Abstract
A heterogeneous copper-catalyzed A3 coupling reaction of aldehydes, amines, and alkynes for the synthesis of propargylamines and benzofurans has been developed. Here, the modified metal–organic framework MIL-101(Cr)-SB-Cu complex was chosen as the heterogeneous copper catalyst and prepared via post-synthetic modification of amino-functionalized MIL-101(Cr). The structure, morphology, thermal stability, and copper content of the catalyst were determined by FT-IR, PXRD, SEM, TEM, EDX, TGA, XPS, and ICP-OES. The catalyst shows high catalytic activity for the aforementioned reactions under solvent-free reaction conditions. High yields, low catalyst loading, easy catalyst recovery and reusability with not much shrink in catalytic activity, and a good yield of 82% in gram-scale synthesis are some of the benefits of this protocol that drove it towards sustainability.
Similar content being viewed by others
Introduction
Propargylamines have wide applications in organic synthesis as they are used as an essential intermediate for the synthesis of various biologically active compounds such as peptides, β-lactams, allylamines, natural products, drug molecules, agrochemical products, etc1,2,3,4. They are also used as precursors for the synthesis of a variety of heterocyclic compounds such as quinolines5, phenanthrolines6, pyrroles6, etc. In addition, propargylamine scaffolds are found in commercially available drugs such as rasagiline and deprenyl and are also used for the treatment of Parkinson’s and Alzheimer’s disease7,8,9. On the other hand, benzofurans are significant oxygen-containing heterocyclic scaffolds that exhibit immense biological and pharmaceutical activities such as anti-inflammatory10, anticancer11, antifungal12, antitumor13, etc. They are not only pivotal structural subunits in naturally occurring bioactive compounds but also act as a useful synthons in the synthesis of many natural products14,15,16. Moreover, benzofurans have several applications in cosmetic formulations and optical brighteners17. Therefore, efforts have been made by researchers to develop methodologies to synthesize propargylamines and benzofurans moieties. Some of the reported synthetic procedures for the synthesis of benzofurans are reactions of 2-chlorophenols with terminal alkynes18, cyclizations of ketones19, the sigmatropic rearrangement of arene20, palladium-catalyzed cyclizations of phenols21, etc. A recent synthetic approach is the transition-metal catalyzed coupling of aldehydes, amines, and alkynes (known as the A3 coupling reaction) which generally produce propargylamines22,23,24,25,26,27. However, benzofurans can also be synthesized by an A3 coupling reaction followed by intramolecular cyclizations28,29,30,31,32,33,34,35. Therefore, sustainable development of methodologies for the synthesis of these important moieties is of significant interest to organic chemists. Some of the biologically significant molecules containing propargylamines (a) and benzofurans (b) moieties are shown in Fig. 1.
In the meantime, metal–organic frameworks (MOFs) are notable porous materials that have gained much attention due to their high surface area, ultra-high porosity, thermal stability, tuneable pore size, etc. MOFs have wide applications in drug delivery36, sensing37, gas storage38, waste and water treatment39,40,41,42, biodiesel production43, catalysis44,45,46,47, etc. The main feature of MOFs is that they can be easily functionalized as per their application by post-synthetic modification (PSM) using suitable organic linkers, keeping the MOF structure intake48,49,50,51. Among other PSM of MOFs, the condensation between free amine of the MOF and aldehydes to create a Schiff base moiety for incorporation of active metal is the most convenient method52,53,54,55,56,57. Recently, several MOFs have been developed for application in the field of heterogeneous catalysis58,59,60. Therefore, in this study amino-functionalized MIL-101(Cr) has been synthesized and further 2-pyridine-carboxaldehyde was used to create a Schiff base moiety [MIL-101(Cr)-SB] in the framework for anchoring Cu metal with MIL-101(Cr)-SB to make the MIL-101(Cr)-SB-Cu complex, which was then employed for the synthesis of propargylamines and benzofurans via A3 coupling and intramolecular cyclization reaction.
Result and discussion
Post-synthetic modification of MIL-101(Cr)-NH2
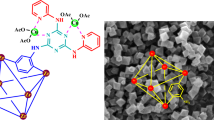
The schematic representation for the preparation of MIL-101(Cr)-SB-Cu (III) is illustrated in Fig. 2. Initially, MIL-101(Cr)-NH2 (I) was prepared using Cr(NO3)3.9H2O and 2-aminobenzene-1,4-dicarboxylic acid (H2N-BDC) by solvothermal method61. Thereafter, the functionalization of the free amino group present in the MIL-101(Cr)-NH2 (I) framework was accomplished by post-synthetic modification (PSM). Accordingly, MIL-101(Cr)-NH2 (I) was reacted with 2-pyridine-carboxaldehyde for constructing a 2-pyridyl-imine (Schiff base) moiety in the framework for incorporation of Cu species to give MIL-101(Cr)-SB (II). Finally, MIL-101(Cr)-SB (II) was then treated with Cu(OAc)2 to get the expected MIL-101(Cr)-SB-Cu complex (III).
