Abstract
Planting alfalfa in grey desert soil can have significant effects on soil nutrient levels, microbial communities, and overall soil improvement. High-throughput sequencing technology was used to explore the relationship between the rhizosphere microbial community structure of grey desert soil planted with different alfalfa varieties (Aohan, WL525HQ, Knight2, Kangsai, Victoria, and WL712), alfalfa characteristics and rhizosphere soil physicochemical properties. Alfalfa planting increased the nitrogen and organic matter in the grey desert soil, and the effects in Victoria, Kangsai, and Aohan were relatively better than those in the unplanted areas and other alfalfa areas. The Chao1 and Shannon indexes showed that the diversity and relative abundance of bacteria and fungi in Kangsai were significantly higher than those in the unplanted areas and other alfalfa areas. Redundancy analysis showed that available nitrogen and phosphorus, as well as fresh weight, significantly affected the changes in fungal and bacterial communities. Variance partitioning analysis showed that soil and alfalfa growth characteristics explained 50.04% and 51.58% of the structural changes in the bacteria and fungi, respectively. Therefore, planting alfalfa changed the community structure of bacteria and fungi, as well as the content of soil nutrients, and different varieties of alfalfa had different effects on soil improvement.
Similar content being viewed by others
Introduction
Grey desert soil is a slightly moist gypsum-salt layer that develops on the fine soil material at the edges of temperate deserts1. It is distributed in the southern Junggar Basin, northern Tianshan Mountains in Xinjiang, and Hexi Corridor in Gansu, China. The gypsum content of grey desert soil in the northern Tianshan Mountains in Xinjiang is between 2 and 8%2. The total area of grey desert soil in Xinjiang is 1.7895 × 106 ha, of which cultivated land accounts for 5.75 × 105 ha; the area of effective cultivated land accounts for 32%, and the salt content is between 0.5 and 2%. Xinjiang is part of a slightly to moderately saline region, which seriously affects or restricts crop growth3.
Alfalfa possesses a developed root system, strong regeneration ability, and perennial nature4. Its potential application for improving soil environment is promising due to its ability for heavy metal enrichment, windproofing, and sand-fixing5. With the increasing need and awareness of environmental protection, the ecological benefits of alfalfa planting have garnered attention6. Several research findings support this notion, including its ability to reduce the content of high-molecular-weight polycyclic aromatic hydrocarbons in coal mining areas soils7, its potential as a remediation material for Cd polluted soil8, its efficacy in soil remediation in oil-mining areas9, and its ability to enhance soil structure and productivity in saline-alkali lands through continuous cropping10. In addition, alfalfa has been divided into different dormancy levels according to the difference of growth period and cold resistance. The dormancy characteristics of alfalfa have a crucial influence on its productivity and adaptability (e.g., wintering ability and cold resistance), and the cold resistance of low-dormancy alfalfa is higher than that of non-dormant alfalfa11,12. Considering soil improvement and ecological restoration, soil microorganisms have become a focal point of research and play a crucial role as a medium for alfalfa’s effectiveness.
Soil microorganisms play a vital role in the soil ecosystem, and their diversity is closely linked to soil nutrients and overall soil health13. Their abundance reflects and determines the ecological characteristics of the soil14. These microorganisms are involved in organic matter, degradation, humus formation15, and the transformation and cycling of soil nutrients, making them essential for improving soil structure16. Therefore, understanding the diversity and community composition of soil microorganisms is relevant. Soil parent materials and nutrients are the primary factors influencing soil microbial communities17. The rhizosphere, which refers to the thin layer of soil surrounding the root system, is a critical zone for interactions between microorganisms and plants18. Research has demonstrated that AMF in alfalfa symbionts can enhance plant nutrient status and improve soil conditions19. The rhizosphere microbial community exhibits high diversity and plays significant role in increasing nutrient availability to plant roots20. Rhizosphere microorganisms release metabolize acids that dissolve insoluble minerals, which plants can then absorb through their roots21. They also secrete indoleacetic acid to stimulate plant growth22. Studies have indicated that environmental changes can disrupt the balance of normal flora, leading to reduced efficient utilization of soil nutrients23. Apart from soil physicochemical characteristics, the microbial structure of the rhizosphere is influenced by plant strains and physiological conditions24. For instance, studies have observed significant variations in the soil microbial structure in rubber tree plantations over the years25. However, the impact of different alfalfa varieties on the rhizosphere soil flora structure in grey desert areas remains unclear.
In this study, the microbial community structure of alfalfa rhizosphere soil in a grey desert area was comprehensively analyzed and compared using high-throughput sequencing technology (partial bacterial 16s rRNA and fungal ITS genes). Our primary objective was to investigate the impact of different alfalfa varieties on the nutrient content and spatial distribution of microbial communities in grey desert soil. By doing so, we aimed to elucidate relationships between alfalfa traits, microorganisms, and soil physicochemical properties. Ultimately, our findings aim to provide valuable insights and guidance for maintaining the sustainable development of grey desert soil ecosystems.
Results
Soil properties
The soil in the control group (CK) of the experimental area was weakly alkaline (pH 7.26). The pH of the rhizosphere in the alfalfa area decreased significantly (p < 0.05), and the Kangsai soil had the lowest pH. The contents of soil organic matter (SOM), available nitrogen (AN), and total nitrogen (TN) in the rhizosphere soil of the different alfalfa varieties were significantly higher than that of CK (p < 0.05). Total potassium (TK) and available potassium (AK) in the Victoria and Kangsai areas were significantly lower than that of CK (p < 0.05). Total phosphorus (TP) and available phosphorus (AP) in the Kangsai area were higher than that of CK (p < 0.05). The TN and AN contents in the Kangsai and Aohan areas were significantly higher than those in other alfalfa areas. The SOM content in the Victoria and Kangsai areas was significantly higher than that the other alfal areas (p < 0.05) (Table 1).
