Abstract
High concentrations of potentially toxic elements (PTEs) in potable water can cause severe human health disorders. Present study examined the fitness of groundwater for drinking purpose based on the occurrence of nine PTEs in a heavy pilgrim and tourist influx region of the Garhwal Himalaya, India. The concentrations of analyzed PTEs in groundwater were observed in the order of Zn > Mn > As > Al > Cu > Cr > Se > Pb > Cd. Apart from Mn and As, other PTEs were within the corresponding guideline values. Spatial maps were produced to visualize the distribution of the PTEs in the area. Estimated water pollution indices and non-carcinogenic risk indicated that the investigated groundwater is safe for drinking purpose, as the hazard index was < 1 for all the water samples. Assessment of the cancer risk of Cr, As, Cd, and Pb also indicated low health risks associated with groundwater use, as the values were within the acceptable range of ≤ 1 × 10−6 to 1 × 10−4. Multivariate statistical analyses were used to describe the various possible geogenic and anthropogenic sources of the PTEs in the groundwater resources although the contamination levels of the PTEs were found to pose no serious health risk. However, the present study recommends to stop the discharge of untreated wastewater and also to establish cost-effective as well as efficient water treatment facility nearby the study area. Present work’s findings are vital as they may protect the health of the massive population from contaminated water consumption. Moreover, it can help the researchers, governing authorities and water supplying agencies to take prompt and appropriate decisions for water security.
Similar content being viewed by others
Introduction
Despite being a significant resource, water is needed for everyone to survive on the planet. It is also required for all living beings, like plants. The sustainable development of a country primarily depends on the availability of freshwater sources. Factors such as limited availability, accessibility, and increased contamination of surface water sources are responsible for the dependency of the public on groundwater sources. Moreover, such water supplies are inadequate, particularly during lean seasons, forcing residents to use groundwater for domestic purposes1. Like surface water sources, groundwater also covers a significant part of the overall supply in many countries for drinking, domestic, commercial and agricultural purposes. Globally, 70% of withdrawn groundwater is used in the agriculture sector of arid and semi-arid counties2. Approximately one-third of human population across the globe mainly relies upon groundwater sources to meet their basic needs of drinking, domestic, agricultural, and commercial uses, etc3,4. As a matter of fact, more than 1.5 billion people in the world mainly depend on groundwater sources for drinking5,6.
Human activities have affected the quality of various groundwater sources due to the addition of domestic, municipal, agricultural, industrial, outdoor, and commercial pollutants to the environment. Further, groundwater is polluted by various contaminants, out of which the PTEs are the major contaminants7. Landfill leachate, application of pesticides, and phosphate fertilizers are some of the anthropogenic activities that can also significantly actuate fluoride contamination in groundwater8. In a recent report, out of about 200 million people, more than 50% are affected only in India, China, and Pakistan by mild to severe dental or skeletal fluorosis4. As per a previous report, ~ 50–60% of the world's population is suffering from health disorders because of either consumption of polluted water or deficiency in the supply of clean drinking water9. From both biological and chemical perspectives, contaminated drinking water may cause severe issues regarding the survival of humans and animals. Unfortunately, groundwater sources are getting adversely affected and enormously polluted occasionally. Hence, many places worldwide are experiencing the adverse effects of groundwater depletion and contamination, including the Indian sub-continent. According to a report, over a billion people are affected due to the unavailability of safe water10. Approximately 25,000 people die per annum due to the unavailability of safe water in developing countries11. The appraisal of water quality focusing on the prevalence of PTEs and associated health risks has been recently carried out in different parts of the world12,13,14,15. Studies have also been carried out in the alluvial plains of Northern India, considering the occurrence of anions and cations and associated health implications16,17,18.
In Garhwal Himalaya, Gangotri is the origin place of the Bhagirathi River. In contrast, Yamunotri is famous for originating from the Yamuna River (also known as the sister river of Bhagirathi), later known as Ganga after its confluence with the Alaknanda River at Devprayag). These shrines attract millions of pilgrims and floating tourists every year due to the mythological beliefs of the Hindu religion and the scenic beauty of the locations, respectively. Nevertheless, the Gangotri shrine always has a massive influx of pilgrims and tourists. In addition to the local population, this influx of pilgrims and tourists always has a water requirement for different purposes. The massive influx of pilgrims increases the water requirement in this region. It may pose excellent stress and adverse severe impacts on available limited potable water sources due to the generation of high amounts of municipal trash. Most of the previous studies in the Uttarakhand State have focused on surface water quality evaluation19,20 and morphological characteristics21 of the hilly terrain of Garhwal and Kumaun Himalaya (India). In addition, some of the studies also considered PTEs occurrence in snow-fed perennial rivers of the Himalayan region22,23,24,25. Some studies have also evaluated the water quality and health implications of spring sources in various parts of Uttarakhand State26,27,28,29,30.
Similarly, the water sources in the plain areas of Uttarakhand State have also been studied with respect to water contamination assessment31,32, geochemical characterization, and environmental risk analysis33. However, assessing the drinking water quality of groundwater sources of the mountainous Garhwal Himalaya region has not received much attention in the literature. There are currently gaps in knowledge of the groundwater quality for human use in the residential, agricultural and tourist areas of this region. Undoubtedly, a detailed investigation of the occurrences of PTEs in the groundwater sources of this important part of the Himalayan region of India is missing.
Moreover, this region is one of the holiest Hindu places in India, and because of this, the floating population of Hindus is remarkably high. These people depend on such water resources for domestic uses, including drinking water. Hence, to protect public mass, this study may provide a better overview to conduct future relevant research to maintain water quality for domestic purposes. With the above aims, the paper focuses on a comprehensive investigation of the groundwater quality of the Bhagirathi valley region in the Garhwal Himalayan region. In this study, the following objectives are targeted to: (i) analyze the concentrations of PTEs in potable groundwater sources; (ii) spatially analyze the distribution of PTEs in the groundwater; (iii) evaluate pollution levels and water quality for drinking and domestic usages through indexing approaches; (iv) assess non-carcinogenic and carcinogenic health impacts due to the PTEs’ occurrences; and (v) identify the possible sources of contaminants in the groundwater using multivariate statistical analysis. It is hoped that the baseline information on the groundwater quality of this area given in this study would enhance managerial strategies toward effective and sustainable protection of groundwater systems. Also, some cost-effective technologies suggested in this study would ensure the safe removal of PTEs from contaminated water before drinking.
Sources of PTEs in water
The surrounding environment of available water sources, the efficiency of treatment technologies, treated water quality, constituting materials of water-carrying pipes due to water stagnation, leakage in pipelines, and plumbing pipes (PP) may contribute to the PTEs content in drinking water34. The PTEs can also release from soil layers through bio-geochemical processes to the surface water bodies with the help of soil runoff and groundwater sources through leaching and percolation processes35. Besides, inorganic fertilizers, surface run-off, partially treated/untreated municipal wastewater, slope factor, land use pattern, excessive withdrawal of groundwater also contaminate drinking water. Further, industrial discharges, applying chemical pesticides, municipal wastewater, and effluents of tanneries are also among the primary sources of Cd, Cu, Ni, Pb, and Cr in potable water34. Several PTEs with their source, associated health implications, and guideline values are reported in potable water (Table 1).
