Abstract
The Kidneys remove toxins from the blood and move waste products into the urine. However, the accumulation of toxins and fluids in the body leads to kidney failure. For example, the overuse of acrylamide and titanium dioxide nanoparticles (TiO2NPs) in many food and consumer products increases human exposure and risks; however, there are almost no studies available on the effect of TiO2NPs coadministration with acrylamide on the integrity of genomic and mitochondrial DNA. Accordingly, this study was conducted to estimate the integrity of genomic and mitochondrial DNA in the renal tissue of mice given acrylamide and TiO2NPs. To achieve this goal, mice were administrated orally TiO2NPs or/and acrylamide at the exposure dose levels (5 mg/kg b.w) and (3 mg/kg b.w), respectively, five times per week for two consecutive weeks. Concurrent oral administration of TiO2NPs with acrylamide caused remarkable elevations in the tail length, %DNA in tail and tail moment with higher fragmentation incidence of genomic DNA compared to those detected in the renal tissue of mice given TiO2NPs alone. Simultaneous coadministration of TiO2NPs with acrylamide also caused markedly high elevations in the reactive oxygen species (ROS) production and p53 expression level along with a loss of mitochondrial membrane potential and high decreases in the number of mitochondrial DNA copies and expression level of β catenin gene. Therefore, from these findings, we concluded that concurrent coadministration of acrylamide with TiO2NPs augmented TiO2NPs induced genomic DNA damage and mitochondrial dysfunction through increasing intracellular ROS generation, decreasing mitochondrial DNA Copy, loss of mitochondrial membrane potential and altered p53 and β catenin genes expression. Therefore, further studies are recommended to understand the biological and toxic effects resulting from TiO2NPs with acrylamide coadministration.
Similar content being viewed by others
Introduction
The incredible rapid increase in industrial progress and various human activities have led to heavily discharge of many chemicals into the environment and increasing their concentration above the natural background levels along with frequent human exposure to chemical pollutants such as heavy metals, acrylamide, and various nanoparticles through various ways such as food and water, threatening human life1,2.
Acrylamide is a thermal pollutant formed during heating processing of proteins and carbohydrates rich food at high temperatures, for example during cooking of potatoes and other food products rich in carbohydrates and poor in proteins (cakes, breads and chips) at a temperature higher than 120 °C. Heated carbohydrates and proteins rich foods found to contain about 5–50 mg/kg of acrylamide as well as drinking water contains about 0.5 mg/L3. Accordingly, human heavily exposed to acrylamide from eating fried potato products, bread and biscuits, as well as, excessive drinking of coffee increase human intake of acrylamide4,5.
The toxicity of acrylamide has been demonstrated in numerous in vitro and in vivo studies. Acrylamide reacts with biological moieties including nucleic acids, proteins, and other large molecules, causing neurotoxic, carcinogenic and reproductive toxic effects6,7,8,9,10. Genotoxicity, clastogenicity and mutagenicity of acrylamide have been proven by high elevations in frequency of chromosomal abnormalities, DNA damage and mutations observed after acrylamide treatment5,10,11,12,13. The study of Alzahrani14, also found that the oral acrylamide single administration (10, 20, or 30 mg/kg or repeated administration (10 mg/kg) for one and two weeks significantly increased the frequency of micronuclei and chromosomal abnormalities induction compared to the negative control values.
Unfortunately, humans are exposed to many other chemicals such as nanoparticles together with acrylamide. Titanium dioxide nanoparticles (TiO2NPs) are one of the widest used nanoparticles in many food, medical and consumer products such as pharmaceuticals, toothpastes, sweets, chewing jam and cosmetics15, as well as, nanoparticles are extensively used in wastewater treatment to remove harmful and xenobiotic chemicals due to their corrosion resistance and high stability16,17. All these applications along with the high uses of TiO2NPs in increasing crop yield by accelerating photosynthesis of and increasing agricultural productivity specific crops15 increase human exposure to TiO2NPs and require studying the effect of exposure of nanoparticles on human health.
