabil. Rehabil. 35(18), 1509–1512 (2013).
Ozyemisci-Taskiran, O., Batur, E. B., Yuksel, S., Cengiz, M. & Karatas, G. K. Validity and reliability of fatigue severity scale in stroke. Top. Stroke Rehabil. 26(2), 122–127 (2019).
Webster, K., Cella, D. & Yost, K. The functional assessment of chronic illness therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 1(1), 79 (2003).
Cella, D. et al. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J. Rheumatol. 32(5), 811–819 (2005).
Kosinski, M., Gajria, K., Fernandes, A. & Cella, D. Qualitative validation of the FACIT-Fatigue scale in systemic lupus erythematosus. Lupus 22(5), 422–430 (2013).
Acaster, S. et al. Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health Qual. Life Outcomes 13(1), 60 (2015).
Müller, S. Measuring Fatigue/tiredness in cancer patients in German-speaking populations: The development of the Fatigue Assessment Questionnaires. Pflege 14(3), 161–170 (2001).
Beutel, M. E., Hinz, A., Albani, C. & Brähler, E. Fatigue assessment questionnaire: Standardization of a cancer-specific instrument based on the general population. Oncology 70(5), 351–357 (2006).
Kos, D. et al. Evaluation of the modified fatigue impact scale in four different European countries. Mult. Scler. J. 11(1), 76–80 (2005).
Junker, C. I., Duch, K., Dreyer, L., Gregersen, J. W. & Kristensen, S. FRI0583 validation of the modified fatigue impact scale in Danish patients with systemic lupus erythematosus. Ann Rheum Dis. 79(Suppl 1), 895–896 (2020).
Smets, E. M. A., Garssen, B., Bonke, B. & De Haes, J. C. J. M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 39(3), 315–325 (1995).
Schneider, R. A. Reliability and validity of the Multidimensional Fatigue Inventory (MFI-20) and the Rhoten Fatigue Scale among rural cancer outpatients. Cancer Nurs. 21(5), 370–373 (1998).
Elbers, R. G., van Wegen, E. E. H., Verhoef, J. & Kwakkel, G. Reliability and structural validity of the Multidimensional Fatigue Inventory (MFI) in patients with idiopathic Parkinson’s disease. Parkinson. Relat. Disord. 18(5), 532–536 (2012).
Tseng, B. Y., Gajewski, B. J. & Kluding, P. M. Reliability, responsiveness, and validity of the visual analog fatigue scale to measure exertion fatigue in people with chronic stroke: A preliminary study. Stroke Res. Treat. 2010, 1–7 (2010).
Stein, K. D., Jacobsen, P. B., Blanchard, C. M. & Thors, C. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manage. 27(1), 14–23 (2004).
Pan, Y., Huang, Y., Zhang, H., Tang, Y. & Wang, C. The effects of Baduanjin and yoga exercise programs on physical and mental health in patients with Multiple Sclerosis: A randomized controlled trial. Complement. Ther. Med. 2022, 70 (2022).
Trieste, L., Cannizzo, S., Palla, I., Triulzi, I. & Turchetti, G. State of the art and future directions in assessing the quality of life in rare and complex connective tissue and musculoskeletal diseases. Front. Med. (Lausanne) [Internet] 9, 986218 (2022). https://www.proquest.com/scholarly-journals/state-art-future-directions-assessing-quality/docview/2723483540/se-2?accountid=12860.
Oken, B. S. et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 62(11), 2058–2064 (2004).
Newman, M. A. et al. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: A pilot study. Mult. Scler. J. 13(1), 113–119 (2007).
Alentorn-Geli, E., Padilla, J., Moras, G., Haro, C. L. & Fernández-Solà, J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. J. Altern. Complement. Med. 14(8), 975–981 (2008).
Coe, S. et al. Physical activity, fatigue, and sleep in people with parkinson’s disease: A secondary per protocol analysis from an intervention trial. Parkinson. Dis. 2018, 1517807 (2018).
Owen, L., Pennington, B., Fischer, A. & Jeong, K. The cost-effectiveness of public health interventions examined by NICE from 2011 to 2016. J. Public Health (Bangkok) 40(3), 557–566 (2018).
Mostert, S. & Kesselring, J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult. Scler. J. 8(2), 161–168 (2002).
Huisinga, J. M., Filipi, M. L. & Stergiou, N. Elliptical exercise improves fatigue ratings and quality of life in patients with multiple sclerosis. J. Rehabil. Res. Dev. 48(7), 881 (2011).