Spectroscopic studies
After the preparation of the MIL-101(Cr)-SB-Cu (III), it was well characterized by various spectroscopic techniques such as Fourier transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray (EDX), X-ray photoelectron spectroscopy (XPS). Thermal stability was determined by thermogravimetric analysis (TGA), and the copper content in the catalyst was examined by inductively coupled plasma optical emission spectroscopy (ICP-OES).
The FT-IR spectra of the MIL-101(Cr)-NH2 (I), MIL-101(Cr)-SB (II), and MIL-101(Cr)-SB-Cu (III) are presented in Fig. 3. In Fig. 3a–c, the characteristic absorption band at around 1390 cm−1 is attributed to the O–C–O symmetric vibrations indicating the presence of dicarboxylate linker in all MOF structures. The absorption band at around 1570 cm−1 is associated with the C=C stretching vibration of the benzene ring. Further, the band observed at around 1695 cm−1 (Fig. 3c) and 1698 cm−1 (Fig. 3b) corresponds to the azomethine group (> C=N–) present in the framework. However, a slight shifting of > C=N– band (Fig. 3c) to a lower value endorses the coordination of the Cu2+ to the azomethine-N of the Schiff base moiety62.
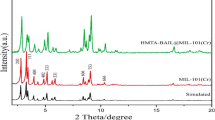
The PXRD patterns of MIL-101(Cr), MIL-101(Cr)-NH2 (I), and MIL-101(Cr)-SB-Cu (III) are shown in Fig. 4. In the PXRD pattern of MIL-101(Cr), the characteristic diffraction peaks at 2θ values 5.4°, 8.9°, 10.3, and 17.3° correspond to (135), (195), (2210), and (4416) planes respectively. Further, the characteristics diffraction peaks of MIL-101(Cr)-NH2 at 2θ values of 5.5°, 8.8°, and 16.9° correspond to (135), (195), and (4416) planes respectively, which are in accordance with that of the literature and therefore, confirm its successful preparation55,63,64,65,66. In the PXRD pattern of MIL-101(Cr)-SB-Cu (III), the presence of peaks at 2θ values of 5.1°, 8.6°, and 17.5° corresponding to MIL-101(Cr)-NH2 peaks implies that the structure of the parent MOF is retained even after the post-synthetic modification including functionalization and metal complexation.
The structural morphology of the prepared MIL-101(Cr)-SB-Cu (III) catalyst was investigated using SEM and TEM analysis. The SEM image as depicted in Fig. 5a, shows irregular agglomerated morphology of the prepared MIL-101(Cr)-SB-Cu (III) catalyst. The TEM images from low to high magnifications are illustrated in Fig. 5b–c. In the TEM image, the black spherical shape indicates the agglomeration of the Cu in the MOF. The EDX spectrum (Fig. 5d) confirms the presence of all requisite elements such as C, N, O, Cr, and Cu in the prepared MIL-101(Cr)-SB-Cu (III) catalyst.
The oxidation state of Cu species in the MIL-101(Cr)-SB-Cu (III) catalyst was determined by XPS analysis. The XPS spectra of the MIL-101(Cr)-SB-Cu (III) catalyst are demonstrated in Fig. 6. The survey spectrum as shown in Fig. 6a displays the peaks for C 1s, N 1s, O 1s, Cr 2p, and Cu 2p indicating the successful formation of MIL-101(Cr)-SB-Cu. In the Cu 2p scan (Fig. 6b), the peaks around 933.9 and 953.7 eV corresponds to Cu 2p3/2 and Cu 2p1/2 respectively, which indicates that the oxidation state of Cu is + 2. Besides these, the satellite peaks at 942.7 and 962.6 eV in Cu 2p also indicate the presence of Cu(II) species33,67.
The thermal stability of the prepared MIL-101(Cr)-SB-Cu (III) catalyst was studied using thermogravimetric analysis. The TGA thermogram of MIL-101(Cr)-NH2 (I) and MIL-101(Cr)-SB-Cu (III) are demonstrated in Fig. 7. The TGA thermogram of the MIL-101(Cr)-NH2 (I) exhibits weight loss at around 100 °C which is due to the loss of absorbed water. A major weight loss process is observed within the temperature 250–500 °C which may be due to the decomposition of the MOF structure. The data is similar to that of the reported value which indicates that the MIL-101(Cr)-NH2 (I) is stable up to 250 °C68. Similarly, the TGA thermogram of catalyst (III) is also consistent with that of the parent MIL-101(Cr)-NH2 (I) suggesting that the thermal stability of the catalyst (III) is sustained even after the post-synthetic modification and functionalization of the MOF. Furthermore, ICP-OES analysis was carried out to check the Cu content in the prepared MIL-101(Cr)-SB-Cu (III) catalyst which was found to be 4.23 wt%.