Upon ingestion of pharmaceuticals, food, water and other consumer products containing TiO2NPs nanoparticles such as chewing gums, candies, sweets and artificial flavors, the TiO2NPs can diffuse throughout the circulation and enter cells of various organs by endocytosis or/and free diffusion causing gastric toxicity, hepatotoxicity, neurotoxicity and nephrotoxicity18,19,20,21,22. Nano-TiO2 particles can also directly or indirectly interact with genomic DNA disrupting the integrity of genomic DNA through induction of chromosomal aberrations, DNA breakages, mutations and alterations of apoptotic genes expressions in various experimental models19,20,22,23,24,25.
As seen, all of the above studies demonstrated toxicity after exposure to acrylamide or TiO2NPs separately and almost no studies have investigated the genotoxicity of both acrylamide and TiO2NPs although human exposure to acrylamide and TiO2NPs together can be done simultaneously. Therefore, the current focused on studying the effect of concurrent simultaneous administration of TiO2NPs and acrylamide on genomic and mitochondrial DNA integrity in the kidney tissues of mice. Genomic DNA integrity was assessed using alkaline comet and laddered DNA fragmentation assays, while, mitochondrial membrane potential and level of intracellular reactive oxygen species (ROS) were studied using Rhodamine and 2,7 dichlorofluorescin diacetate dyes, respectively. Moreover, quantitative real time polymerase chain reaction (qRT-PCR) was done to measure the number of mitochondrial DNA copy and expression level of apoptotic genes.
Materials and methods
Animals
Swiss Webster male mice weighting 20–25 gm and aging 10–12 weeks were purchased from Animal House of the National Organization for Drug Control and Research (NODCAR). For acclimatization mice were kept in the animal house conditions at the Department of Zoology, Faculty of Science Cairo University under standard dark/light cycle and supplied with standard diet pellets and water that were given ad libitum.
Chemicals
Acrylamide and TiO2NPs were purchased as white powder from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Powder of TiO2NPs < 100 nm was immediately suspended in deionized distilled water prior to use for preparing the given dose of 5 mg/kg b.w equivalent to the human exposure dose26,27, while, acrylamide was dissolved in deionized distilled water to prepare the tested dose of 3 mg/kg b.w equivalent to the human exposure dose3. Other used chemicals and reagents used in the experiments of this study were of analytical and molecular biology grade.
Characterization of TiO2NPs
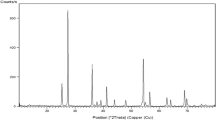
Powder of TiO2NPs purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA) with CAS number 13463-67-7 and a purity 99.5% has been characterized in recent study13 using X-ray diffraction (XRD) to ensure the purity of the TiO2NPs crystallites and transmission electron microscopy (TEM) to detect the shape and average particle size of suspended TiO2NPs in aqueous media.
Ethical consideration and treatment schedule
The experimentations and study plan were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Cairo University with accreditation number (CU/I/F/15/18). This study was reported according to ARRIVE guidelines and also Animal handling and experimentations were conducted in accordance with the Guidelines of the National Institutes of Health (NIH) regarding the care and use of animals for experimental procedures.
Twenty-four male mice were acclimated for one week and then randomly divided into four groups, six animals per group. The negative control group (group I) was orally given distilled deionized water and the three treated groups (Groups II to IV) were orally administered acrylamide at a dose level of 3 mg/kg body weight3 or/and TiO2NPs at a dose level of 5 mg/kg body weight26,27 separately or simultaneously five times per week for two successive weeks. Mice from all groups were sacrificed 24 h after the last administration and dissected. The obtained kidney tissues were stored at − 80 °C for further molecular studies.
Estimation of genomic DNA integrity
To assess the effect of acrylamide or/ and TiO2NPs administration on the integrity of genomic DNA laddered DNA fragmentation and alkaline comet assays were conducted in the renal tissues of all groups.
Ladder DNA fragmentation assay
Laddered DNA fragmentation assay was done by gently homogenizing small pieces of renal tissues in cold Tris EDTA (TE) and sodium dodecyl sulfate lysis buffer, RNase A then added and incubating samples for 1 h in lysis buffer at 37 °C. After lysis, proteinase K was added to samples, incubated at 50 °C, and precipitated genomic DNA using cold absolute ethanol. The precipitated genomic DNA was dissolved in deionized dist. water and 15 µl of the dissolved genomic DNA (3 µg) was electrophoresed in 1% agarose gel at 70 V, visualized and photographed using a UV trans-illuminator28.