Human exposure to PTEs and associated health risks
PTEs such as Zn, B, Mo, Cu, Fe, and Co at their lower concentrations act as cofactors in various metabolic and other biological processes35. The human body requires more than 65 heavy metals, whereas a few others, such as Pb, Hg, Al, As, Cd, Ni are toxic to the human body44. PTEs are found at a trace level in the environment. The body cannot metabolize these trace elements and thus can be very toxic in nature27. These elements are also characterized as potentially toxic (PTEs) due to associated health risks. Arsenic and cadmium have been recognized as carcinogenic chemical agents for human beings45. Moreover, skin and kidney damage are associated with As and Cd exposure, respectively. The adverse human health effects include heart diseases and high blood cholesterol levels due to Sb, anemia due to Pb, renal and liver disorders due to Hg, and Cu is responsible for gastric problems reported by ATSDR46 and USEPA47. The As, Cd, Pb, Cr, Cu, Hg, and Ni are among various PTEs which can create several human health disorders after their high concentrations through potable water46 as arsenic, cadmium, and lead have been extensively studied on risks associated with them on human population48.
Antagonistic and synergistic human health effects
Various international agencies have suggested the threshold concentration values (guideline values) for a few PTEs in potable water to protect the health of human beings36,47. The guideline values are estimated based on the lowest concentrations with a non-observed-adverse effect limit (NOAEL). However, the guideline values prescribed by various drinking water regulatory agencies are considered health protective. Nevertheless, the co-exposures of multiple PTEs may also need further attention from the researchers in this issue. The co-exposure to various metals in potable water may change the level of toxicity in users. Due to the prevalence of a toxic element, another metal could show the effect at up to one-twentieth of the prescribed WHO value49. Antagonism is a process when the combined effect of two or more elements is lower than the suggested toxic effects of one element. While in the case of synergism, the impact of the combination is more significant than suggested by one element’s toxic effects50,51. Therefore, it is important to examine the synergistic or antagonistic consequences of co-exposure to more PTEs via drinking water. In the case of the presence of multiple PTEs in potable water, the impact on human health might be observed below the regulatory limits of respective metals34. Despite a low level of a particular element, the combined risk of multiple metals may exceed the allowable risk level. For instance, the risk of As in reference to genotoxicity and metabolism may be intensified due to the co-presence of Sb52. In contrast, it has also been observed that the co-exposure to As and Se led to significantly low toxicity53. Similarly, Zn is also observed to decrease As toxicity54.
Materials and methods
Description of the study area
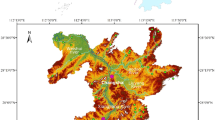
The area under the present study lies along the Bhagirathi valley of Garhwal Himalaya, India, approximately 3065 m above the mean sea level. This area is inhabited by the local population living in different patches. Usually, millions of tourists and pilgrims visit the famous Gangotri Dham (located near the study area) every year. These pilgrims generally stay in hotels lying in the Bhagirathi valley region. The ‘Gangotri Dham’ is a famous religious place for Hindu pilgrims near the origin of the Bhagirathi River (Gomukh). Figure 1a and b show the geological pattern and geographical map showing the sampling sites of the study area, respectively. A length of 2500 km of the Himalayan Arc, with a width of 300 km, defines the Tibetan plateau's southern boundary and the Indian-Eurasian collision zone 55,56. The collision of the Eurasian and Indian tectonic plates, which began around 55 million years ago, results in the rising of the Tibetan plateau and the formation of the Himalayan orogeny55,57. The Garhwal Himalaya is a Himalayan orogeny limited in the north by the Indus-Tsangpo suture and in the south by the HFT, composed of many litho-tectonic units. The dendritic and sub-dendritic drainage patterns are predominant in the studied area. A central trunk stream and lesser tributaries that join it are characteristic of a dendritic drainage pattern in streams, as shown in Fig. 1(c). These tributaries are scattered across the terrain in a manner that resembles the branching structure of tree roots. For instance, local topography and subsurface geology affect the pattern’s shape and texture. The Bhagirathi River is the primary river in the study region.
(a) Geological map (prepared with ArcGIS, version 10.8.0.12790, URL: https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources) of the study area. (b) Geographical map (prepared with ArcGIS, version 10.8.0.12790, URL: https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources) of the study area showing sampling sites. (c): Map showing the topography and drainage pattern of the study (The map was prepared with ArcGIS, version 10.8.0.12790, URL: https://www.esri.com/en-us/arcgis/products/arcgis-desktop/resources).
Sampling procedure and measurements of trace elements
Thirty-three geographical locations were selected for water sampling in the Bhagirathi valley of Garhwal Himalaya, India, considering the general public's utilization of a particular water source for drinking and other purposes. The samples were collected from bore wells (average depth 30–200 m) and springs. The sampling sites belong to residential and agricultural areas. The samples were taken in PET bottles of one-liter capacity. The sampling bottles were cleaned with 10% (v v-1) HNO3 before the sampling, followed by proper rinsing with deionized water. The water samples collected in the pre-processed bottles were further acidified in situ with HNO3 (pH < 2). The water samples were brought to the laboratory by maintaining a cold chain (4 °C) for elemental analysis. Inductively coupled plasma-mass spectroscopy (ICP–MS, Make: Perkin Elmer, Model: ELAN DRCe) instrument was used for the determination of the concentrations of different types of PTEs (Al, Cr, Mn, Cu, Zn, As, Se, Cd, and Pb) in water samples.
Quality control (QC) and quality assurance (QA)
Quality control and quality assurance are critical parameters in the authenticity of experimental data. Therefore, the proper sampling and measurement protocols were followed in different stages of the study. Detailed information about the preparation of water samples for qualitative and quantitative analysis of trace elements, along with calibration verification standard procedures and accuracy of the elemental analysis, are described elsewhere37. The detection limits of Al, Cr, Mn, Cu, Zn, As, Se, Cd and Pb are 100, 20, 10, 10, 30, 5, 0.5, 0.1 and 5 mg L-1, respectively.
Indexical analysis for water quality assessment
The fitness of a water source for drinking is defined in terms of permissible limits fora particular PTE by national and international drinking water regulating agencies and concerned governments. Nevertheless, the effects of several non-hazardous and hazardous PTEs in a collective way on human health are not yet defined by the regulatory bodies or governments. Therefore, the potability of groundwater concerning various trace elements is generally assessed by the researchers in terms of pollution indices as given below58.
Heavy metal pollution index (HPI)
The HPI is defined as the ratio of the sum of the products of the unit weightage factors of different elements and their sub-indices to the sum of unit weightage factors. This index describes the total impact of PTEs on groundwater quality in terms of chemical pollution. The Eqs. (1) and (2) are used to determine the HPI:
In the present study, the BIS guideline values59 for drinking water for PTEs were used in calculations of HPI.
Heavy metal evaluation index (HEI)
The HEI describes the overall groundwater quality concerning PTEs concentration and is estimated using the Eq. (3)60:
Contamination index (CI)
The contamination index (CI) is applied to measure the extent of contamination by considering the overall effects of various PTEs assumed to be harmful to an individual58. It is computed using Eqs. (4) and (5)61. The terminology of different terms used in the above equations is described in Table 2.
Assessment of human health hazards
The risk assessment is commonly characterized as a strategy for determining the potential of any given amount of heavy metals harming human health over a particular period62. The risk on human health (may be categorized as non-carcinogenic and carcinogenic) evaluation is often based on an estimate of its related risk level63.