Phenotypic analysis of six alfalfa varieties
The stem diameter (SD) of WL712 and Kangsai was significantly higher than that of the other alfalfa varieties (p < 0.05). The plant height (PHT) of Victoria, Kangsai, and Aohan was significantly lower than that of WL712 (p < 0.05). The internode length (ILH) of WL712 and WL525HQ was significantly higher than that of the other alfalfa varieties (p < 0.05). The number of branches (NOBs) of Kangsai, Knight2, and Aohan were significantly higher than those of WL712 and WL525HQ (p < 0.05). The fresh weight (FW) of Kangsai was significantly higher than that of the other alfalfa varieties (p < 0.05) (Table 2).
Sequencing analysis
The Shannon–Wiener curve showed that the diversity analysis dataset was sufficiently large to reflect the diversity of bacteria and fungi in the sample (Fig. S1A, B). The end of the rarefaction curve tended to saturate gradually, indicating that the sequencing data reasonably covered microbial diversity (Fig. S1C, D). Coverage reflects the coverage of the microbial community. Table 3 shows that the coverage was ≥ 97%, which can represent the real situation of microorganisms in the sample, indicating that most of the types of bacteria and fungi were detected in the sample.
We obtained 1,179,163 high-quality 16s rRNA sequences from 18 alfalfa rhizosphere samples and five CK samples, and 33,656–68,183 analysis sequences (average length of 416.9 bp) were obtained from each sample (Table S1). The bacterial OTUs were compared and annotated in the SILVA database, and the bacteria were divided into 38 phyla, 130 classes, 305 orders, 475 families and 888 genera. In total, 1,613,617 high-quality ITS sequences were obtained from 23 soil samples, and 69,194–82,152 analysis sequences (average length of 236.0 bp) were obtained from each sample (Table S2). Fungal OTUs were compared and annotated in the UNITE database, and the fungi were divided into 14 phyla, 41 classes, 85 orders, 173 families and 354 genera.
The groups common among the soils were regarded as core communities. Figure S2 shows that the number of OTUs shared by all soil bacterial and fungal samples was 2034 and 198, respectively.
Diversity of the soil microbial community in alfalfa
Homogenization was performed with the minimum number of samples, and diversity was statistically analyzed based on the homogenized output data. The diversity and relative abundance indices of the rhizosphere microbial communities of the alfalfa varieties differed (Table 3). The Chao1 index of the bacteria was the highest in Kangsai, followed by Victoria, Knight2, Aohan, and CK; the lowest was in WL525HQ. The Chao1 index of the fungi was the highest in Kangsai, followed by WL712 and Knight2; the lowest was in CK (p < 0.05).
The Shannon index showed that the Kangsai bacterial diversity was significantly higher than that of CK, and the lowest was in WL525HQ (p < 0.05). The diversity of soil fungi in the alfalfa varieties was significantly higher than that in CK; the diversity of soil fungi in Kangsai was the highest, and the diversity of soil fungi in WL712 was the lowest (p < 0.05).
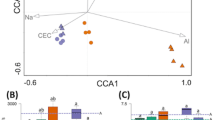
β-diversity was used to reflect the differences in microbial composition among the groups. Non-metric multidimensional scaling (NMDS) analysis showed that the bacterial communities in the Victoria, Knight2, Kangsai, and Aohan alfalfa soils were the closest. CK, WL525HQ, and WL712 had the largest distances between sequences (Fig. 1A). The different alfalfa varieties of soil fungal communities were the closest, but the distance between the CK and the rhizosphere fungal communities in the alfalfa area was large (Fig. 1B). NMDS revealed a unique structure of soil microorganisms between the alfalfa planting area and CK.
Non-metric multidimensional scaling (NMDS) analysis of bacteria (A) and fungi (B). Points with different colors or shapes represent the samples in different groups. The distance of the sample points represents the similarity of the composition of the sample species. Stress lower than 0.1 shows that NMDS analysis is representative. The closer the samples in the graph, the higher their similarity.
Composition and relative abundance of core microorganisms
The bacterial phyla with high relative abundances were Actinobacteria, Proteobacteria, Chloroflexi, and Acidobacteria, followed by Gemmatimonadetes and Firmicutes (Fig. 2A). More than 82% of these bacteria were detected in different groups, and the bacterial phyla with significant differences were Firmicutes and Methylomirabilota (p < 0.05) (Fig. 2A, Table S3). The dominant genera were Vicinamibacterales, JG30-KF-CM45, Gemmatimonadetes, and Arthrobacter among the groups (Fig. 2B). The bacterial genera with significant differences were Arthrobacter, Bacillus, Sphingomonas, Gaiella, and Skermanella (the top 20 in relative abundance) (p < 0.05) (Fig. 2B, Table S4).
The fungal phyla with relatively high abundance were Ascomycota, Basidiomycota, and Mortierellomycota (Fig. 3A). More than 96% of these fungi were detected in different groups, and the fungal phyla with a significant difference was Basidiomycota (p < 0.05) (Fig. 3A, Table S5). Additionally, the dominant genera were Fusarium, Cladosporium, Paracylindrocarpon, Mortierella, and Cephalotrichum among the groups (Fig. 3B). The fungal genera with significant differences were Fusarium, Lectera, Gibberella, Paracylindrocarpon, Cladosporium, Cephalotrichum, Alternaria, Gibellulopsis, Vishniacozyma, Talaromyces, Pseudombrophila, and Paramyrothecium (Fig. 3B, Table S6).