Alkaline comet assay
According to the protocol described by Tice et al.,29 a mixture of renal cell suspension and low melting agarose was spread onto a fully frosted slide pre-coated with normal melting agarose (1%), left to dry and placed in cold lysis buffer (2.5 M NaCl, 100 mM EDTA and 10 mM Tris, pH 10) with freshly added 1% Triton X-100 and 10% DMSO for 24 h in the dark at 4ºC. Slides were incubated in alkaline buffer for 15 min and then denatured single-stranded DNA was electrophoresed at 25 V and 300 mA (0, 90 V/cm) for 30 min. Slides after electrophoresis were neutralized in Trizma base to reanneal denatured single stranded DNA, fixed in cold absolute ethanol, dried and stored at room temperature. Re-annealed double stranded DNA was stained with ethidium bromide, examined and photographed using epi-fluorescent microscope. Fifty nuclei were analyzed for each sample using TriTek Comet ScoreTM Freeware v1.5 scoring software and the three parameters: Tail length, % DNA in tail and tail moment were used as indicators for the DNA breakages in renal cells.
Estimation of mitochondrial DNA integrity
The impact of acrylamide or/and TiO2NPs oral administration on the integrity of mitochondrial DNA was evaluated by studying the mitochondrial membrane potential and measuring the number of mitochondrial DNA copy in renal tissues of all groups.
Mitochondrial membrane potential
The mitochondrial membrane permeability potential was studied in the renal tissues of control and treated mice with the fluorescent Rhodamine-123 dye30. Small pieces of renal tissues were gently homogenized in phosphate buffered saline (PBS), the clear cell suspension was mixed with Rhodamine-123 fluorescent dye and left for 1 h at 37 °C in the dark. Renal cells were then washed twice with PBS and the intensity of fluorescent light emitted from cells stained with Rhodamine-123 imaged and analyzed using an epi-fluorescence microscope at 20 × magnification.
Mitochondrial DNA copy
To quantify the number of mitochondrial DNA copy in renal tissues of negative control and treated mice, a quantitative real time polymerase chain reaction (RT-PCR) was done: total RNA and DNA was first extracted from renal tissues using GeneJET Purification Kit (Thermo Scientific, USA) and then the RNA was reversely converted into complementary DNA (cDNA) using Oligo(dT)18 and Random Hexamer primers according to the manufacturer's instructions for the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). A separate RT-PCR was then conducted in the 7500 Fast system thermal cycler (Applied Biosystem 7500, Clinilab, Egypt) to amplify the mitochondrial 12S ribosomal RNA (12S rRNA) gene using the pre-deigned primers shown in Table 131. The obtained data were standardized against the nuclear 18S rRNA gene as a housekeeping gene and the number of mitochondrial DNA copies was then determined using a comparative Ct (DDCt) method. Results were expressed as mean ± S.D.
mRNA expression of p53 and β-catenin genes
To measure the mRNA expression level of p53 and β-Catenin genes in the renal tissues of negative control and treated mice; whole RNA was extracted and reversely transcribed into complementary DNA (cDNA) using the GeneJET RNA Purification and Revert Aid First Strand cDNA Synthesis Kits (Thermo Scientific, USA), respectively. For cDNA synthesis each a µg of template RNA and 1 µl of primer were added to a sterile nuclease free water to complete the final volume to 12 µl in nuclease free tube on ice. Then added reaction buffer, RNase Inhibitor, nucleotides and Revert Aid M-MuLV RT completed the final volume to 20 µl. Samples were mixed gently, incubated 42 °C for 60 min and terminated the reaction by heating at 70 °C for 5 min. Amplification of p53 and β-Catenin genes was then conducted using the primers' sequences listed in Table 1 and previously designed by32,33. The results obtained from RT-PCR were then normalized to the housekeeping β-actin gene and the comparative Ct (DDCt) method was used to calculate the fold changes in the expression level of p53 β-Catenin genes.