Non-carcinogenic risk
Non-carcinogenic risk due to the ingestion of analyzed PTEs in potable groundwater was calculated using Eqs. (6) and (7) in terms of Chronic Daily Intake (CDI) through ingestion (CDIing) and dermal (CDIder) pathways. Hazard Quotients (HQs) were further estimated for each PTE via ingestion (HQing) and dermal (HQder) modes using Eqs. (8) and (9), respectively64,65 Two population sub-groups, i.e., adults and children considered for risk estimation in this study.
Further, the hazard index (HI) for all the analyzed PTEs in potable water can be computed from the following Eqs. (11), (12), and (13):
The terminology of different input parameters used in evaluating non-carcinogenic human health hazard risk is given below in Table 3.
Carcinogenic risk
Continuous ingestion and dermal exposure to certain PTEs in water can potentially bring carcinogenic disorders in humans. The carcinogenic risk (CR) via oral and dermal exposure to PTEs in potable groundwater was evaluated using the Eqs. (14), (15), and (16). It is defined as the incremental risk of a person developing cancer over their lifetime exposure to a possible carcinogen65,68. The CRing and CRder were estimated for As, Cr, Cd, and Pb in water using the following equations:
The cancer slope factors (CSFs) values of 0.5, 1.5, 0.0061, and 0.0085 were used to compute CRing and CRder for As, Cr, Cd, and Pb, respectively.
Results and discussions
Occurrence of PTEs in the groundwater of the study area
The statistical information about analyzed PTEs estimated in different groundwater sources in Bhagirathi Valley is depicted in Table 4. The contents (in μg L−1) of the analyzed PTEs in different water sources were found in the order: Zn (1041.436) > Mn (178.443) > As (19.734) > Al (17.449) > Cu (10.138) > Cr (9.705) > Se (1.966) > Pb (0.366) > Cd (0.141).The contour maps created in ArcGIS 10.8 showing the variation of analyzed PTEs contents over the sampling sites are demonstrated in Fig. 2a–i. The point dataset of PTEs concentration was imported into ArcGIS software to prepare a contour map, and Spatial Analyst extension was enabled in it. The Kriging interpolation method was used to create a continuous raster surface from the point data because it produces the best illustrations of spatially distributed data.
The Al, Cr, Cu, Zn, Se, Cd, and Pb concentrations were not found to exceed the corresponding acceptable limits recommended by regulatory agencies for drinking water. The sources of origins and possible human health effects of these PTEs are portrayed in Table 1.
Al concentration in studied groundwater was not observed to be more than the acceptable limit prescribed by BIS or WHO. All the water sources were observed safe with respect to Cr contamination according to the guidelines of BIS and WHO. Mn concentration exceeded the BIS limit of 100 µg/L in 9% of analyzed water samples.None of the samples was observed to have Mn concentration exceeding WHO guideline value of 400 µg/L (Table 4). Thus, contamination due to Mn is almost negligible in the region. The Cu and Zn values were observed to be relatively lower than those reported in previous investigations in Uttarakhand, as shown in Table 5. The concentrations of Mn and Asin in the analyzed groundwater samples exceeded their respective BIS acceptable limits in 9% and 4% samples, respectively (Table 4). The analyzed groundwater samples of Bhagirathi valley were observed to show Se well below the acceptable limit of 10 µg/L36,59. The concentrations of Cd and Pb in the analyzed groundwater samples were also found to lie below their safe limits.
In brief, the concentrations of analyzed PTEs (except Mn and As) in all the analyzed water samples were found below the safe limits suggested by various drinking water regulating agencies. Further, the pollution level of Mn and As in groundwater sources is marginal in the study area. More accurately, the concentrations of other PTEs except for Mn and As in this study are observed far below the acceptable limits, ensuring that groundwater is safe for drinking (Table 4). However, these findings are inconclusive, and comprehensive coverage of more water sources may provide a representative picture of the PTEs occurrence in different kinds of water bodies in the study area.
Comparison of PTEs concentrations with other studies
The findings of the current work were compared with other studies in different water sources of the mountain and plain areas of the Uttarakhand state (Table 5). Gaur et al. reported elevated concentrations of Al, Cr, Cd, and Pb in the groundwater of Haridwar and Dehradun districts, Uttarakhand, with the range of 56–58, 94–98, 130–133, and 84–90 µg L−1, respectively69. Similarly, Cu and Pb contents were also reported to be very high in the range of 140–240 and 240–340 µg L−1, respectively, in the Mallital portion of Spring-Fed Naini Lake70. The concentration of Cu fluctuated between BDL-200 and BDL-125 µg L−1 in the Eastern and Western basins of Sattal Lake of the Nainital district, which is under the safe limit 71. The concentration of Cd in Naini Lake of the Nainital region was reported by CGWB72 up to 1000 μg L−1, which is several times above than guideline limit of 3 μg L−1.
In the research by Chhimwal et al27, the Mn concentration in groundwater of the Naini lake basin was noted in a range from 10 to 150 μg L−1, which is above than acceptable level of 100 μg L−1. The same study also obtained even high Cu concentrations ranging from BDL (below detection limit) to 240 μg L−1. Similarly, Cu, among other analyzed trace metals, was observed above the desirable limit of BIS (2012) in the groundwater of the Haridwar district and ranged from BDL to 73 μg L−179. Matta et al.76 have also reported the high occurrence of Ni and Cd contents in the Haridwar region ranging from 2.233–48.583 and 1.642–3.367 μg L−1 compared to other analyzed PTEs in the surface water system. Haritash et al.80 also reported elevated Ni (BDL-36.7 μg L−1) and Cu (32.1–58.1 μg L−1) concentrations in the surface water of the Rishikesh region. Similarly, measurements for Mn, Ni, Cu, As, and Pb were also observed to be very high except for Cu and fluctuated in the range of 0.19–2086, 0.04–129, 0.03–50.9, 0.10–102, and BDL-36.8 µg L−1, respectively by Khan and Rai 32 in the groundwater sources of Haridwar district. The exceeded contents of Ni, As, and Hg in the surface water of Garhwal region, Uttarakhand, were reported by Kumar et al.78 with a range of0.734–23.280, 2.511–16.142, and 5.164–7.820 μg L−1, respectively. Among these elevated concentrations, only Hg was observed to be several times higher than the safe limit of 1 μg L−1. The concentrations of Mn and Pb in groundwater sources were previsouslynotedas0.001–140 and 0.003–11.3 μg L−1, respectively26. Thus, most of the surface and groundwater samples exceeded the safe limit of various analyzed PTEs. It is also observed that compared to other studies, the results of the present study show lower concentrations of PTEs except for Mn and As in drinking water sources compared to their corresponding acceptable and permissible limits advocated by various national and international regulating agencies. It indicates that although the possibility of significant risk due to the above-mentioned PTEs are less, the public drinking water supplying agencies need to act on effective treatment process, exploring alternate water sources, or regular monitoring of the water supply schemes in the Bhagirathi valley of Garhwal Himalaya in India.
The quantile–quantile (Q-Q) plots for the observed water quality data are shown through Fig. 3a–i. It shows non-normal behaviour of all analyzed PTEs concentration in groundwater sources. It indicated that 8 out of 9 analyzed metals (such as Al (Ҝ = 7.156), Mn (Ҝ = 12.348), Cu (K = 1.047), Zn (K = 9.514), As (Ҝ = 10.918), Se (Ҝ = 3.865), Cd (Ҝ = 5.166), and Pb (Ҝ = 24.716) were found as Leptokurtic behaviours with heavy-tails. However, only Cr (Ҝ = − 0.029) exhibit Platykurtic behaviour with flat tails. All analyzed PTEs concentrations in groundwater of Bhagirathi valley demonstrated positively skewed (Al = 2.609, Cr = 0.841, Mn = 3.500, Cu = 1.303, Zn = 2.909, As = 2.923, Se = 1.603, Cd = 2.366, and Pb = 4.705) frequency distribution signifying their non-normal behaviour.