Factors related to the change in soil microbial community diversity
Alfalfa planting changed the soil microbial community structure and physicochemical properties. Changes in the grey desert soil microbial community structure were mediated by differences in alfalfa growth and soil properties. RDA is mainly used to reflect the relationship between the flora and other factors. After removing of redundant variables, six influential factors were selected for RDA. AN (R2 = 0.2835, p = 0.04) and FW (R2 = 0.6812, p = 0.001) significantly affected the bacterial community structure (Fig. 4A, Table S7). TP (R2 = 0.6111, p = 0.001), AP (R2 = 0.3354, p = 0.022), TK (R2 = 0.8784, p = 0.001), AK (R2 = 0.5524, p = 0.002), and FW (R2 = 0.8735, p = 0.001) also significantly affected fungal community structure (Fig. 4B, Table S8).
Redundancy analysis (RDA) of sequencing data (symbols) and environmental characteristics (arrows). Bacterial and fungal communities are shown in (A and B) respectively. Values of axes 1 and 2 are the percentages explained by the corresponding axis. Analysis of the level of contribution of the soil and plant characteristics to changes in bacterial (C) and fungal (D) communities.
Variance partition analysis (VPA) evaluates the contribution of environmental characteristics to the structures of fungi and bacteria (Fig. 4). The environmental characteristics explained 50.04% and 51.58% of bacterial and fungal structural changes, respectively (Fig. 4C, D). Alfalfa and soil characteristics explained 11.70% and 24.13% of bacterial structure changes and 8.90% and 14.19%, respectively, of fungal structure changes. Notably, the common explanations of environmental factors with respect to bacteria and fungi were 11.70% and 28.49%, respectively, which revealed the close interaction between alfalfa and the soil microbes in grey desert.
There was a significant correlation between the microorganisms and environmental factors at the phylum level (Fig. 5). Figure 5A shows that Firmicutes, Actinobacteria, Acidobacteria, Bacteroidetes, Gemmatimonadota, Myxococcus, Proteobacteria, Methylomirabilota, and Chloroflexi were significantly negatively correlated with FW. In addition, Firmicutes showed a significant positive correlation with TK. Myxococcus were significantly negatively correlated with AK. Planctomycetota, Myxococcota, and Methylomirabilota were significantly positively correlated with AN (Table S9). Figure 5B shows that Mortierellomycota and Calcarisporiellomycota were significantly and negatively correlated with FW. Basidiomycota and Olpidiomycota were significantly and positively correlated with AN. Mortierellomycota, Blastocladiomycota, and Ascomycota were significantly and positively correlation with TP. Chytridiomycota and Ascomycota were significantly and positively correlated with TK. Blastocladiomycota and Chytridiomycota were significantly and positively correlated with AP (Table S10).
Heatmap shows relationship between bacterial (A) and environmental variables and relationship between fungi (B) and environmental variables. The X and Y axes represent environmental factors and species, respectively. R is shown in different colors. P values less than 0.05 are marked with *. * 0.01 pP < 0.05, ** 0.001 p < 0.01, or *** p < 0.001.
Analysis of significant differences among soil microbes
Identifying specific biomarkers in the samples holds greater significance than analyzing the overall abundance and diversity of microorganisms. Through LefSe analysis, we discovered 48 distinct bacterial taxa (Fig. 6A) and 87 unique fungal taxa (Fig. 6B) that exhibited significant differences among the soil samples from the experimental areas.
Cladogram showing phylogenetic distribution of bacterial and fungal lineages in the soil. Circles indicate phylogenetic levels from domain to genus. The diameter of each circle is proportional to the abundance of the group. Nodes with different colors represent significantly enriched microbial groups. Light yellow nodes indicate microbial groups without significance.
Figure 7A and B show the linear discriminant analysis (LDA) scores of the bacterial and fungal communities with significant differences among the groups (Tables S11 and S12). Sphingomonas and Bacillus were significantly enriched in WL712 group. Gaiella, Rokubacteriales, and MND1 were significantly enriched in Victoria group. Arthrobacter, Skermanella, and Microvirga were significantly enriched in the WL525HQ group. Rhizobium was significantly enriched in the Aohan samples (Fig. 7A).
At the fungal genus level, Fusarium, Gibellulopsis, Podospora, Rhizophagus, and Stemphylium were significantly enriched in WL712. Cephalotrichum, Lectera, Phoma, Chrysosporium, Scutellinia, and Neosetopyrena were significantly enriched in Victoria group. Gibberella, Preussia, Purpureocillium, Schizothecium, and Chaetopyrena were significantly enriched in the WL525HQ group. Chordomyces, Glomerellales, Talaromyces, Beauveria, Lecanicillium, Coprinopsis, and Neonectria were significantly enriched in the Knight2 group. Alternaria, Simplicillium, and Stephanonectria were significantly enriched in the Kangsai samples. Collarina, Paraphoma, Acremonium, Paramyrothecium, Vishniacozyma, Rhizoctonia, and Cladosporium were significantly enriched in the Aohan samples. Paracylindrocarpon, Pseudogymnoascus, and Pseudombrophila were significantly enriched in CK (Fig. 7B). The relative abundances of bacterial and fungal communities with significant differences among the groups are shown in Figs. S3 and S4, respectively.