Generation of intracellular ROS
The generation level of ROS within renal cells was evaluated by mixing renal cells suspension with 2,7 dichlorofluorescin diacetate dye that enters passively. Mixed cells with dye were left in dark for thirty minutes to allow interaction between dye and generated ROS within cells. After incubation cells were spread on a clean slide, visualized and photographed the forming the highly fluorescent dichlorofluorescein compound under epi-fluorescence microscope at 20 × magnification34.
Statistical analysis
Results obtained from Comet assay and RTPCR were analyzed using the Statistical Package for the Social Sciences (SPSS) (version 20) at the significance level p < 0.05. One way analysis of variance (ANOVA) and Duncan's test were done to compare between the negative control and three treated groups. All results this study are expressed as mean ± Standard Deviation (S.D).
Results
Characterization of TiO2NPs
Characterization of TiO2NPs in our recent study13 confirmed the purity of purchased TiO2NPs powders through the appearance of unique bands for TiO2NPs crystals and also demonstrated the polyhedral shape the of well dispersed TiO2NPs with about 60 nm median particles size shown in TEM image.
Integrity of genomic DNA
Laddered DNA fragmentation
As displayed in Fig. 1 oral administration of TiO2NPs (5 mg/kg) and acrylamide (3 mg/kg) separately or simultaneously together caused dramatic damage to renal genomic DNA as displayed by the seen smeared fragmentized pattern of DNA running on agarose gel compared to the intact DNA pattern of the negative control mice. However, the degree of DNA damage observed after administration of TiO2NPs alone was lower than that observed after administration of acrylamide alone or in combination with TiO2NPs as displayed in Fig. 1).
Alkaline comet assay
Results of Comet assay demonstrated the induction of DNA breakages by TiO2NPs (5 mg/kg) and acrylamide (3 mg/kg) administered separately or simultaneously together by the statistically significant (p < 0.001) elevations in tail length, %DNA in tail and tail moment noticed in renal tissues of mice given TiO2NPs or/and acrylamide compared to the negative control values (Table 2). Otherwise, the DNA damage induced by administration of acrylamide alone or simultaneously with TiO2NPs was significantly (p < 0.001) higher than that induced by administration of TiO2NPs alone. This was manifested by the statistical significant (p < 0.001) elevations in the three Comet parameters: tail length, %DNA in tail and tail moment observed after administration of acrylamide alone or in combination with TiO2NPs compared to their values in renal tissues of mice given TiO2NPs alone (Table 2). Examples for the scored Comet nuclei in the renal tissues of the negative control group and mice given acrylamide or/and TiO2NPs are displayed in Fig. 2.
Integrity of mitochondrial DNA
Mitochondrial membrane potential
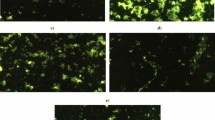
Staining the renal cells with Rhodamine-123 dye showed that oral administration of acrylamide and TiO2NPs separately or together simultaneously caused a large reduction in the integrity of the mitochondrial membrane permeability potential as manifested by the greater decreases in the intensity of the fluorescence light emitted from the treated renal cells compared with the light emitted from the untreated control renal cells (Fig. 3). Moreover, oral administration of TiO2NPs alone caused less reduction in the integrity of mitochondrial membrane potential compared to reductions resulting from administration of acrylamide alone or in combination with TiO2NPs (Fig. 3).
Mitochondrial DNA copy
The copy number of mitochondrial DNA was statistically significantly (p < 0.001) decreased after multiple oral administration of acrylamide or/and TiO2NPs compared to the copy of the negative control mitochondrial DNA as seen in Fig. 4. Otherwise, oral administration of acrylamide alone resulted in less reduction in mitochondrial DNA copy than those resulting from administration of TiO2NPs alone or in combination with acrylamide (Fig. 4).
Variation in the copy of mitochondrial DNA in the renal cells of the negative control mice and mice given orally acrylamide and TiO2NPs separately or simultaneously together. Six mice were analyzed for each group. Results are expressed as mean ± SD and analyzed using one-way analysis of variance followed by Duncan’s test to test the similarity between the control and three treated groups. Means with different letters indicates statistical significant difference between the compared groups at a significant level p < 0.001.