Catalytic activity studies
The prepared MIL-101(Cr)-SB-Cu was then utilized for catalytic application. At first, its catalytic activity was studied for the synthesis of propargylamines via A3 coupling reaction where 4-methylbenzaldehyde (1a), morpholine (2a), and phenylacetylene (3a) were used as the model substrates for screening different reaction conditions. Initially, the reaction was carried out with MIL-101(Cr)-SB-Cu (15 mg) in toluene under refluxed condition, and the propargylamine product 4a was obtained in 82% yield within 1 h (Table 1, entry 1). When the reaction was performed in solvent-free reaction condition (SFRC) at 100 °C, product 4a was obtained in 86% yield (Table 1, entry 2). Further, screening different solvents such as EtOH, CH3CN, DCE, and H2O afforded 39%, 47%, 35%, and 19% yields respectively (Table 1, entries 3–6). However, CHCl3 did not produce the desired product 4a (Table 1, entry 7). Therefore, SFRC was chosen as the optimum condition. Next, the model reaction was performed with different catalyst amounts from 0 to 20 mg under SFRC (Table 1, entry 8–11). The best yield of 86% of product 4a was obtained with 15 mg of the catalyst. Furthermore, increasing the amount of catalyst to 20 mg, there was no increment in the yield of product 4a. On the other hand, a lower amount of catalyst (< 15 mg) furnished a lower yield of the product (Table 1, entries 8 and 9). As expected, product 4a was not formed in the absence of a catalyst (Table 1, entry 11). Eventually, at lower temperatures such as 60 °C, and 80 °C, the lower yield of product 4a was obtained (Table 1, entries 12 and 13). Thereafter, increasing the temperature to 120 °C also did not enhance the yield of product 4a (Table 1, entry 14). Moreover, MIL-101(Cr)-NH2 and MIL-101(Cr) also did not produce the desired product 4a (Table 1, entry 15).
After optimization of the suitable reaction condition, the generality of the methodology was explored using different aryl aldehydes. Aryl aldehydes containing –CH3, –OCH3, –F, –Cl, –Br afforded the corresponding products in good to excellent yields (85–96%) (Fig. 8, 4a–4k). Further, 2-furaldehyde and trans-cinnamaldehyde also produced the desired products in good yields (Fig. 8, 4l, and 4m). Morpholine as the secondary base and phenylacetylene as the alkynes afforded good yields. However, when primary amine such as aniline was used, no desired product was obtained. Further, piperidine and diethyl amine produced a trace amount of products.
The catalytic activity of MIL-101(Cr)-SB-Cu was further studied for the synthesis of benzofurans where 2-hydroxybenzaldehyde (1d), morpholine (2a), and phenylacetylene (3a) were chosen as the model substrates for optimization of the reaction condition. Therefore, to optimize the reaction condition, different solvents, temperature, and catalytic loading were investigated and the results are summarized in Table 2. Initially, the reaction was performed with MIL-101(Cr)-SB-Cu (15 mg) in the presence of DMAP in toluene under refluxed condition, and the benzofuran 5a was obtained in 84% yield with a trace amount of propargylamine 4d (Table 2, entry 1). However, in the absence of DMAP, the propargylamine 4d was obtained in 87% yield and failed to produce the benzofuran 5a (Table 2, entry 2). This might be due to the low nucleophilicity of the hydroxyl group of 2-hydroxybenzaldehyde. Hence, DMAP acts as the base which facilitates the intramolecular nucleophilic attack by abstracting the proton from the hydroxyl group to give the benzofuran products. To inspect the effect of solvent on the model reaction, several solvents including solvent-free reaction conditions (SFRC) were screened. Fortunately, an excellent yield of 92% of product 5a was achieved in solvent-free reaction condition in 6 h (Table 2, entry 3). Further, EtOH afforded product 5a in trace amount and the uncyclized product 4d in 42% yield (Table 2, entry 4). In the case of CHCl3, product 5a was not formed and only a trace amount of 4d was observed in TLC (Table 2, entry 5). However, solvents like CH3CN, DCE, and H2O provided 4d in 45%, 37%, and 20% yields respectively with a trace amount of 5a (Table 2, entries 6–8). Henceforth, the reactions were further performed in SFRC. Thereafter, different catalyst concentration was also examined. In the absence of a catalyst, no product formation was detected (Table 2, entry 9). When the reactions were carried out with 5, 10, 15, and 20 mg of the catalyst, product 5a was obtained in 74%, 86%, 92%, and 92% yields respectively (Table 2, entries 10–12). The best result was obtained with 15 mg of the catalyst and further increasing the amount of catalyst no increment in the yield of the product was observed. After that, the effect of different amounts of DMAP on the model reaction was checked and the best result was obtained with 0.5 equiv. of DMAP (Table 2, entries 13 and 14). Other bases like Na2CO3, K2CO3, and Cs2CO3 were also screened. Na2CO3 afforded 26% yield of the product 5a along with 67% yield of 4d (Table 2, entries 15). In case of K2CO3 and Cs2CO3, the products 5a were obtained in 67% and 81% yields respectively along with trace amount of 4d in both the cases (Table 2, entries 16 and 17). Finally, the impact of temperature was investigated and the highest yield of the desired product 5a was achieved at 100 °C. Lowering the temperature from 100 to 60 °C afforded a lower yield of the product whereas high temperature (120 °C) did not improve the yield of the product 5a (Table 2, entries 18–20). In summary, the optimum reaction condition for this reaction is 15 mg of the catalyst, 0.5 equiv. of DMAP, SFRC, and 100 °C. Moreover, MIL-101(Cr)-NH2 and MIL-101(Cr) did not furnish any cyclized (5a) or uncyclized (4d) products under the optimized reaction condition. (Table 2, entries 21 and 22).