Discussion
At the experimental station, we established five control plots and 18 alfalfa plots. Among the different alfalfa varieties, Kangsai alfalfa demonstrated a significant decreased in soil pH, while its fresh weight was higher than that of the other varieties (Table 1). This finding aligns with the understanding that high-yield alfalfa varieties tend to be more tolerant to low soil pH26. Additionakky, the biological nitrogen fixation process in alfalfa root nodules can enhance soil nitrogen fertility27. Compared with the CK, alfalfa planting significantly increased the TN, AN, and SOM content of the soil (Table 1), similar to the results in the literature28.
An appropriate supply of nitrogen can promote the vigorous growth of crops and increase crop yield29,30. P and K can enhance the adaptability of crops to the external environment and the robustness of crop stalks, respectively31. In this study, AN and AP in WL712 and Kangsai were higher than those in CK and the other alfalfa areas, and the FW and SD of WL712 and Kangsai were significantly better than those of the other alfalfa areas (Tables 1 and 2). These results may be the result of plant-soil-microbial interactions, which is similar to results in the literature.
The Chao1 and Shannon indices revealed that the diversity and relative abundance of fungi in the alfalfa planting area were significantly higher than those in the CK, and the relative abundance and diversity of the bacteria and fungi in Kangsai were the highest (Table 3). Moreover, the TN, AN, and SOM contents in Kangsai were higher than those in the CK and other alfalfa areas. These finding are consistent with previous studies32, suggesting that alfalfa may improve substance circulation o and ecological structure of the soil by altering the structure of the microbial community.
The histogram shows that Actinobacteria, Proteobacteria, Gemmatimonadetes, Chloroflexi, Acidobacteria, and Firmicutes were the dominant bacterial phyla (relative abundance was higher than 1%), while Ascomycota, Basidiomycota, and Mortierellomycota were the dominant fungal phyla. Regardless of whether alfalfa was planted, the composition of the soil microbial communities was basically the same, but the relative abundances were significantly different (Figs. 2A and 3A). Typically, according to the literature, there is no difference in the diversity of microbial communities between natural and agricultural soils33. Thus, using alfalfa to improve the grey desert soil structure does not necessarily mean that the diversity of bacteria and fungi will decrease or disappear34. Bi et al.35 found that Proteobacteria and Acidobacteria were the main groups observed during the transformation of soil types. In this study, the relative abundances of Proteobacteria and Acidobacteria were 19.01% and 14.58%, respectively. Additionally, Acidobacteria belong to acidophilic and oligotrophic flora, which mostly grow in acidic and nutrient-deficient soils36. We found that the relative abundance of Acidobacteria in the alfalfa planting area increased significantly, which may be related to soil acidity. Actinobacteria grow in neutral or weakly acidic soils rich in organic matter37. We found that the abundance of Actinobacteria in the alfalfa cultivation area was higher than that in CK. These results may also be related to an increase in organic matter and a decrease in soil pH.
The cultivation of different alfalfa varieties contributed differently to the soil microbial communities (Fig. 4A, B). Research has reported that alfalfa cultivation changes soil microbial community structure and soil characteristics, which might be the result of interactions between plants and microorganisms25. Notably, the diversity of soil microorganisms may be related to the plant cultivars38. The β-diversity results were consistent with those in the literature (Fig. 1). Changes in microbial communities were closely related to soil characteristics (particularly soil N content) and plant growth39. The cultivation of alfalfa significantly increased the nitrogen content of the soil, which may be related to the nitrogen-fixing ability of leguminous forage (Table 1). Nitrogen is important for maintaining the life activities of plants; therefore, plant growth may consume soil nitrogen, and microbial activities may also utilize and transform soil nitrogen40. These results may also be one of the reasons for the difference in the nitrogen content among the different alfalfa soils (Table 1). Additionally, more than 50% of the microbial community changes were explained by the characteristics of alfalfa and soil, which indicated that environmental factors were the main factors affecting the microbial community structure. The cultivation of alfalfa improved soil structure, which was beneficial for soil nutrient circulation and carbon and nitrogen storage27. In this study, the cultivation of Kangsai, Victoria, and Aohan improved the grey desert soil nutrients (TN, AN, and SOM). Kangsai, Victoria, and Knight 2 alfalfa had the highest abundance and diversity of soil microbial communities (Table 3).
LefSe analysis revealed that Bacillus and Sphingomonas were special bacteria with significant differences between WL712 and the other experimental groups at the genus level (Fig. 6, Table S11). Bacillus spp. can produce a range of antibiotics that inhibit the propagation of harmful microorganisms and maintain soil health41. Sphingomonas reduce proline content in plant roots and weaken plant stress resistance42. Cold stress increases the proline content of alfalfa roots, with low dormancy43. We found that the relative abundance of Sphingomonas in WL712 was higher than that in the other areas (Fig. 2B). The interaction between alfalfa and soil microorganisms may be one reason for the good growth performance and low overwintering rate of WL712 plant. Arthrobacter and Microvirga were significantly enriched in the WL525HQ soil. In addition to denitrification, Arthrobacter inhibits the reproduction of pathogenic bacteria44. Microvirga ia an alkalophilic bacterium that plays a role in denitrification and reduction of nitrates45. These bacteria might play a role in the improvement and maintenance of grey desert soil. Gaiella, Rokubacteriales, and MND1 were significantly enriched in Victoria soil. The abundance of the rhizosphere growth-promoting bacterium MND1 positively correlated with crop yield and SOM content46. Rokubacteriales were significantly enriched in farmland soils polluted with heavy metals47. Rhizobium was significantly enriched in Aohan soil. Rhizobium fixes biological nitrogen, which can improve soil nutrients by increasing soil ammonium nitrogen in soil48. Our results are consistent with those in the literature (Table 1).