Dalgas, U. et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult. Scler. J. 16(4), 480–490 (2010).
Llewellyn-Bennett, R., Bowman, L. & Bulbulia, R. Post-trial follow-up methodology in large randomized controlled trials: A systematic review protocol. Syst. Rev. 5(1), 214 (2016).
Foote, K. & Tsui, A. Information Systems and Measurement for Assessing Program Effects (National Academies Press, 1994).
Alingh, R. A. et al. Protocol of a longitudinal cohort study on physical activity behaviour in physically disabled patients participating in a rehabilitation counselling programme: ReSpAct. BMJ Open 5(1), e007591 (2015).
Brandenbarg, P. et al. Physical activity behaviour up to 1 year post-rehabilitation among adults with physical disabilities and/or chronic diseases: Results of the prospective cohort study ReSpAct. BMJ Open 12(6), e056832 (2022).
Andrews, N. E., Strong, J. & Meredith, P. J. activity pacing, avoidance, endurance, and associations with patient functioning in chronic pain: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 93(11), 2109-2121.e7 (2012).
Murphy, S. L., Alexander, N. B., Smith, D. M. & Alexander, N. B. Measuring activity pacing in women with lower-extremity osteoarthritis: A pilot study and research health science specialist. Geriatr. Res. 62(3), 329–334 (2008).
Edwards, A. et al. Practical and clinical approaches using pacing to improve self-regulation in special populations such as children and people with mental health or learning disabilities. J. Rehabil. Med. Clin. Commun. 4(1), jrmcc00057 (2021).
Murphy, S. L. & Kratz, A. L. Activity pacing in daily life: A within-day analysis. Pain 155(12), 2630–2637 (2014).
Umstattd, M., Wilcox, S., Saunders, R., Watkins, K. & Dowda, M. Self-regulation and physical activity: The relationship in older adults. Am. J. Health Behav. 32, 2 (2008).
Nijs, J., Paul, L. & Wallman, K. Chronic fatigue syndrome: An approach combining self-management with graded exercise to avoid exacerbations. J. Rehabil. Med. 40(4), 241–247 (2008).
Wilson, I. B. & Cleary, P. D. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273(1), 59–65 (1995).
Megari, K. Quality of life in chronic disease patients. Health Psychol. Res. 1(3), 27 (2013).
Engberg, I., Segerstedt, J., Waller, G., Wennberg, P. & Eliasson, M. Fatigue in the general population- associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: The northern Sweden MONICA study 2014. BMC Public Health 17(1), 654 (2017).
Basu, N. et al. Fatigue is associated with excess mortality in the general population: Results from the EPIC-Norfolk study. BMC Med. 14(1), 122 (2016).
Schwarz, R., Krauss, O. & Hinz, A. Fatigue in the general population. Oncol. Res. Treat. 26(2), 140–144 (2003).
Watt, T. et al. Fatigue in the Danish general population. Influence of sociodemographic factors and disease. J. Epidemiol. Commun. Health 54(11), 827–833 (2000).
Fieo, R. A., Mortensen, E. L., Lund, R. & Avlund, K. Assessing fatigue in late-midlife. Assessment 21(6), 706–712 (2014).
Galland-Decker, C., Marques-Vidal, P. & Vollenweider, P. Prevalence and factors associated with fatigue in the Lausanne middle-aged population: A population-based, cross-sectional survey. BMJ Open 9(8), e027070 (2019).
Acknowledgements
This review was supported by the UK Research and Innovation, and Economic and Social research Council funded NINE Doctoral Training Partnership (grant number: ES/P000762/1).
Funding
The PhD project of IB is funded by Economic and Social research Council funded NINE Doctoral Training Partnership (grant number: ES/P000762/1). UK Research and Innovation funded the publication of this review.
Author information
Authors and Affiliations
Contributions
I.B., K.E.S., U.S.A., T.F., K.L.H., and F.J.H. contributed to the conception of the work. I.B. and K.E.S. conducted the search strategy and data extraction. I.B., K.E.S. and U.S.A. assisted with the meta-analysis. All authors drafted the work revising it critically for important intellectual content and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barakou, I., Sakalidis, K.E., Abonie, U.S. et al. Effectiveness of physical activity interventions on reducing perceived fatigue among adults with chronic conditions: a systematic review and meta-analysis of randomised controlled trials. Sci Rep 13, 14582 (2023). https://doi.org/10.1038/s41598-023-41075-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41075-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.