Expression of p53 and β-Catenin genes
As noted in Table 3, multiple oral administration of acrylamide and TiO2NPs caused statistical significant (p < 0.001) elevations in the expression level of p53 gene compared with that of the negative control renal tissue. Meanwhile, the expression level of the p53 gene in renal cells of mice orally given TiO2NPs alone was significantly (p < 0.001) lower than that expressed in the renal tissues of mice given acrylamide alone or simultaneously with TiO2NPs (Table 3).
On the contrary, several oral administration of acrylamide and TiO2NPs caused statistically significant (p < 0.001) decreases in the expression level of the β-Catenin gene compared to that of the negative control (Table 3). On the other hand, the expression level of β-Catenin gene in renal tissue of mice administered TiO2NPs alone was statistically (p < 0.001) higher than that of mice given acrylamide alone or simultaneously with TiO2NPs (Table 3).
Generation of intracellular ROS
Examination of renal cells stained with the 2,7 dichlorofluorescin diacetate (DCFH-DA) dye using epi-fluorescent microscope showed that ROS are highly generated after oral administration of TiO2NPs or/and acrylamide as the intensity of the fluorescent light emitted from the stained renal cells was remarkably higher compared to those emitted from the negative control renal cells (Fig. 5).
Discussion
Intensive uses of various consumer and food products containing acrylamide or TiO2NPs increase the risk of human exposure to acrylamide TiO2NPs together. However, almost no studies have investigated the effect of acrylamide and TiO2NPs coadministration on the integrity of genomic and mitochondrial DNA in kidney tissues. Therefore, this study was done to estimate the effect of simultaneous co-administration acrylamide and TiO2NPs on the integrity of genomic and mitochondrial DNA in renal tissues of mice.
In this study, acrylamide and TiO2NPs in this study were orally administered to mice at the dose level 3 mg/kg and 5 mg/kg, respectively, equivalent to the human exposure dose, because human excessive consumption of high temperature cooked starchy foods and other acrylamide containing foodstuffs reach human daily intake of acrylamide to 3 mg/kg by3,35,36. Similarly, the massive use of TiO2NPs in plastics, cosmetics toothpaste, Candy, sweets, gums, artificial flavors and other foods due to their high stability and anti-corrosion properties increases human ingestion of a large amount of TiO2NPs exceeding 5 mg/kg26,27,37.
Our results demonstrated the genotoxicity of acrylamide (3 mg/kg) or/and TiO2NPs (5 mg/kg) given orally daily over two weeks through the noticed fragmented smeared pattern of genomic DNA electrophoresed on ethidium bromide stained agarose gel compared to the intact appearance of the negative control genomic DNA. Similarly, the noticed remarkable elevations in measured Comet parameters: tail length, %DNA in tail and tail moment confirmed the induction of DNA breakage after multiple administration of the tested low doses of acrylamide or/and TiO2NPs in kidney tissues of mice because alkaline Comet assay sensitively detects single and double stranded DNA breakages29. These findings thus supported the demonstrated genotoxicity of acrylamide and TiO2NPs administered separately in previous studies19,20,23,24,38,39,40.
The generation of ROS normally occurs within cells during numerous metabolic processes. However, exposure to various toxic agents leads the production of intracellular ROS over the normal level leading to a disruption of the balance between ROS generation and antioxidant defense system inducing oxidative stress13,41,42,43. Excessive generation of ROS by acrylamide or/and TiO2NPs was manifested in this study by marked increases in the intensity of fluorescent light emitted from renal cells stained with 2,7 Dichlorofluorescein diacetate dye consistent with the previous suggestion of oxidative stress induction through ROS generation as an accepted mechanism of acrylamide or TiO2NPs induced toxicity13,20,22,44,45. Therefore, the increased DNA damage induction observed in the renal tissues of mice orally given low doses of acrylamide and TiO2NPs together may result from the observed higher ROS generation that attacks cellular proteins, lipids, carbohydrates, and DNA causing cell damage and necrosis making renal cells more susceptible to DNA damage than that induced after administration of acrylamide or TiO2NPs alone. These results are consistent with our recent study showing increased TiO2NPs genotoxicity when TiO2NPs coadministered with acrylamide through increased ROS generation in mice brain tissue13.