The Box and Whisker plots for the obtained dataset are shown in Fig. 4a–i. It points out that Mn and Zn have been detected to have higher values than other evaluated PTEs. These plots display umpteen outliers in all analyzed PTEs due to concentration disparity.
Correlation analysis for contaminant source apportionment
In general, the interpolation methods may not accurately predict the variability of PTEs concentration. Therefore, applying a suitable correlation method is imperative for a better understanding of PTEs variation and source apportionment4. Spearman correlation analysis based on the non-normal distribution of the dataset was further calculated for the determination of particular PTE(s) in an analyzed groundwater sample which helps to know the prevalence of another PTE(s) along with their coexistence due to anthropogenic reasons in the studied area81. Table 6 demonstrates the correlation test with statistical significance between analyzed PTEs. The dependency was found between PTEs displayed in Table 6, in which Spearman coefficient ‘r’ of Al against Cr is 0.508 at p = 0.003, showing dependence between Al and Cr. Similarly, the coefficient ‘r’ of Mn against Zn (0.741, p = 0.000); and Cu against Pb (0.599, p = 0.000) exhibits strong (> 0.50) by Bangotra et al81 but a positive relationship between (Al—Cr), (Mn –Zn), and (Cu—Pb). However, Spearman coefficient ‘r’ of Zn against Cd (0.460 at p = 0.007); and Se against Pb (0.478 at p = 0.005) indicates low (< 0.50) and positive correlation between the pairs of Zn and Cd, and Se & Pb. In contrast, the negative coefficient ‘r’ of Al against Mn (− 0.578, p = 0.000) and Zn (− 0.646, p = 0.000); Cr verses Mn (− 0.575, p = 0.000), Zn (− 0.540, p = 0.001) shows a strong correlation between (Al–Mn), (Al–Zn), (Cr–Mn), and (Cr–Zn). Consistent with the line above, Mn established an opposite weak correlation with As (− 0.441, p = 0.010). Therefore, the above Spearman correlation test revealed that variation of one of the analyzed PTEs showed a corresponding change in another. However, the nonappearance of correlations between any two different PTEs suggests that a single factor is not responsible for the control of concentrations of such PTEs, a combination of different phases supported by various geochemical substances may be responsible81.
The significant positive and negative correlations between various elements in the groundwater data point towards potential sources of contamination that may have both natural and anthropogenic origins. For instance, the positive correlation between Al and Cr suggests a common source of pollution, such as industrial activities or natural weathering of geological formations rich in both elements. Chromium is often associated with various industrial processes, such as metal plating, leather tanning, and stainless-steel production82,83. Aluminum can also stem from industrial activities, but it is also naturally present in the Earth's crust84. Thus, the nearby industrial sites and geological formations in the study area are potential sources of contamination. The positive correlation between Zn and Mn indicates a possible relationship between these two elements' sources. Zinc contamination can arise from industrial activities, including metal smelting, galvanization, and battery production85,86. Manganese contamination can also result from industrial discharges or runoff from agricultural activities87. Both elements might originate from common anthropogenic activities or geological sources, necessitating further investigations into the potential industrial and agricultural activities in the vicinity of the groundwater sites. The significant positive correlations between Cd and Zn, as well as Pb and Cu, could be indicative of similar pollution sources. Cadmium is commonly found in batteries, pigments, and industrial processes such as metal plating82,88. It can contaminate the environment alongside zinc, which is also utilized in galvanization and other industrial applications. Similarly, copper and lead often co-occur in industrial settings, including plumbing, electronics, and mining82,88,89. These correlations suggest that anthropogenic activities in the area may be contributing to the co-contamination of these elements in the groundwater.
On the other hand, the observed significant negative correlations between certain elements in the groundwater data also provide important clues about potential contamination sources. The negative correlations between Mn and Cr, Mn and Al, Zn and Al, Zn and Cr, and As and Mn suggest contrasting sources or processes that influence their concentrations in the groundwater83,88. The negative correlation between Mn and Cr and Mn and Al may indicate that different industrial activities or geological sources are contributing to the presence of these elements in the groundwater. For example, while Cr may arise from industrial processes like metal plating, Mn could originate from the natural weathering of rocks or agricultural runoff82,87,88. Aluminum, on the other hand, might come from both industrial sources and natural occurrences. Similarly, the negative correlations between Zn and Al, Zn and Cr, and As and Mn also suggest the possibility of diverse sources affecting the concentrations of these elements. Arsenic contamination is often linked to natural sources like geothermal activity or mineral deposits89, whereas Zn and Al contamination can have both natural and anthropogenic origins83,88.
Principal component analysis for contaminant source apportionment
The principal component analysis (PCA) was performed on the groundwater quality data, in order to complement the results of Spearman's correlation analysis, using IBM SPSS software (version 22). The scree plot of the analysis and the 3D visualization of the principal components are presented in Fig. 5. On the other hand, the principal component extractions, alongside their percentages of variance and eigenvalues, are shown in Table 6. Five principal components with eigenvalues greater than 1 were selected. Cumulatively, they explained about 79.915% of the variabilities in the groundwater quality. The PC 1, explaining 26.226% variance, has significant negative loadings on Mn, Zn, and Cd. However, Cr had significant positive loading in the PC 1. The PC 2, which had 19.226% variability, has significant positive loadings on pH, EC, and TDS. However, Al and Pb were negatively loaded in PC 2 (Table 6). In PC 3, with percentage of variance of 15.160%, Cu has positive loading whereas As has negative loading. In PC 4 (variability = 10.320%), Pb was negatively loaded while Se was positively loaded. Finally, the PC 5, with 8.577% variance, has significant positive loading on As (Table 7).
In addition to the correlation analysis, the results of the PCA provide valuable insights into the associations between the groundwater quality parameters and their potential contamination sources. In the first principal component (PC 1), the significant negative loadings of Mn, Zn, and Cd suggest that these elements may have a common contamination source. The positive loading of Cr indicates a separate source of contamination for this element in the PC 1. This differentiation in loadings suggests that there might be natural geological processes or anthropogenic activities contributing to the presence of Mn, Zn, and Cd in the groundwater, while Cr might be associated with a distinct pollution source. Moving to the second principal component (PC 2), the positive loadings of pH, EC, and TDS indicate a common association between these parameters, possibly related to natural mineral dissolution or leaching processes90,91. Conversely, negative loadings of Al and Pb suggest that they might have a different contamination source. The presence of Al and Pb in groundwater can often be linked to industrial discharges, mining, agricultural runoff, or urban activities82,83,84,88.
Similarly, the associations observed in PC 3, PC 4, and PC 5, point to unique contamination patterns for Cu, As, Pb, Se, and As. Cu's positive loading in PC 3 could be attributed to industrial or agricultural activities82,87,89, while As's negative loading may indicate its presence from natural geological sources89. Pb's negative loading in PC 4 suggests a potential anthropogenic origin, possibly from industrial practices or old water supply infrastructure90,91. Meanwhile, Se’s positive loading in PC 4 could be linked to natural geological processes89. Finally, the significant positive loading of As in PC 5 could be associated with a specific natural or anthropogenic contamination source in the area. Geological formations rich in arsenite have been reported to contribute to enrichment of groundwater with arsenic89. Human activities like mining also play important role in arsenic contamination89. Overall, the findings of the PCA provided crucial information for understanding the contamination sources of various groundwater quality parameters. The separation of loadings across different principal components suggested that multiple sources, both natural (geological) and anthropogenic, contributed to the overall groundwater quality variabilities. Therefore, more investigations may be necessary to pinpoint the exact sources of these PTEs in the groundwater and subsequently implement effective measures for contamination prevention and remediation.