Fusarium and Gibellulopsis were special fungi with significant differences between WL712 and other experimental areas at the dominant genus level (Fig. 7, Table S12). Most Fusarium species can synthesize toxic mycotoxins that threaten the health of the soil and plants49. The increased abundance of Gibellulopsis had a certain effect on slowing own soil salinization50. The relative abundance of Fusarium in Kangsai and Knight 2 was lower than that in other alfalfa areas. Gibberella and Chaetopyrena were significantly enriched in WL525HQ soil. Gibberella can produce gibberellins to improve the growth and salt tolerance of crops51. Cephalotrichum and Phoma were significantly enriched in Victoria soil. Cephalotrichum is considered a potential strain for remediation as pollution in soil52. Phoma can improve the tolerance of crops53. Sordariomycetes and Chordomyces are strongly alkali-tolerant and effective alkalophilic bacteria54. Talaromyces is a salt-tolerant fungi fungus that produce chitinases and chitosanases55. The relative abundances of Sordariomycetes, Chordomyces, and Talaromyces in the Kangsai and Knight 2 areas were higher than those in the other areas. The relative abundance of Cladosporium in Kangsai and Aohan was higher than that in other areas (Fig. 3B, Table S6). Cladosporium effectively removed imazalil from industrial wastewater56. In summary, almost all significantly enriched microbial communities play a role in the improvement of grey desert soil structure and healthy plant growth.
Conclusion
High-throughput sequencing showed that alfalfa cultivation changed the microbial community structure in grey desert soil, and Kangsai alfalfa increased the diversity and relative abundance of bacterial and fungal communities. The alfalfa varieties had different effects on soil nutrient content, and the improved effects of Victoria, Kangsai, Knight2, and Aohan were better than those of the other varieties. Additionally, Changes in the microbial community were mainly attributed to AN, AP, and FW. Considering the soil nutrients, microbial community structure, and alfalfa growth characteristics, the Kangsai alfalfa variety is more suitable for grey desert soil structure improvement. Our research provides a new direction for selecting alfalfa varieties suitable for grey desert soil improvement.
Materials and methods
Sample collection
The study site is located at an experimental station of Shihezi University, Xinjiang (N44.20°, E88.30°, altitude 420 m), with a temperate continental arid climate and an annual average temperature of 8.1 °C. Varieties of alfalfa seeds were donated by the Grassland Research Institute of the China Academy of Agricultural Sciences. Before seeding, the study area was plowed and harrowed without applying organic fertilizers or pesticides. During alfalfa growth, field management comprised manual weeding and timely watering (after mowing, before winter, and after returning to green).
The study area measured 150 m × 200 m. In May 2020, 18 experimental plots (alfalfa planting areas) and five control plots (cultivated soil without any vegetation, CK) were established (20 m × 30 m), and a 10 m buffer zone was established between the experimental plots. Six alfalfa varieties (Aohan, Victoria, Kangsai, Knight2, WL525HQ, and WL712) (Table 4) were planted in the experimental area, with a 0.25 m plant spacing and 0.3 m row spacing. Three experimental plots not adjacent to each other were randomly selected for each alfalfa variety. In August 2021, 50 alfalfa plants were randomly selected from each experimental site by using the “S” sampling method, the aboveground section (with a stubble of 5 cm) was mowed, and the whole root system was dug out. 50 rhizosphere soil samples were obtained by using the “root shaking” method57 and uniformly mixed as a biological duplication, and three biological duplicate soil samples were obtained for each alfalfa variety. The soil sampling method for the control plot was similar to that aforementioned, and five biological duplicate soil samples were obtained. The obtained samples were screened for impurities. One part was placed in a sealed bag for high-throughput sequencing. The other part was air-dried and preserved to determine soil physicochemical properties. In addition, number of branches (NOB), fresh weight (FW), internode length (ILH), plant height (PHT), and stem diameter (SD) were measured for 50 alfalfa plants (in each experimental plot).
Determination of soil physicochemical properties and phenotypic traits
Soil and water were mixed at a ratio of 1:2.5. Soil pH was measured by using a pH acidity meter (Model PHS-3C pH Meter, China). AN and SOM contents were determined using alkali solution diffusion and potassium dichromate oxidation-oil bath heating, respectively. We used 0.5 mol/L sodium bicarbonate as the extractant. The AP content was determined using molybdenum antimony colorimetry. Using 1 mol/L NH4OAc as the extractant, AK content in the soil was detected using the flame photometric method. The TN in the soil was determined using the Kjeldahl method. TP in the soil was determined using the HCIO4-H2SO4 digestion method. The TK in the soil was determined using sodium hydroxide melting and flame photometry. The detection and calculation of plant agronomic traits (PHT, FW, ILH, SD, and NOB) were based on a report in the literature58.
High-throughput sequencing
Total DNA was obtained from the soil samples by using a soil DNA extraction kit (E.Z.N.A.® Soil DNA Kit, USA). PCR (ABI GeneAmp® 9700, USA) chain reactions were used to amplify these genes. Primers targeting the V3–V4 regions of 16s rRNA (338F: ACTCCTACGGGAGGCAGCAG; 806R: GGACTACHVGGGTWTCTAAT) containing barcodes were used59. Primers targeting the ITS1F-ITS2R regions (ITS1F: CTTGGTCATTTAGAGGAAGTAA; ITS2R: GCTGCGTTCTTCATCGATGC) containing barcodes were used. The amplified products were detected using 2% agarose gel electrophoresis. Next, we used a DNA gel recovery kit to recover the target band (Axygen Biosciences, USA) and verified this result by using 2% gel electrophoresis again. PCR products were quantified by a Quantus™ Fluorometer (Promega, USA), and the samples were adjusted for sequencing according to the required sequencing amount of each sample. Sequencing was performed by Shanghai Majorbio Biopharm Technology (Shanghai, China), which used the Illumina MiSeq platform.