After optimization of the suitable reaction condition, the versatility of the present protocol was explored with various substituted 2-hydroxybenzaldehyde containing electron-withdrawing groups and electron-donating groups (Fig. 9, 5a–5k). In all the cases, the products were obtained in good to excellent yields (86–95%). Further, electron-withdrawing groups provided slightly better yields of the products (5f and 5k) than electron-donating groups (5b, 5c, 5i, and 5j). However, aliphatic alkynes such as 1-octyne, and 1-pentyne did not produce the target products. Morpholine as the secondary amine afforded the desired products in good to excellent yields but aniline, diethyl amine, and piperidine were unable to produce the desired products.
Plausible mechanism
A schematic representation of a plausible mechanism for the synthesis of propargylamines and benzofurans is presented in Fig. 1034. Initially, the alkyne 3 is activated by coordination with the Cu-center of the catalyst to form the copper acetylide intermediate V. The catalyst also promotes the condensation reaction of salicylaldehydes 1 and secondary amine 2 by activating the carbonyl carbon of the aldehyde which gives the iminium intermediate VI. Then, the iminium intermediate VI interacts with the copper acetylide intermediate V to produce the propargylamine 4. In the presence of a base, it abstracts the proton from the phenolic hydroxyl group and facilitates intramolecular cyclization to afford the intermediate VIII. Finally, tautomerization furnishes the desired benzofuran 5.
Scale-up reaction
Gram-scale synthesis was carried out to check the possibility of industrial application (Fig. 11). Therefore, the reaction was performed using 2-hydroxybenzaldehyde (1d, 9 mmol, 1.080 g), morpholine (2a, 10.8 mmol, 0.940 g), phenylacetylene (3a, 13.5 mmol, 1.377 g), DMAP (4.5 mmol, 0.549 g) and MIL-101(Cr)-SB-Cu (135 mg) under the optimized reaction condition. After that, the reaction mixture was purified and the desired product was obtained in 82% yield. This good result refers the protocol for industrial application.
Reusability of the catalyst
Easy separation and reusability of the catalyst are important parameters of a heterogeneous catalyst. Therefore, the reusability of the catalyst was studied for the synthesis of benzofuran (5a) using 2-hydroxybenzaldehyde (1d), morpholine (2a), and phenylacetylene (3a) under the optimized reaction condition. After 6 h, ethyl acetate was added to the reaction mixture, and the catalyst was separated by centrifugation followed by filtration. The recovered catalyst was washed with ethyl acetate, ethanol, and diethyl ether and dried properly. Thereafter the recovered catalyst was used in another set of reactions. The catalyst was reused for up to five consecutive runs. It was noticed that the recovered catalyst offered good yield till five runs (Fig. 12).
The structural morphology and stability of the reused MIL-101(Cr)-SB-Cu (III) catalyst (after 5th run) were studied using various spectroscopic techniques such as FT-IR (Fig. 13), SEM (Fig. 14a), TEM (Fig. 14b), TGA (Fig. 15), and ICP-OES. From the FT-IR spectrum (Fig. 13) of the reused catalyst no notable changes in the characteristic bands were observed. The SEM (Fig. 14a) and TEM (Fig. 14b) images of the reused catalyst give evidence of some aggregation of the Cu particles. Further, the TGA thermogram (Fig. 15) of the reused catalyst was analogous to that of the fresh catalyst. The leaching of Cu after the 5th cycle was examined by ICP-OES analysis and it was found that only 0.562 ppm Cu leached out.
Comparative study
The present protocol for the synthesis of benzofurans has been compared with those of previously reported methods in the literature. The comparative summary is illustrated in Table 3. From Table 3, it can be concluded that the present protocol also shows better result as that of the reported methods.