Human health risk assessment
Pollution Indices
The estimated pollution indices represent the combined effects of PTEs on quality of studied water sources and such methods are globally recognized used in the comprehensive water quality evaluation of different potable water sources58,61. The HPI, HEI, and CI were determined separately during this study for each sample site by incorporating individually measured concentrations of analyzed PTEs and using their corresponding standard limits (Table 4). The value of HPI was found to range from 0.117 to 4.233 with a mean of 1.354 ± 0.922. The indices (Table 8) showed that HPI for all the drinking water sources were far below the critical limit of 100 reported by Prasad and Bose92.
There is an inverse relationship between computed HEI and water quality. It means that lower HEI affirms the better quality of the water source. The mean value of HEI was computed as 0.333 ± 0.144 with a marginal range between 0.150 and 0.870 for the analyzed water sources. The obtained present level of HEI shows that water quality falls far within the first category of the low pollution range. Moreover, the CI was used as a reference to estimate the extent of overall metal pollution in the concerned water source58. The estimated CI may further be grouped into three categories as follows: CI < 1 (low), Cd = 1–3 (moderate) and Cd > 3 (high). The mean CI value was − 8.667 ± 0.144 with marginal fluctuation from -8.850 to -8.130 for water sources. The indices showed that CI for all the potable water sources were observed below the critical limit of 3 reported by Backman et al58.This suggests that high-quality water for drinking about the analyzed PTEs in the studied region of Bhagirathi valley region. The computed carcinogenic risk assessments have good stability and indicate a unique and natural source of PTEs in the study area, somewhat related to the soil-geochemical background than to the anthropogenic origin of these analyzed PTEs. Present study demonstrated a ‘nil to very low’ degree of water contamination concerning the analyzed PTEs. Thus, the studied Bhagirathi valley zone is not subjected to pollution concerning PTEs and hence has a low potential for carcinogenic risk to the inhabitants in Garhwal Himalaya.
Non-carcinogenic and carcinogenic risks
Non-carcinogenic risk
To explore the effect of PTEs on human health, hazard risk assessment has also been carried out due to ingestion and dermal pathways on two sub-population groups, e.g. adults and children. The non-carcinogenic CDI and HQ values for ingestion and dermal routes about each analyzed PTE for adults and children are presented in Tables 9 and 10. Similarly, the estimated HI values for ingestion and dermal routes for adults and children are shown in Table 9 and Fig. 5a and b. The human body exposure through oral and dermal pathways was estimated in terms of CDIingestion and HQingestion values using Eqs. (6) and (8). Tables 9 and 10 indicates that for both population sub-groups, such as adults and children, the average HQingestion values exhibited the following sequence: Al < Pb < Cu < Cd < Se < Zn < Mn < As < Cr. For adults, the HQingestion values for Zn showed wide range (5.63 × 10−4 to 1.64 × 10−1) with a mean value of 2.14 × 10−2 ± 3.37 × 10−2. Similarly, in the case of children, a wide range (8.18 × 10−4 to 2.38 × 10−1) is also characterized by Zn with a mean value of 3.11 × 10−2 ± 4.90 × 10−2 (Table 9).
However, none of the studied water samples is characterized by a high HQingestion value greater than the unity in Table 9. Therefore, without any exception, the HQingestion of studied groundwater sources corresponding to each analyzed PTE is estimated to be less than 1. Further, the HQingestion values in children are always high compared to those of adults (Table 9).
Further, CDIdermal and HQdermal values were also calculated using Eqs. (7) and (9), respectively and the values of HQder values corresponding to nine PTEs are presented in Table 10. It is observed that the HQder values corresponding to Mn exhibits wide variation (Table 10). Further, none of the analyzed PTEs is observed to be greater than unity. Thus, there are negligible potential human health risks from groundwater sources in the Bhagirathi valley of the Garhwal Himalaya region that is contaminated with dissolved PTEs.
Human exposure to multiple chemicals was estimated in terms of hazard index (HI). The HI is the measure of an overall effect posed by analyzed non-carcinogenic PTEs via ingestion and dermal pathways. Figure 6a and b exhibit the HIing and HIder values for each sampling location corresponding to analyzed PTEs of the groundwater sources. The estimated HIing and HIder were observed < 1, showing insignificant risks to the inhabitants of the study area. Moreover, based on obtained HIing and HIder values, the total Hazard Index (HItot) was further estimated by adding together all HIs via ingestion and dermal modes. The estimated average values of HIing, HIder and HItotal obtained for adults were found to be 0.204, 0.005, and 0.209, respectively. These indices were obtained as 0.297, 0.022 and 0.319 for children in the study zone. The high values of HI at sites 18 and 25 are due to high concentrations of Mn and As, respectively (Table 11).
Carcinogenic risk
The estimated carcinogenic human health risks (CRing and CRder) associated with analyzed PTEs viz., Cr, As, Cd, and Pb in groundwater sources are shown in Table 12. The carcinogenic risks associated with other analyzed PTEs were not estimated due to the unavailability of corresponding cancer slope factors (CSFs).
The cancerous risks are considered safe in case of ≤ 1.0 × 10−6 according to the prescription limit of USEPA47 for various PTEs. The computed CRing values for adults follow the sequence: As > Cr > Pb > Cd, while dominance for children sub-group under the same ingestion category is found in the frequency: Cd < Cr < As < Pb. The adults may be at high cancer risk due to daily dermal exposure route to the high values of As, Cr, Pb, and Cd through groundwater. The potential risks ascribed due to As and Cr through the dermal pathway for children are high owing to value (> 1.0 × 10−6) due to their carcinogenic natures displayed in Table 12. The carcinogen to children only due to high levels of As and Cr. The estimated high CRing and CRder values (> 1.0 × 10−6) may suggest an individual's lifetime risk of acquiring cancer47,81. For example, CRing and CRder of 10−4 indicate that in cancer may be developed in 1 among 10,000 people64. Furthermore, children are more susceptible to Pb ingestion through drinking water.
Removal of PTEs from drinking water
Numerous studies are reported on the primary investigation of the prevalence of PTEs in potable water sources of different underdeveloped nations34. Significant research has been carried out on treatment techniques of removing PTEs including chlorination, adsorption, ion-exchange, boiling, and solar disinfection. However, some of them are not much effective for PTEs removal96. Removing PTEs from potable water is essential to protect human health. Several studies exist on the removal of on As, Cd, Cr, Cu, Fe, Hg, Ni, Pb, and Zn46,48. The treatment techniques e.g. adsorption, chemical precipitation, electrochemical, ion exchange, membrane filtration, biological, hybrid method etc. are recognized as effective methods for PTEs removal from water97,98. Among these, adsorption is trusted to be advantageous for PTEs removal from water99. The combined approach of chemical precipitation and biological treatment performed by Ahmed et al.98 report a successful recovery of 99.3 and 98.4% of total Cr and Cr(VI), respectively from tannery effluent. The coagulation-flocculation for PTEs removal emphasized that apart from Cu2+, Pb2+, and Ni2+, other metals such as As2+, Se2+, Cr2+, Sb3+, Sb5+, Ag2+ also removed efficiently100.