Oxidizing cellular macromolecules and depleting antioxidants by over ROS generation also promotes mitochondrial DNA damage and triggers apoptosis46,47. Our finding of Loss of mitochondrial membrane potential integrity and remarkable reduction in number of mitochondrial DNA copies demonstrated the induction of mitochondrial dysfunction by multiple administrations of the tested low doses of TiO2NPs or acrylamide. Moreover, dramatic reduction of mitochondrial DNA copies and impairment of mitochondrial membrane potential noticed in the mice renal cells after simultaneous coadministration of TiO2NPs with acrylamide can be attributed to the induced increased extra ROS generations that highly disrupted mitochondrial membrane and mitochondrial DNA integrity hampering mitochondrial DNA biogenesis43,48,49.
Apoptosis occurs naturally during aging and development to preserve cell populations in the tissues. However, inappropriate apoptosis can be induced by excessive ROS generation and DNA damage induction50. Consequently, our findings on the marked induction of DNA damage, ROS generation and mitochondrial dysfunction after chronic administration of TiO2NPs or/and acrylamide may serves as a signal triggering apoptosis of renal cells because even a single double-stranded DNA break is sufficient to induced cellular death51. Moreover, the remarkable elevations in the expression level of the apoptotic p53 gene and significant reductions in the expression level of β-Catenin gene noticed after administration of TiO2NPs or/and acrylamide confirmed the induction of apoptosis by TiO2NPs or/and acrylamide administration because overexpression of the p53 gene mediates and stimulates apoptosis by reducing the expression of β-Catenin as well as accelerated degradation of the β-Catenin protein itself52,53.
Conclusion
Based on the above findings, it was concluded that the coadministration of TiO2NPs with acrylamide increased the genomic DNA damage and mitochondrial dysfunction induced by administration of TiO2NPs or acrylamide alone by significantly increasing the generation of ROS and the expression of p53 and β-catenin genes. Therefore, further studies are recommended to understand the biological and toxic effects of coadministration of TiO2NPs with acrylamide.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mathiesen, L., Buerki-Thurnherr, T., Pastuschek, J., Aengenheister, L. & Knudsen, L. E. Fetal exposure to environmental chemicals; insights from placental perfusionstudies. Placenta 106, 58–66 (2021).
Wang, Z., Walker, G. W., Muir, D. C. G. & Nagatani-Yoshida, K. Toward a global understanding of chemical pollution: A first comprehensive analysis of national and regional chemical inventories. Environ. Sci. Technol. 54(5), 2575–2584 (2020).
World Health Organization. Guidance document for WHO monographers and reviewers evaluating contaminants in food and feed (2016)
Stadler, R. H., & Lineback, D. R. Process-Induced Food Toxicants: Occurrence, Formation, Mitigation, and Health Risks (Hoboken, NJ, 2009) https://doi.org/10.5860/choice.46-6765
European Food Safety Authority (EFSA), Benford, D., Bignami, M., Chipman, J. K. & Ramos Bordajandi, L. Assessment of the genotoxicity of acrylamide. EFSA J. 20(5), e07293 (2022).
Pennisi, M. et al. Neurotoxicity of acrylamide in exposed workers. Int. J. Environ. Res. Public Health 10(9), 3843–3854 (2013).
Uthra, C. et al. Therapeutic potential of quercetin against acrylamide induced toxicity in rats. Biomed. Pharmacother. 86, 705–714 (2017).
Kacar, S. & Sahinturk, V. A multiple organ toxicant: Acrylamide. Osmangazi J. Med. 40(1), 94–100 (2018).
Kumar, J., Das, S. & Teoh, S. L. Dietary acrylamide and the risks of developing cancer: Facts to ponder. Front. Nutr. 5, 14–26 (2018).
Kacar, S. & Sahinturk, V. The protective agents used against acrylamide toxicity: An in vitro cell culture study-based review. Cell J. 23(4), 367–381 (2021).
Nixon, B. J., Stanger, S. J., Nixon, B. & Roman, S. D. Chronic exposure to acrylamide induces DNA damage in male germ cells of mice. Toxicol. Sci. 129(1), 135–145 (2012).
Pelucchi, C. et al. Dietary acrylamide and the risk of pancreatic cancer in the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann. Oncol. 28(2), 408–414 (2017).