Sequence bioinformatics analysis
According to the overlapping relationship between PE reads, FLASH software (https://ccb.jhu.edu/software/FLASH/index.shtml) was used to merge pairs of reads into a sequence. The quality of the reads and the effects of merging were filtered using quality control. Sequences shorter than 200 bp, sequences containing N bases, and reads with an average mass of less than 20 were removed. Effective sequences were obtained by distinguishing the samples based on the barcode and primer sequences at both ends of the sequence, and the direction of the sequence was corrected. USEARCH v7.1 software was used to eliminate chimeras60. Operational taxonomic units (OTUs) of sequences with a similarity of 97% were determined using UPARSE software (version 7.1 http://www.drive5.com/uparse/)61,62. Rarefaction and Shannon curves were generated using OTUs. The SILVA and UNITE databases were used to classify the sequences of bacteria and fungi, respectively63,64. Relative abundance was calculated as the percentage of the sequence number of microbial taxa in the total sequence number of the samples.
Statistical analysis
One-way ANOVA in SPSS software (version 26; Chicago, USA) was used to compare samples. MOTHUR software was used to calculate the diversity indexes of the microbial community (version v.1.30.2 https://mothur.org/wiki/calculators/). Based on the OTU Bray–Curtis algorithm, non-metric multidimensional scaling (NMDS) analysis was conducted using Vegan software to evaluate changes in the microbial community composition. Changes in the relative microbial abundance are represented by histograms and heatmaps. Redundancy analysis (RDA) and variation distribution were used to analyze the relationship between influential factors and the microbial community structure. Redundant variables that depended on other measurement variables were removed using RDA, and variables with a strong influence were selected automatically. The redundant parameters were removed from the variance expansion factor values. The LDA (LDA ≥ 3.5) of the groups of samples was performed using LefSe to identify meaningful organisms with significant differences. A heatmap was constructed using the R vegan software package (version 3.3.1). Spearman correlation analysis of the microbial community structure and environmental factors was performedusing SPSS software.
Ethical approval
We confirm that the use of plants in the present study complies with international, national and/or institutional guidelines.
Data availability
The data is available at the Sequence Read Archive (SRA) of NCBI: https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA893894 (bacteria) and https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA894156 (fungi).
References
Lebedeva, M. P. & Shishkov, V. A. A Comparative analysis of the microfabrics of surface horizons and desert varnish in extremely arid soils of the mojave (USA) and trans-altai gobi (Mongolia) deserts. Eurasian Soil Sci. 49(2), 163–179 (2016).
He, T., Jessica, G. & Gu, Y. Dataset of mineral contents of fine-sand and silt-sized soil particles from deserts in China and Mongolia. Data Brief. 35, 106879–106884 (2021).
Guo, H. N., Shi, X. D., Ma, L. J., Tao, Y. & Wei, M. Long-term irrigation with saline water decreases soil nutrients, diversity of bacterial communities, and cotton yields in a gray desert soil in China. Pol. J. Environ. Stud. 29(6), 4077–4088 (2020).
Corentin, C. et al. Comparing the deep root growth and water uptake of intermediate wheatgrass (Kernzar) to alfalfa. Plant Soil. 472(1–2), 369–390 (2022).
Shi, H. F., Sun, G. L., Gou, L. M. & Guo, Z. F. Rhizobia-legume symbiosis increases aluminum resistance in alfalfa. Plants-Basel 11(10), 1275 (2022).
David, G. P., Beatriz, A. & Manuel, B. M. The adequacy of alfalfa crops as an Agri-Environmental Scheme: A review of agronomic benefits and effects on biodiversity. J. Nat. Conserv. 69, 126253–126265 (2022).
Shi, W. et al. Remediation of soil polluted with HMW-PAHs by alfalfa or brome in combination with fungi and starch. J. Hazard. Mater. 36, 115–121 (2018).
Li, Y. J. et al. Nitrogen addition facilitates phytoremediation of PAH-Cd cocontaminated dumpsite soil by altering alfalfa growth and rhizosphere communities. Sci. Total Environ. 806, 150610 (2022).
Panchenko, L., Muratova, A. & Turkovskaya, O. Comparison of the phytoremediation potentials of Medicago falcata L. and Medicago sativa L. in aged oil-sludge-contaminated soil. Environ. Sci. Pollut. Res. 24, 3117–3130 (2017).
Li, F. X., Guo, Y. Z., Wang, Z. J. & Mu, Y. X. Influence of different phytoremediation on soil microbial diversity and community composition in saline-alkaline land. Int. J. Phytoremediat. 24(5), 507–517 (2022).
Sledge, M. K., Bouton, J. H. & Kochert, G. Shifts in pest resistance, fall dormancy, tolerant synthetics derived yeild in 12-, 24-, and 120-parent grazing from CFU 101 alfalfa. Crop Sci. 13, 1736–1740 (2003).
Haagenson, D. M., Cunningham, S. M., Joern, B. C. & Volence, J. J. Autumn defoliation effects on alfalfa winter survival, root physiology, and gene expression. Crop Sci. 43(4), 1340–1348 (2003).