It is noteworthy that some methods, e.g., ion exchange, membrane filtration, etc. are not feasible in low- and medium-income states owing to high cost. For such countries, the proposed treatment technologies should be less costly and also easy to adopt. Further, it should be developed using readily available local resources by local workers so that less skilled persons can also handle the system. Moreover, such treatment systems should require low operating and maintenance costs. All the above discussed approaches are having their own pro and cons depending upon local factors. However, among these, adsorption process has been considered relatively better than others in terms of cost and easy handling. Further, low-cost adsorbent development is still a challenge for the scientific society and it requires extensive research yet101. However, ion exchange can also be good for the removal of PTEs, if high costs can be beard. Otherwise adsorption can be given preference over most of the water purification techniques after considering local needs and challenges.
Conclusions and recommendations
Based on the present work’s findings, it was found that the concentrations of PTEs viz. Al, Cr, Cu, Zn, Se, Cd, and Pb were not present above their acceptable limits prescribed by the BIS and WHO in the water of studied area for drinking purpose. However, the concentrations of Mn and As in a few samples were found to be greater than their prescribed limits. The relatively high values of Mn in groundwater may be ascribed to the Mn-containing agrochemicals release of untreated domestic sewage sludge in the study area. On the other hand, high value of As in groundwater may be attributed to pipeline corrosion and groundwater dynamics. The estimated values of pollution indices (HPI, HEI, and CI) were observed well below the threshold values which indicates groundwater is not polluted in the investigated region. However, if the partially treated or untreated domestic and municipal sewages are continuously discharged, need of a effective and efficient water treatment becomes mandatory to check the movement of toxic elements into the groundwater aquifer. The present study is an indicative work in nature as a pilot project and it is very significant in terms of public health protection. One of the limitations of this study is the absence of uncertainty analysis in the human health risk assessment, which could have provided valuable insights into the reliability and robustness of the risk estimates. Therefore, we recommend that future studies in this field incorporate uncertainty analysis to enhance the accuracy and comprehensiveness of risk assessments. In this geographical area, the floating population of pilgrimage is very high so that it is vital to conduct such type of studies in near future. . Thus, it can be inferred that such type of pilot research works can be the basis for the future extensive studies. The results presented in this study can provide better insight to the local administration, government, public water supply agencies to ensure the optimum water quality of the study area.
Data availability
The data generated and analyzed in this study are available from the corresponding author upon reasonable request.
References
Mukherjee, I. & Singh, U. K. Delineation of groundwater potential zones in a drought-prone semi-arid region of east India using GIS and analytical hierarchical process techniques. CATENA 194, 104681 (2020).
Mukherjee, I., Singh, U. K. & Chakma, S. Evaluation of groundwater quality for irrigation water supply using multi-criteria decision-making techniques and GIS in an agroeconomic tract of Lower Ganga basin, India. J. Environ. Manag. 309, 114691 (2022).
FAO (Food and Agriculture organization of United Nations). Aquastate website (2016). Retrieved on May 10, 2023.
Mukherjee, I. & Singh, U. K. Environmental fate and health exposures of the geogenic and anthropogenic contaminants in potable groundwater of Lower Ganga Basin, India[J]. Geosci. Front. 13(3), 101365 (2022).
Adimalla, N. & Wu, J. Groundwater quality and associated health risks in a semi-arid region of South India: Implication to sustainable groundwater management. Hum. Ecol. Risk. Assess. 25(1–2), 191–216 (2019).
Mukherjee, I. & Singh, U. K. Groundwater fluoride contamination, probable release, and containment mechanisms: A review on Indian context. Environ. Geochem. Health 40, 2259–2301 (2018).
Mahajan P. & Kaushal J. Role of Phytoremediation in Reducing Cadmium Toxicity in Soil and Water. J. Toxico. 4864365 (2018).
Mukherjee, I. & Singh, U. K. Fluoride abundance and their release mechanisms in groundwater along with associated human health risks in a geologically heterogeneous semi-arid region of east India. Microchem. J. 152, 104304 (2019).
Kazmi, S. S. & Khan, S. A. Level of nitrate and nitrite contents in drinking water of selected samples received at AFPGMI, Rawalpindi. Pak. J. Physiol. 1, 1–2 (2005).
Bresline, E. Sustainable water supply in developing countries. Geol. Soc. Amer. Pap. No.194–1 (2007).
Li, E. X. & Ling, B. Effect of water pollution on human health. Sanit. Engg. Ch. 5(1), 3–5 (2006).
Ayejoto, D. A., Agbasi, J. C., Egbueri, J. C. et al. Assessment of oral and dermal health risk exposures associated with contaminated water resources: an update in Ojoto area, southeast Nigeria. Int. J. Environ. Analyt. Chem. (2022).
Agbasi, J. C. & Egbueri, J. C. Assessment of PTEs in water resources by integrating HHRISK code, water quality indices, multivariate statistics, and ANNs. Geocar. Int. 2034990 (2022).
Unigwe, C.O., Egbueri, J. C. & Omeka, M. E. Geospatial and statistical approaches to nitrate health risk and groundwater quality assessment of an alluvial aquifer in SE Nigeria for drinking and irrigation purposes. J. Ind. Chem. Soc. 99(6), (2022).
Onjia, A. et al. Editorial: Chemometric approach to distribution, source apportionment, ecological and health risk of trace pollutants. Front. Environ. Sci. 10, 1107465. https://doi.org/10.3389/fenvs.2022.1107465 (2022).
Singh, G., Rishi, M. S., Herojeet, R. et al. Evaluation of groundwater quality and human health risks from fluoride and nitrate in semi-arid region of northern India. Environ. Geochem. Health (2019).
Kaur, L., Rishi, M. S. & Siddiqui, A. U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pol. 259, 113711. https://doi.org/10.1016/j.envpol.2019.113711 (2020).
Rishi, M. S., Kaur, L. & Sharma S. Groundwater quality appraisal for non-carcinogenic human health risks and irrigation purposes in a part of Yamuna sub-basin, India. Hum. Eco. Risk Assess An Int. J. 254, 1682514 (2019). https://doi.org/10.1080/10807039.2019.1682514.
Tiwari, M. P. & Tiwari, P. Assessment of water quality status and Zooplanktons of river Bhagirathi in Uttarkashi. J. Water Environ. Nanotechnol. 5(1), 92–101 (2020).
Kumar, A. et al. Long term trend analysis and suitability of water quality of river Ganga at Himalayan hills of Uttarakhand, India. Environ. Tech. Inn. 22, 101405 (2021).
Bhattacharjee, D. Morphological characteristics of Gangotri glacier area, Uttarakhand using GIS and Remote Sensing techniques. Am. Int. J. Res. Human. Art. Soc. Sci. 11(1), 11–16 (2015).
Rai, U. N. et al. Biomonitoring of metals in Ganga water at different ghats of Haridwar: Implications of constructed wetland for sewage detoxification. Bull. Environ. Contam. Toxicol. 89, 805–810 (2012).
Kansal, A., Siddiqui, N. A. & Gautam, A. Assessment of heavy metals and their interrelationships with some physicochemical parameters in eco-efficient rivers of Himalayan region. Environ. Monit. Assess. 185, 2553–2563 (2013).
Seth, R., Mohan, M., Singh, P. et al. Water quality evaluation of Himalayan Rivers of Kumaun region, Uttarakhand, India. Appl. Water Sci. (2014).