Safwat, G., Mohamed, A. A. & Mohamed, H. R. H. Estimation of genotoxicity, apoptosis and oxidative stress induction by TiO2 nanoparticles and acrylamide subacute oral coadministration in mice. Sci. Rep. 12(1), 18648 (2022).
Alzahrani, H. A. Protective effect of l-carnitine against acrylamide-induced DNA damage in somatic and germ cells of mice. Saudi J. Biol. Sci. 18(1), 29–36 (2011).
Waghmode, M. S. et al. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Appl. Sci. 1, 310–319 (2019).
Theron, J., Walker, J. A. & Cloete, T. E. Nanotechnology and water treatment: Applications and emerging opportunities. Crit. Rev. Microbiol. 34(1), 43–69 (2010).
Ijadpanah-Saravy, H., Dehestaniathar Khodadadi, A. & Safari, M. Optimization of photocatalytic degradation of β-naphthol using nano TiO2-activated carbon composite. Desalin. Water Treat. 57, 4708–4719 (2016).
Vevers, W. & Jha, A. N. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology 17, 410–420 (2008).
El-Ghor, A. A., Noshy, M. M., Galal, A. & Mohamed, H. R. Normalization of nano-sized TiO2-induced clastogenicity, genotoxicity and mutagenicity by chlorophyllin administration in mice brain, liver, and bone marrow cells. Toxicol. Sci. 142(1), 21–32 (2014).
Mohamed, H. R. Estimation of TiO2 nanoparticle-induced genotoxicity persistence and possible chronic gastritis-induction in mice. Food Chem. Toxicol. 83, 76–83 (2015).
Song, B., Liu, J., Feng, X., Wei, L. & Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 10(1), 1042. https://doi.org/10.1186/s11671-015-1042-9 (2015).
Mohamed, H. R. H. et al. Accumulative persistence of the genotoxic and mutagenic effects induced by low doses of TiO2 nanoparticles increases the incidence of hepatocellular carcinoma in mice. Recent Res. Genet. Genom. 1(1), 29–47 (2019).
Mohamed, H., & Hussien, N. Genotoxicity studies of titanium dioxide nanoparticles (TiO2NPs) in the brain of mice. Scientifica 6710840 (2016).
Shabbir, S., Kulyar, M. F. & Bhutta, Z. A. Toxicological consequences of titanium dioxide nanoparticles (TiO2NPs) and their jeopardy to human population. BioNanoSci. 11, 621–632 (2021).
Ling, C. et al. Genotoxicity evaluation of titanium dioxide nanoparticles in vitro: a systematic review of the literature and meta-analysis. Biol. Trace Elem. Res. 199, 2057–2076 (2021).
Weir, A. A. TiO2 Nanomaterials: Human exposure and environmental release. M.Sc. thesis. Arizona State University (2011).
Shahare, B., Yashpal, M. & Gajendra,. Toxic effects of repeated oral exposure of silver nanoparticles on small intestine mucosa of mice. Toxicol. Mech. Methods 23(3), 161–167 (2013).
Sriram, M. I., Kanth, S. B. M., Kalishwaralal, K. & Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 5, 753–762 (2010).
Tice, R. R. et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35(3), 206–221 (2000).
Zhang, Y. et al. Possible involvement of oxidative stress in potassium bromate-induced genotoxicity in human HepG2 cells. Chem. Biol. Int. 189, 186–191 (2011).
Chen, R. et al. Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicol. Rep. 1, 450–458 (2014).
Gutierrez, M. I. et al. Infrequent p53 mutation in mouse tumors with deregulated myc. Cancer Res. 52, 1032–1035 (1992).
Takahashi, M., Nakatsugi, S., Sugimura, T. & Wakabayashi, K. Frequent mutations of the β-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis 21(6), 1117–1120 (2000).
Siddiqui, M. A. et al. Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells. Toxicol. In Vitro 24(6), 1592–1598 (2010).
Chen, J. H. & Chou, C. C. Acrylamide inhibits cellular differentiation of human neuroblastoma and glioblastoma cells. Food Chem. Toxicol. 82, 27–35 (2015).
Yilmaz, B. O., Yildizbayrak, N., Aydin, Y. & Erkan, M. Evidence of acrylamide- and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum. Exp. Toxicol. 36(12), 1225–1235 (2017).