Conclusion
In conclusion, an efficient and sustainable protocol for the synthesis of propargylamines and benzofurans via A3 coupling and cycloisomerization reaction of aldehydes, amines, and alkynes has been developed utilizing MIL-101(Cr)-SB-Cu as an easily recoverable and reusable heterogeneous catalyst. A series of propargylamine and benzofuran derivatives were synthesized bearing different electron-donating and electron-withdrawing groups. High yields, operational simplicity, and solvent-free reaction condition are some of the advantages of this methodology. The catalyst could be easily separated by centrifugation followed by filtration and shows excellent catalytic activity up to five consecutive runs. The gram-scale synthesis also provided a high yield of 82% implying its possibility for application at the industrial level.
Experimental section
General information
All the chemicals required were obtained from Sigma-Aldrich, Alfa Aesar, Spectrochem, and TCI and used without further purification. FT-IR spectra were recorded on a Bruker Alpha II system (ν max in cm−1) on KBr disks. 1H NMR and 13C NMR (400 MHz and 100 MHz respectively) spectra were recorded using a Bruker Avance II-400 spectrometer using CDCl3 as the solvent (chemical shifts in δ with TMS as internal standard). Powder XRD analyses were carried out using a Bruker D8 Advance and Rigaku Ultima IV XRD instrument. Transmission Electron Microscopy (TEM) analysis was carried out using a JEOL JSM 100CX system. Scanning electron microscopy (SEM) and Energy Dispersive X-ray (EDX) analysis were carried out using a JSM-6360 (JEOL) system. X-Ray Photoelectron Spectroscopy (XPS) was performed using a PHI 5000 VersaProbe III system. Thermogravimetric analysis (TGA) was carried out using a Perkin Elmer Precisely STA 6000 simultaneous thermal analyzer. Inductively coupled plasma optical emission spectroscopy (ICP-OES) was carried out using Thermo Scientific™ iCAP™ 7600 instrument. TLC Silica gel 60 F254 (Merck) was used for TLC analysis. Hexane refers to the fraction boiling between 60 and 80 °C.
Synthesis of MIL-101(Cr)-NH2 (I),61 MIL-101(Cr)-SB (II), and MIL-101(Cr)-SB-Cu (III)
A solution of NaOH (0.40 g) in deionized H2O (30 mL) was taken in a round bottom flask. To that, 1.60 g of Cr(NO3)3.9H2O and 0.72 g of 2-aminobenzene-1,4-dicarboxylic acid (NH2-H2DBC) were added slowly under constant stirring at room temperature for 30 min. After that, the mixture was transferred into a 50 mL Teflon-lined stainless steel autoclave and kept at 150 °C in a muffle furnace for 12 h. Then, the obtained green powder was collected by filtration and washed thoroughly with H2O, DMF, and ethanol respectively. Eventually, the MIL-101(Cr)-NH2 was dried at 100 °C. This dried 0.40 g of MIL-101(Cr)-NH2 (I) was dispersed in 30 mL of ethanol. Then, pyridine-2-carboxaldehyde (0.57 mL) was added dropwise to the mixture and refluxed for 24 h with constant stirring. Finally, the MIL-101(Cr)-SB (II) was obtained by centrifugation, filtration and washed properly with ethanol, diethyl ether, and dried. Thereafter, 0.40 g of MIL-101(Cr)-SB (II) was dispersed in 30 mL of ethanol. To that mixture, Cu(OAc)2·H2O (0.25 g) was added and the solution was refluxed for 24 h with constant stirring. After that, the obtained solid materials were centrifuged, filtered, and washed with ethanol, and diethyl ether, and it was dried to get the resulting MIL-101(Cr)-SB-Cu (III) complex.
General procedure for the synthesis of propargylamines
In a 25 mL round bottom flask, aryl aldehydes (1, 1 mmol), secondary amines (2, 1.2 mmol), and MIL-101(Cr)-SB-Cu (III) [15 mg] were taken and stirred at 100 °C under SFRC for 15 min. Then, aryl acetylenes (3, 1.5 mmol) were added dropwise to the reaction mixture and stirring was continued for 45 min. Then, 10 mL of ethyl acetate was added to the reaction mixture and the catalyst was separated by centrifugation followed by filtration. The organic solvent was evaporated under reduced pressure and the crude products were further purified by column chromatography (silica gel 100–200 mesh) using ethyl acetate/hexane (1:19) as eluent.
General procedure for the synthesis of benzofurans
A mixture of substituted 2-hydroxybenzaldehydes (1, 1 mmol), secondary amines (2, 1.2 mmol), and MIL-101(Cr)-SB-Cu (III) [15 mg] was taken in a 25 mL round bottom flask and stirred at 100 °C under SFRC for 15 min. Then, aryl acetylenes (3, 1.5 mmol) were added dropwise into the reaction mixture and continued the stirring for another 45 min. Then, DMAP (0.5 mmol) was added to the reaction mixture, and the stirring was continued for 5 h. After that, ethyl acetate (10 mL) was added to the reaction mixture, and the catalyst was separated by centrifugation followed by filtration. The organic solvent was washed with H2O (2 × 5 mL), and brine (1 × 5 mL), and dried over anhydrous Na2SO4. The organic solvent was evaporated under reduced pressure and the crude products were further purified by column chromatography (silica gel 100–200 mesh) using ethyl acetate/hexane as eluent.