Zhang, M. J., Jia, J. Q., Lu, H., Feng, M. C. & Yang, W. D. Functional diversity of soil microbial communities in response to supplementing 50% of the mineral N fertilizer with organic fertilizer in an oat field. J. Integr. Agri. 20(8), 2255–2264 (2021).
Song, Y. Y. et al. Microbial abundance as an indicator of soil carbon and nitrogen nutrient in permafrost peatlands. Eco. Indic. 115, 106362–106372 (2020).
Chen, Q. Y., Niu, B., Hu, Y. L., Luo, T. X. & Zhang, G. X. Warming and increased precipitation indirectly affect the composition and turnover of labile-fraction soil organic matter by directly affecting vegetation and microorganisms. Sci. Total Environ. 714, 136787–136795 (2020).
Yan, J., Han, X. Z., Lu, X. C., Chen, X. & Zou, W. X. Land use indirectly affects the cycling of multiple nutrients by altering the diazotrophic community in black soil. J. Sci. Food Agric. 102(9), 3788–3795 (2022).
Guo, J. X., Zhou, Y. X., Guo, H. J. & Wei, M. Saline and alkaline stresses alter soil properties and composition and structure of gene-based nitrifier and denitrifier communities in a calcareous desert soil. BMC Microbiol. 21, 1–22 (2021).
Verma, K. K. et al. Silicon and soil microorganisms improve rhizospheric soil health with bacterial community, plant growth, performance and yield. Plant Signal. Behav. 17(1), e2104004–e2104418 (2022).
Rahman, M. A. et al. Arbuscular mycorrhizal symbiosis mitigates iron (Fe)-deficiency retardation in alfalfa (Medicago sativa L.) through the enhancement of Fe accumulation and sulfur-assisted antioxidant defense. Int. J. Mol. Sci. 21(6), 2219–2234 (2020).
Xia, X. Y., Zhang, P. P. & He, L. L. Effects of tillage managements and maize straw returning on soil microbiome using 16S rDNA sequencing. J. Integr. Plant Bio. 61, 765–777 (2019).
Weiman, S. Microbes help to drive global carbon cycling and climate change: Although global balancing of carbon dioxide is more complex, in the short term some marine microbes trap it, while soil microorganisms release it. Microbe 10(6), 233–238 (2015).
Kang, S. M. et al. Inoculation with indole-3-acetic acid producing rhizospheric rhodobacter sphaeroides KE149 augments the growth of adzuki bean plants under water stress. J. Microbiol. biotechnol. 30(5), 717–725 (2020).
Zhang, X. M. et al. Distinct drivers of core and accessory components of soil microbial community functional diversity under environmental changes. mSystems 4(5), e00374-e419 (2019).
Wesley, A. B., Deborah, E. G., Donald, R. Z., Danielle, S. S. & Kurt, P. K. Plant effects on and response to soil microbes in native and non-native Phragmites australis. Ecol. Appl. 32(4), e2565–e2583 (2022).
Zhou, Y. J., Li, J. H., Friedman, C. R. & Wang, H. F. Variation of soil bacterial communities in a chronosequence of rubber Tree (Hevea brasiliensis) plantations. Front. Plant Sci. 8, 849 (2017).
Lakic, Z., Antic, M., Duric, I. & Popovic, V. Morphological characteristics of alfalfa genotypes tolerant to low soil pH. Genetika-Belgrade 51(3), 907–922 (2019).
He, G. X., Song, J. C., Wen, Y. J., Liu, C. T. & Qi, J. Effects of different rhizobium fertilizers on alfalfa productivity and soil fertility(Article). Acta Pratacul. Sin. 29(5), 109–120 (2020).
Song, X., Fang, C., Yuan, Z. Q. & Li, F. M. Long-term growth of alfalfa increased soil organic matter accumulation and nutrient mineralization in a semi-arid environment. Front. Environ. Sci. 9, 1–12 (2021).
Heiskanen, J., Ruhanen, H. & Hagner, M. Effects of compost, biochar and ash mixed in till soil cover of mine tailings on plant growth and bioaccumulation of elements: A growing test in a greenhouse. Heliyon 8(2), e08838–e08847 (2022).
Adebayo, A. R., Kutu, F. R. & Sebetha, E. T. Effect of different nitrogen fertilizer rates and plant density on growth of water efficient maize variety under different field conditions. Indian J. Agric. Res. 55(1), 81–86 (2021).
Luan, M. D. et al. Transport and homeostasis of potassium and phosphate: Limiting factors for sustainable crop production. J. Exp. Bot. 68(12), 3091–3105 (2017).
Hu, H., Chen, X. J., Hou, F. J., Wu, Y. P. & Cheng, Y. X. Bacterial and fungal community structures in Loess Plateau Grasslands with different grazing intensities. Front. Microbiol. 8, 606 (2017).
Tripathi, B. M. et al. Tropical soil bacterial communities in Malaysia: pH dominates in the equatorial tropics too. Microb. Ecol. 64, 474–484 (2012).
Lan, G. Y., Li, Y. W., Wu, Z. X. & Xie, G. S. Soil bacterial diversity impacted by conversion of secondary forest to rubber or eucalyptus plantations: A case study of Hainan Island, South China. For. Sci. 63, 87–93 (2017).
Bi, B. Y. et al. Plants use rhizosphere metabolites to regulate soil microbial diversity. Land Degrad. Dev. 32(18), 5267–5280 (2021).
Johannes, S. et al. The evolution of ecological diversity in acidobacteria. Front. Microbiol. 13, 715637–715650 (2022).