Aithani, D., Kushawaha, J. & Naik, S. R. S. Water quality of Himalayan rivers in Uttarakhand. Taloor, A. K. et al. (eds.), Water, Cryosphere, and Climate Change in the Himalayas, A Geospatial Approach pp. 247–258.
Prasad, M. et al. Health risks associated with the exposure to uranium and heavy metals through potable groundwater in Uttarakhand state of India. J. Radio. Nucl. Chem. 319(1), 13–21 (2019).
Chhimwal, M. et al. Water quality of springs and lakes in the Kumaon lesser Himalayan region of Uttarakhand, India. J. Water Health 20(4), 737 (2022).
Chauhan, J. S., Badwal, T. & Badola, N. Assessment of potability of spring water and its health implication in a hilly village of Uttarakhand, India. Appl. Water Sci. 10, 73 (2020).
Prasad, M. et al. Radiological impact assessment of soil and groundwater of Himalayan regions in Uttarakhand, India. J. Radioanal. Nucl. Chem. 323, 1269–1282 (2020).
Prasad, M. et al. A comprehensive study of radon levels and associated radiation doses in Himalayan groundwater. Acta Geophys. 66, 1223–1231 (2018).
Yasir & Srivastava, S. Monitoring of ground water quality in the province of district Dehradun (Uttarakhand), India. Arch. Agri. Environ. Sci. 1(1), 43–48.
Khan, M. U. & Rai, N. Arsenic and selected heavy metal enrichment and its health risk assessment in groundwater of the Haridwar district, Uttarakhand, India. Environ. Ear. Sci. 81, 337 (2022).
Bhutiani, R. et al. Geo-chemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar. India. Energ. Ecol. Environ. 2(2), 155–167 (2017).
Chowdhury, S. et al. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Tot. Environ. 569–570, 476–488 (2016).
Mukherjee, I., Singh, U. K. & Singh, R. P. An overview on heavy metal contamination of water system and sustainable approach for remediation. Water Pol. Manage. Prac., edited by Singh, A., Agrawal, M. & Agrawal, S. B. pp. 255–278 (2021).
World Health Organization (WHO). Guidelines for Drinking-water Quality. Fourth ed. World Health Organization, Geneva (2011).
Prasad, M. et al. A systematic study on occurrence, risk estimation and health implications of heavy metals in potable water of Garhwal Himalaya, India. Scienti. Repor. 12, 20419 (2022).
Vidu, R. et al. Removal of heavy metals from wastewaters: A challenge from current treatment methods to nanotechnology applications. Tox. 8, 101. https://doi.org/10.3390/toxics8040101 (2020).
Röllin, H. B. &Nogueira, C. M. C. A. Manganese: Environmental Pollution and Health Effects, Editor(s): J.O. Nriagu, Encycl. Environ. Health, Elsevier, 617–629 (2011). https://doi.org/10.1016/B978-0-444-52272-6.00540-7.
Obasi, P. N. & Akudinobi, B. B. Potential health risk and levels of heavy metals in water resources of lead-zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 10, 184 (2020).
Mohammed Abdul, K. S. et al. Arsenic and human health effects: A review. Environ. Toxico. Pharmaco. 40, 828–846 (2015).
Genchi, G. et al. The effects of cadmium toxicity. Int. J. Environ. Res. Pub. Health 17, 3782 (2020).
World Health Organization (WHO). Guidelines for drinking-water quality: Fourth edition incorporating the first addendum. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum (2014).
Mahajan, P., Singla, S. & Kaushal, J. Phytoremediation of heavy metals using Brassica juneca-a Review. J. Chem., Environ. Sci. Its Applica. 2(2), 157–173 (2016).
International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risks to humans. 1–115 (2016).
Agency for Toxic Substances and Disease Registry (ATSDR). Toxicologial Profiles, Toxic Substances Portal (2015).
United States Environmental Protection Agency (USEPA). Regulated drinking water contaminants (2015).
Fu, H. Z., Wang, M. H. & Ho, Y. S. Mapping of drinking water research: A bibliometric analysis of research output during 1992–2011. Sci. Tot. Environ. 443, 757–765 (2013).
Escher, B. I. & Hermens, J. L. M. Modes of action in ecotoxicology: Their role in body burdens, species sensitivity, qsars, and mixture effects. Environ. Sci. Technol. 36(20), 4201–4217 (2002).
Mauderly, J. L. et al. Is the air pollution health research community prepared to support a multi pollutant air quality management framework?. Inhal. Toxicol. 22, 1–19 (2010).
United States Environmental Protection Agency (USEPA). Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures; EPA: Washington, DC, USA (2000).
Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 107(3), 131–144 (1997).
Biswas, S., Talukder, G. & Sharma, A. Prevention of cytotoxic effects of arsenic by short-term dietary supplementation with selenium in mice in vivo. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 441(1), 155–160 (1999).
Buschmann, J. B. et al. Contamination of drinking water resources in the Mekong delta floodplains: Arsenic and other trace metals pose serious health risks to population. Environ. Int. 34, 756–764 (2008).
Patel, N. K. et al. Seismicity around the Mahendragarh-Dehradun basement fault in the western Ganga plain, India: A neotectonic perspective. Int. J. Earth Sci. 109, 689–706 (2020).
Patel, N. K. & Pati, P. Evolution of terminal fans in the Indo-Gangetic foreland basin: A process-response model. Geomorp. 419 (2022).
Hodges, K. V. Tectonics of the Himalaya and southern Tibet from two perspectives. Geol. Soc. Am. Bull. 112, 324–350 (2000).
Backman, B. et al. Application of a ground water contamination index in Finland and Slovakia. Environ. Geo. 36, 55–64 (1998).
Bureau of Indian Standards (BIS). Indian standard for drinking water-specification, second edition, IS 10500: 2012, Drinking Water Sectional Committee, FAD 25. New Delhi, India (2012).
Singh, K. R. et al. Review of existing heavy metal contamination indices and development of an entropy-based improved indexing approach. Environ. Dev. Sustain. A Multidisci Appr. Theo. Prac. Sustain. Dev. 22, 7847–7864 (2019).
Ravindra, K. & Mor, S. Distribution and health risk assessment of arsenic and selected heavy metals in Groundwater of Chandigarh, India. Environ. Pollut. 250, 820–830 (2019).
Bempah, C. K. & Ewusi, A. Heavy metals contamination and human health risk assessment around Obuasi gold mine in Ghana. Environ. Monit. Assess. 188, 261 (2016).
Wongsasuluk, P. et al. Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in UbonRatchathani province, Thailand. Environ. Geochem. Health 36, 169–182 (2014).
United State Environmental Protection Agency (USEPA). Risk assessment guidance for superfund Volume I: Human health evaluation manual (Part A). Washington DC, USA. EPA/540/1–89/002; (1989).
Mukherjee, I., Singh, U. K. & Patra, P. K. Exploring a multi-exposure-pathway approach to assess human health risk associated with groundwater fluoride exposure in the semi-arid region of east India. Chemo. 233, 164–173 (2019).
United State Environmental Protection Agency (USEPA). Regulated drinking water contaminants (2015).
United State Department of Energy (USDOE). The risk assessment information system (RAIS). Washington, DC: United State Department of Energy’s Oak Ridge Operations Office (ORO) (2011).
Gržetic, I. & Ghariani, A. R. H. Potential health risk assessment for soil heavy metal contamination in the central zone of Belgrade (Serbia). J. Serb. Chem. Soc. 73, 923–934 (2008).