Data availability
The data used to support this study are included in the article and its supplementary information files.
References
Arefi, E., Khojastehnezhad, A. & Shiri, A. A core-shell superparamagnetic metal-organic framework: A recyclable and green catalyst for the synthesis of propargylamines. New J. Chem. 45, 21342–21349 (2021).
Dulle, J. et al. Efficient three-component coupling catalysed by mesoporous copper–aluminum based nanocomposites. Green Chem. 15, 1238–1243 (2013).
Boulton, A. A. et al. Aliphatic propargylamines: New antiapoptotic drugs. Drug Dev. Res. 42, 150–156 (1997).
Lee, E.-S., Yeom, H.-S., Hwang, J.-H. & Shin, S. A practical gold-catalyzed route to 4-substituted oxazolidin-2-ones from N-boc propargylamines. Eur. J. Org. Chem. 2007, 3503–3507 (2007).
Xiao, F., Chen, Y., Liu, Y. & Wang, J. B. Sequential catalytic process: Synthesis of quinoline derivatives by AuCl3/CuBr-catalyzed three-component reaction of aldehydes, amines, and alkynes. Tetrahedron 64, 2755–2761 (2008).
Shibata, D., Okada, E., Molette, J. & Médebielle, M. Facile synthesis of fluorine-containing 1,10-phenanthrolines by the pyridine-ring formation reaction of N-propargyl-5,7-bis(trifluoroacetyl)-8-quinolylamine with amines: Isolation of the intermediates 1,4-dihydro-1,10-phenanthrolin-4-ols. Tetrahedron Lett. 49, 7161–7164 (2008).
McCormack, P. L. Rasagiline: A review of its use in the treatment of idiopathic Parkinson’s disease. CNS Drugs 28, 1083–1097 (2014).
Miklya, I. The significance of selegiline/(−)-deprenyl after 50 years in research and therapy (1965–2015). Mol. Psychiatry 21, 1499–1503 (2016).
Yu, P. H., Davis, B. A. & Boulton, A. A. Aliphatic propargylamines: Potent, selective, irreversible monoamine oxidase B inhibitors. J Med Chem. 35, 3705–3713 (1992).
Dawood, K. M., Abdel-Gawad, H., Rageb, E. A., Ellithey, M. & Mohamed, H. A. Synthesis, anticonvulsant, and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg. Med. Chem. 14, 3672–3680 (2006).
Flynn, B. L., Hamel, E. & Jung, M. K. One-pot synthesis of Benzo[b]furan and indole inhibitors of tubulin polymerization. J. Med. Chem. 45, 2670–2673 (2002).
Gündoğdu-Karaburun, N., Benkli, K., Tunali, Y. & Üçucu, U. Synthesis and antifungal activities of some aryl[3-(imidazol-1-yl/triazol-1-ylmethyl) benzofuran-2-yl] ketoximes. Eur. J. Med. Chem. 41, 651–656 (2006).
Baraldi, P. G. et al. Synthesis and antitumor activity of new benzoheterocyclic derivatives of distamycin A. J. Med. Chem. 43, 2675–2684 (2000).
Asif, M. Mini review on important biological properties of benzofuran derivatives. J. Anal. Pharm. Res. 3, 11–12 (2016).
Rao, M. L. N. & Murty, V. N. Rapid access to benzofuran-based natural products through a concise synthetic strategy. Eur. J. Org. Chem. 2016, 2177–2186 (2016).
Khodarahmi, G., Asadi, P., Hassanzadeh, F. & Khodarahmi, E. J. Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents: A review. Res. Med. Sci. 20, 1094–1104 (2015).
Safaei-Ghomi, J. & Ghasemzadeh, M. A. Zinc oxide nanoparticle promoted highly efficient one pot three-component synthesis of 2,3-disubstituted benzofurans. Arab. J. Chem. 10, S1774–S1780 (2017).
Wang, J.-R. & Manabe, K. Hydroxyterphenylphoshine-palladium catalyst for Benzo[b]furan synthesis from 2-chlorophenols. Bifunctional ligand strategy for cross-coupling of chloroarenes. J. Org. Chem. 75, 5340–5342 (2010).
Wright, J. B. Some reactions of mannich bases derived from α-phenoxyacetophenone and α-phenoxypropiophenone. J. Org. Chem. 25, 1867–1872 (1960).
Takeda, N., Miyata, O. & Naito, T. Efficient synthesis of benzofurans utilizing [3,3]-sigmatropic rearrangement triggered by N-trifluoroacetylation of oxime ethers: Short synthesis of natural 2-arylbenzofurans. Eur. J. Org. Chem. 2007, 1491–1509 (2007).