Zhang, M., Chen, T. & Wang, Y. Insights into TiO2 polymorphs: Highly selective synthesis, phase transition, and their polymorph-dependent properties. RSC Adv. 7, 52755–52761 (2017).
Ansar, S., Siddiqi, N. J., Zargar, S., Ganaie, M. A. & Abudawood, M. Hepatoprotective effect of Quercetin supplementation against Acrylamide-induced DNA damage in wistar rats. BMC Compl. Altern. Med. 16(1), 327 (2016).
Shimamura, Y., Iio, M., Urahira, T. & Masuda, S. Inhibitory effects of Japanese horseradish (Wasabia japonica) on the formation and genotoxicity of a potent carcinogen, acrylamide. J. Sci. Food Agric. 97, 2419–2425 (2017).
Hagio, S. et al. Effect of sampling time on somatic and germ cell mutations induced by acrylamide in gpt delta mice. Genes Environ. 43(4), 1–12 (2021).
Dizdaroglu, M. Substrate specificities and excision kinetics of DNA glycosylases involved in base-excision repair of oxidative DNA damage. Mutat. Res. 531, 109–126. https://doi.org/10.3233/JAD-2010-100465 (2003).
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S. & Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Org. J. 5(1), 9–19 (2012).
Mohamed, H. R. H. Alleviation of cadmium chloride-induced acute genotoxicity, mitochondrial DNA disruption, and ROS generation by chocolate coadministration in mice liver and kidney tissues. Biol. Trace Elem. Res. 200(8), 3750–3761 (2022).
Zhao, M. et al. The chemoprotection of a blueberry anthocyanin extract against the acrylamide-induced oxidative stress in mitochondria: Unequivocal evidence in mice liver. Food Funct. 6(9), 3006–3012 (2015).
Semla, M., Goc, Z., Martiniaková, M., Omelka, R., & Formicki, G. Acrylamide: A common food toxin related to physiological functions and health. Physiological Research (Czech Academy of Sciences, 2017)
Rothfuss, O., Gasser, T. & Patenge, N. Analysis of differential DNA damage in the mitochondrial genome employing a semi-long run real-time PCR approach. Nucleic Acids Res. 38, e24 (2010).
Cui, H., Kong, Y. & Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Trans. 2012, 646354 (2012).
Muftuoglu M., Mori M. P., & Souza-Pinto N. C. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion (2014)
Mohamed, H. R. H. Estimation of genomic instability and mitochondrial DNA damage induction by acute oral administration of calcium hydroxide normal- and nano- particles in mice. Toxicol Lett 304, 1–12 (2019).
Elmore, S. Apoptosis: A review of programmed cell death. Toxicol Pathol 35(4), 495–516 (2007).
Kaina, B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol 66(8), 1547–1554 (2003).
Sadot, E., Geiger, B., Oren, M. & Ben-Ze’ev, A. Down-regulation of beta catenin by activated p53. Mol Cell Biol 21, 6768–6781 (2001).
Salem, N. S., Mohamed, H. R. H. & Abd-El Razek, A. M. Measurement of DNA damage, oxidative stress, and gene expression of β-Catenin and P53 genes in liver and brain of male mice receiving monosodium L-glutamate monohydrate. Asian J. Pharm. Clin. Res. 13(7), 127–132 (2020).
Acknowledgements
Many thanks and appreciation to the Department of Zoology, Faculty of Science, Cairo University, for providing the chemicals and equipment needed to conduct experiments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The present work was partially funded by Faculty of Science Cairo University and Faculty of Biotechnology, October University for Modern Sciences and Arts (MSA) Egypt.
Author information
Authors and Affiliations
Contributions
H.R.H.M.: designed the study plan and performed the experiments, wrote manuscript and conducted statistical analysis. L.S.T.B. done experimentations and wrote manuscript. H.R.H.M. and A.D. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, H.R.H., Behira, L.S.T. & Diab, A. Estimation of genomic and mitochondrial DNA integrity in the renal tissue of mice administered with acrylamide and titanium dioxide nanoparticles. Sci Rep 13, 13523 (2023). https://doi.org/10.1038/s41598-023-40676-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40676-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.