Gaur, S., Singh, N. & Saxena, S. Status of lead present in ground drinking water samples of Uttarakhand (Garhwal Region) in India. Asian J. Biomed. Pharma. Sci. 1(1), 32–38 (2011).
Sharma, M. &Bradely. Water quality assessment of the Central Himalayan Lake Nainital. Adv. Environ. Chem. 473074 (2014).
Inaotombi, S. & Gupta, P. K. Water quality of a Central Himalayan lake, lake Sattal, Uttarakhand. J. Ecophysiol. Occup. Hlth. 14(1&2), 83–102 (2014).
Central Ground Water Board (CGWB). Report on status of pollution in and around Nainital lake, Nainital district, Uttarakhand. Central ground water board, ministry of water resources, Government of India, Faridabad, India (2010b).
Bahita, T. A. et al. Assessment of heavy metal contamination in livestock drinking water of Upper Ganga Canal (Roorkee City, India). Arab. J. Geosci. 14, 2861 (2021).
Matta, G. et al. Determination of water quality of Ganga river system in Himalayan region, referencing indexing techniques. Arab. J. Geosci. 13, 1027 (2020).
Matta, G., Kumar, A., Tiwari, A. K. et al. HPI appraisal of concentrations of heavy metals in dynamic and static flow of Ganga river system. Environ. Dev. Sustain. (2018).
Matta, G. et al. Assessment of heavy metals toxicity and ecological impact on surface water quality using HPI in Ganga river. INAE Lett. 3, 123–129 (2018).
Tyagi, S. et al. Multivariate statistical analysis of river Ganga water at Rishikesh and Haridwar, India. Analyt. Chem. Lett. 10(2), 195–213 (2020).
Kumar, P. et al. Evaluation of physicochemical, heavy metal pollution and microbiological indicators in water samples of Ganges at Uttarakhand India: An impact on public health. Int. J. Environ. Rehab. Con. IX(SP2), 445–466 (2020).
Deoli, B. K., Bartarya, S. K. & Siddiqui, N. A. Assessment of surface and ground water quality of Haridwar district of Uttarakhand. Int. J. Chem. Tech. Res. 10(10), 95–118 (2017).
Haritash, A. K., Gaur, S. & Garg, S. Assessment of water quality and suitability analysis of river Ganga in Rishikesh. India. Appl. Water Sci. 6, 383–392 (2016).
Bangotra, P. et al. Occurrence and health implications of heavy metals in drinking water of south-western region of Punjab. India. Environ. Mon. Assess. 195, 367 (2023).
Ukah, B. U. et al. Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos. Nigeria. Int. J. Ener. Water Res. 3(4), 291–303 (2019).
Egbueri, J. C. & Mgbenu, C. N. Chemometric analysis for pollution source identification and human health risk assessment of water resources in Ojoto Province, southeast Nigeria. Appl. Water Sci. 10(4), 98 (2020).
Shekhawat, K., Chatterjee, S. & Joshi, B. Chromium toxicity and its health hazards. Int. J. Adv. Res. 3(7), 167–172 (2015).
Guan, Y., Shao, C. & Ju, M. Heavy metal contamination assessment and partition for industrial and mining gathering areas. Int. J. Environ. Res. Publ. Health 11(7), 7286–7303 (2014).
Agbasi, J. C. et al. Water pollution indexing and health risk assessment due to PTE ingestion and dermal absorption for nine human populations in Southeast Nigeria. Groundw. Sustain. Dev. 21, 100921 (2023).
Ezugwu, C. K. et al. Multi-criteria approach to water quality and health risk assessments in a rural agricultural province, southeast Nigeria. Hydro. Res. 2, 40–48 (2019).
Egbueri, J. C. Heavy metals pollution source identification and probabilistic health risk assessment of shallow groundwater in Onitsha. Nigeria. Anal. Lett. 53(10), 1620–1638 (2020).
Egbueri, J. C. & Enyigwe, M. T. Pollution and ecological risk assessment of potentially toxic elements in natural waters from the Amekametallogenic district in southeastern Nigeria. Anal. Lett. 53(17), 2812–2839 (2020).
Egbueri, J. C. & Agbasi, J. C. Data-driven soft computing modeling of groundwater quality parameters in southeast Nigeria: Comparing the performances of different algorithms. Environ. Sci. Poll. Res. 29(25), 38346–38373 (2022).
Karmakar, B., Singh, M. K., Choudhary, B. K. et al. Investigation of the hydrogeochemistry, groundwater quality and associated health risks in industrialized regions of Tripura, northeast India. Environ. Foren., 2006363 (2021).
Prasad, B. & Bose, J. M. Evaluation of heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ. Geol. 41, 183–188 (2001).
Mohan, S. V., Nithila, P. & Reddy, S. J. Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health A Tox. Haz. Subst. Environ. Eng. 31, 283–289 (1996).
Edet, A. E. & Offiong, O. E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower cross river basin southeastern Nigeria. Geo. J. 57, 295–304 (2002).
Prasanna, M. V. et al. Evaluation of water quality pollution indices for heavy metal contamination monitoring: A case study from Curtin Lake, Miri City. East Malaysia. Environ. Earth Sci. 67, 1987–2001 (2012).
De Kwaadsteniet, M. et al. Domestic rainwater harvesting: Microbial and chemical water quality and point-of-use treatment systems. Water, Air, Soil Poll. 224, 1629 (2013).
Kurniawan, T. A. et al. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chemic. Engg. J. 118, 83–98 (2006).
Ahmed, E. et al. Remediation and recycling of chromium from tannery wastewater using combined chemical-biological treatment system. Process Saf. Environ. Protect. 104, 1–10 (2016).
Hua, M. et al. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 211–212, 317–331 (2012).
Qasem, N. A. A., Mohammed, R. H. & Lawal, D. U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water (2021).
Selvi, A. et al. Integrated remediation processes toward heavy metal removal/recovery from various environments-a review. Front. Environ. Sci. 7, 66 (2019).
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
M.P., G.A.K. & R.C.R. designed the study; M.P. and R.S.A. collected and prepared samples, performed field survey; M.P., R.S.A. & G.A.K. performed laboratory work; M.P., R.S.A. & N.K.P. prepared maps; M.P., R.S.A., A.L.S. and J.C.E. wrote, reviewed, and edited the manuscript. All authors contributed extensively to the discussions about the work and in reviewing and revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aswal, R.S., Prasad, M., Patel, N.K. et al. Occurrences, sources and health hazard estimation of potentially toxic elements in the groundwater of Garhwal Himalaya, India. Sci Rep 13, 13069 (2023). https://doi.org/10.1038/s41598-023-40266-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40266-7
This article is cited by
-
Assessment of groundwater quality, source identification, and health risk around oil and gas drilling sites
Environmental Earth Sciences (2024)
-
Contamination and sediment quality evaluation of toxic metals enrichment in heavy mineral-rich beach sands of Arish City, Northeastern Egypt
Euro-Mediterranean Journal for Environmental Integration (2024)
-
Heavy metal pollution and health risk assessment using deterministic and Monte Carlo simulation approaches in the Himalayan spring and surface water systems of Kulgam District, Kashmir valley, India
Environmental Earth Sciences (2024)
-
Physicochemical properties and health risk assessment of selected heavy metals from soil and borehole water in Ifite-Awka, Anambra State, Nigeria
Discover Applied Sciences (2024)
-
Distribution, health effects and source apportionment of uranium and other potentially toxic elements in Ganga water
Journal of Radioanalytical and Nuclear Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.