Bernini, R., Cacchi, S., De Salve, I. & Fabrizi, G. Palladium-catalyzed synthesis of lipophilic benzo[b]furans from cardanol. Synthesis 2007, 873–882 (2007).
Hazarika, R. Magnetically separable ZnFe2O4 nanoparticles: A low cost and sustainable catalyst for propargyl amine and NH-triazole synthesis. Appl. Catal. A 625, 118338 (2021).
Veisi, H., Mohammadi, L., Hemmati, S., Tamoradi, T. & Mahammadi, P. In situ immobilized silver nanoparticles on rubia tinctorum extract-coated ultrasmall iron oxide nanoparticles: An efficient nanocatalyst with magnetic recyclability for synthesis of propargylamines by A3 coupling reaction. ACS Omega 4, 13991–14003 (2019).
Milen, M., Györke, G., Dancsó, A. & Volk, B. Study on the A3-coupling reaction catalyzed by readily available copper-containing minerals. Synthesis of propargylamines. Tetrahedron Lett. 61, 151544 (2020).
Kujur, S. & Pathak, D. D. Reduced graphene oxide-immobilized iron nanoparticles Fe(0)@rGO as heterogeneous catalyst for one-pot synthesis of series of propargylamines. Res. Chem. Intermed. 46, 369–384 (2020).
Mittal, A., Kumari, S., Yadav, P. D. & Sharma, S. K. A new copper complex on graphene oxide: A heterogeneous catalyst for N-arylation and C-H activation. Appl. Organometal. Chem. 34, e5362 (2020).
Zarenezhad, E., Taghavi, R., Kamrani, P., Farjam, M. & Rostamnia, S. Gold nanoparticle decorated dithiocarbamate modified natural boehmite as a catalyst for the synthesis of biologically essential propargylamines. RSC Adv. 12, 31680–31687 (2022).
Sharghi, H., Shiri, P. & Aberi, M. A solvent-free and one-pot strategy for ecocompatible synthesis of substituted benzofurans from various salicylaldehydes, secondary amines, and nonactivated alkynes catalyzed by copper(I) oxide nanoparticles. Synthesis 46, 2489–2498 (2014).
Zhang, X., Li, D., Jia, X., Wang, J. & Fan, X. CuI/[bmim]OAc in [bmim]PF6: A highly efficient and readily recyclable catalytic system for the synthesis of 2,3-disubstituted benzo[b]furans. Catal. Commun. 12, 839–843 (2011).
Sakai, N., Uchida, N. & Konakahara, T. Facile and efficient synthesis of polyfunctionalized benzofurans: three-component coupling reactions from an alkynylsilane, an o-hydroxybenzaldehyde derivative, and a secondary amine by a Cu(I)-Cu(II) cooperative catalytic system. Tetrahedron Lett. 49, 3437–3440 (2008).
Li, H., Liu, J., Yan, B. & Li, Y. New domino approach for the synthesis of 2,3-disubstituted benzo[b]furans via copper-catalyzed multi-component coupling reactions followed by cyclization. Tetrahedron Lett. 50, 2353–2357 (2009).
Abtahi, B. & Tavakol, H. CuI-catalyzed, one-pot synthesis of 3-aminobenzofurans in deep eutectic solvents. Appl. Organomet. Chem. 35, e6433 (2021).
Xu, Z., Xu, J. & Li, Y. CuSO4 nanoparticles loaded on carboxymethylcellulose/polyaniline composites: A highly efficient catalyst with enhanced catalytic activity in the synthesis of propargylamines, benzofurans, and 1,2,3-triazoles. Appl. Organomet. Chem. 35, e6349 (2021).
Sadjadi, S., Heravi, M. M. & Malmir, M. Green bio-based synthesis of Fe2O3@SiO2-IL/Ag hollow spheres and their catalytic utility for ultrasonic-assisted synthesis of propargylamines and benzo[b]furans. Appl. Organometal. Chem. 32, e4029 (2018).
Purohit, G., Rajesh, U. C. & Rawat, D. S. Hierarchically porous sphere-like copper oxide (HS-CuO) nanocatalyzed synthesis of benzofuran isomers with anomalous selectivity and their ideal green chemistry metrics. ACS Sustain. Chem. Eng. 5, 6466–6477 (2017).
Sirajunnisa, P., George, L. H., Manoj, N., Prathapan, S. & Sailaja, G. S. Lawsone derived Zn(II) and Fe(III) metal organic frameworks with pH dependent emission for controlled drug delivery. New J. Chem. 45, 14589–14597 (2021).
Yao, J., Yue, T., Huang, C. & Wang, H. A magnified aptamer fluorescence sensor based on the metal organic frameworks adsorbed DNA with enzyme catalysis amplification for ultra-sensitive determination of ATP and its logic gate operation. Bioorg. Chem. 114, 105020 (2021).