Veillette, M., Bonifait, L., Mbareche, H., Duchaine, C. & Marchand, G. Preferential aerosolization of Actinobacteria during handling of composting organic matter. J. Aerosol. Sci. 116(3), 83–91 (2018).
Yang, Y., Wu, H. & Wang, S. Comparison of microbial communities in the rhizosphere soils from two varieties of camellia sinensis in Yunnan Province, China. Microbiology 90(6), 801–804 (2021).
Miguel, J. B. G. et al. Nitrogen fertilization and stress factors drive shifts in microbial diversity in soils and plants. Symbiosis 84(3), 379–390 (2021).
Xu, H. W. et al. Impact of nitrogen addition on plant-soil-enzyme C-N–P stoichiometry and microbial nutrient limitation. Soil Bio. Biochem. 170, 108714–108724 (2022).
Huang, H. M. et al. Integrating broussonetia papyrifera and two bacillus species to repair soil antimony pollutions. Front. Microbiol. 13, 871581–871595 (2022).
Wang, Q. et al. The endophytic bacterium Sphingomonas SaMR12 alleviates Cd stress in oilseed rape through regulation of the GSH-AsA cycle and antioxidative enzymes. BMC Plant Biol. 20(1), 63–76 (2020).
Liu, Z. Y., Baoyin, T., Li, X. L. & Wang, Z. L. How fall dormancy benefits alfalfa winter-survival? Physiologic and transcriptomic analyses of dormancy process. BMC Plant Biol. 19(1), 1–13 (2019).
Karima, E., Mohammed, M., Fatima, A., Omar, L. & Mohamed, B. Aerobic denitrification using Bacillus pumilus, Arthrobacter sp., and Streptomyces lusitanus: Novel aerobic denitrifying bacteria. Bioresourc. Technol. Rep. 14, 100663–100669 (2021).
Mehmood, M. A. et al. Enrichment of bacteria involved in the nitrogen cycle and plant growth promotion in soil by sclerotia of rice sheath blight fungus. Stress Bio. 2(1), 1–13 (2022).
Wang, Q. et al. Effect of intercropping with Vicia faba on secondary metabolites and rhizosphere soil microbial diversity of Notopterygium incisum, China. J. Chin. Mater. Med. 47(10), 2597–2604 (2022).
Duan, R. Y. et al. Diversity and composition of soil bacteria between abandoned and selective-farming farmlands in an antimony mining area. Front. Microbiol. 13, 953624–953633 (2022).
Ju, W. L. et al. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotox. Environ. Safe. 167, 218–226 (2019).
Rishni, M. et al. Ability of plant pathogenic fungi Gibberella fujikuroi and Fusarium commune to react with airborne methyl jasmonate. Biosci. Biotechnol. Biochem. 83(9), 1650–1654 (2019).
Cai, S. M. et al. Phytoremediation of secondary salinity in greenhouse soil with Astragalus sinicus, Spinacea oleracea and Lolium perenne. Agric.-Basel 12(212), 212–220 (2022).
Chen, L. C. et al. Nitric oxide negatively regulates gibberellin signaling to coordinate growth and salt tolerance in Arabidopsis. J. Genet. Genom. 49(8), 756–765 (2022).
Ceci, A., Spinelli, V., Massimi, L., Canepari, S. & Persiani, A. M. Fungi and arsenic: Tolerance and bioaccumulation by soil saprotrophic species. Appl. Sci. 10(9), 3218 (2020).
Zhou, X. R., Dai, L., Xu, G. F. & Wang, H. S. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 11(1), 1–11 (2021).
Grum-Grzhimaylo, A. A., Georgieva, M. L. & Bondarenko, S. A. On the diversity of fungi from soda soils. Fungal Divers. 76(1), 27–74 (2016).
Paranetharan, M. S., Thirunavukkarasu, N., Rajamani, T., Murali, T. S. & Suryanarayanan, T. S. Salt-tolerant chitin and chitosan modifying enzymes from Talaromyces stipitatus, a mangrove endophyte. Mycosphere 9(2), 25–226 (2018).
Papazlatani, C. V. et al. Isolation, characterization and industrial application of a Cladosporium herbarum fungal strain able to degrade the fungicide imazalil. Environ. Pollut. 301, 119030–119041 (2022).
Gobran, G. R. & Clegg, S. A conceptual model for nutrient availability in the soil-root system. Can. J. Soil Sci. 76, 125–131 (1996).
Qi, J. J., Yu, X., Wang, X. Z., Zhang, F. F. & Ma, C. H. Differentially expressed genes related to plant height and yield in two alfalfa cultivars based on RNA-seq. PeerJ 10, e14096–e14112 (2022).
Li, Z. et al. Responses of spatial-temporal dynamics of bacterioplankton community to large-scale reservoir operation: A case study in the Three Gorges Reservoir, China. Sci. Rep. 7, 42469 (2017).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Stackebrandt, E. & Goebel, B. M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44, 846–849 (1994).
Edgar, R. C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and webbased tools. Nucleic Acids Res. 41, D590–D596 (2013).
Pruesse, E. et al. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 7188–7196 (2007).
Acknowledgements
This work was supported by China Agriculture Research System of MOF and MARA.
Author information
Authors and Affiliations
Contributions
J.J.Q. and C.H.M. wrote the main manuscript text and D.Q.F., X.Z.W., and F.F.Z performed the experiments. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qi, J., Fu, D., Wang, X. et al. The effect of alfalfa cultivation on improving physicochemical properties soil microorganisms community structure of grey desert soil. Sci Rep 13, 13747 (2023). https://doi.org/10.1038/s41598-023-41005-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41005-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.