Zeng, Z. et al. Flexible microporous copper(II) metal-organic framework toward the storage and separation of C1–C3 hydrocarbons in natural gas. Inorg. Chem. 60, 8456–8460 (2021).
Rojas, S. & Horcajada, P. Metal-organic frameworks for the removal of emerging organic contaminants in water. Chem. Rev. 120, 8378–8415 (2020).
Taghavi, R. et al. Magnetite metal-organic frameworks: Applications in environmental remediation of heavy metals, organic contaminants, and other pollutants. Inorg. Chem. 61, 15747–15783 (2022).
Karimi-Maleh, H. et al. State-of-art advances on removal, degradation and electrochemical monitoring of 4-aminophenol pollutants in real samples: A review. Environ. Res. 222, 115338 (2023).
Alamgholiloo, H., Pesyan, N. N., Mohammadi, R., Rostamnia, S. & Shokouhimehr, M. Synergistic advanced oxidation process for the fast degradation of ciprofloxacin antibiotics using a GO/CuMOF-magnetic ternary nanocomposite. J. Environ. Chem. Eng. 9, 105486 (2021).
Xie, W. & Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chem. Eng. J. 365, 40–50 (2019).
Dhakshinamoorthy, A., Li, Z. & Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 47, 8134–8172 (2018).
Bavykina, A. et al. Metal-organic frameworks in heterogeneous catalysis: Recent progress, new trends, and future perspectives. Chem. Rev. 120, 8468–8535 (2020).
Gupta, A. et al. Development of a new catalytic and sustainable methodology for the synthesis of benzodiazepine triazole scaffold using magnetically separable CuFe2O4@MIL-101(Cr) nano-catalyst in aqueous medium. Appl. Organomet. Chem. 34, e5782 (2020).
Taghavi, R. & Rostamnia, S. Four-component synthesis of polyhydroquinolines via unsymmetrical hantzsch reaction employing Cu-IRMOF-3 as a robust heterogeneous catalyst. Chem. Methodol. 6, 639–648 (2022).
Yildiz, C. et al. Post-synthetic modification of DUT-5-based metal organic frameworks for the generation of single-site catalysts and their application in selective epoxidation reactions. ChemCatChem 12, 1134–1142 (2020).
Nouri, F., Rostamizadeh, S. & Azad, M. Post-synthetic modification of IRMOF-3 with an iminopalladacycle complex and its application as an effective heterogeneous catalyst in Suzuki-Miyaura cross-coupling reaction in H2O/EtOH media at room temperature. Mol. Catal. 443, 286–293 (2017).
Kalaj, M. & Cohen, S. M. Postsynthetic modification: An enabling technology for the advancement of metal-organic frameworks. ACS Cent. Sci. 6, 1046–1057 (2020).
Alamgholiloo, H. et al. Formation and stabilization of colloidal ultra-small palladium nanoparticles on diamine-modified Cr-MIL-101: Synergic boost to hydrogen production from formic acid. J. Colloid Interface Sci. 567, 126–135 (2020).
Kaur, M. et al. Post-Synthesis modification of metal-organic frameworks using Schiff base complexes for various catalytic applications. Chem. Eng. J. 423, 130230 (2021).
Hou, J. et al. Synthesis of UiO-66-NH2 derived heterogeneous copper (II) catalyst and study of its application in the selective aerobic oxidation of alcohols. J. Mol. Catal. A: Chem. 407, 53–59 (2015).
Xiong, G. et al. La-metal-organic framework incorporating Fe3O4 nanoparticles, post-synthetically modified with Schiff base and Pd. A highly active, magnetically recoverable, recyclable catalyst for C-C cross-couplings at low Pd loadings. J. Catal. 361, 116–125 (2018).
Huang, K., Guo, L. L. & Wu, D. F. Synthesis of metal Salen@MOFs and their catalytic performance for styrene oxidation. Ind. Eng. Chem. Res. 58, 4744–4754 (2019).
Sen, R., Saha, D., Koner, S., Brandao, P. & Lin, Z. Single crystal to single crystal (SC-to-SC) transformation from a nonporous to porous metal–organic framework and its application potential in gas adsorption and suzuki coupling reaction through postmodification. Chem. Eur. J. 21, 5962–5971 (2015).
Bunchuay, T. et al. Microwave-assisted one-pot functionalization of metal-organic framework MIL-53(Al)-NH2 with copper(II) complex and its application in olefin oxidation. Catal. Sci. Technol. 7, 6069–6079 (2017).
Moghadaskhou, F., Tadjarodi, A., Mollahosseini, A. & Maleki, A. Synthesis of UiO heterogeneous -66-Sal-Cu(OH)2 by a simple and novel method: MOF-based metal thin film as a catalyst for olefin oxidation. ACS Appl. Mat. Interfaces 15, 4021–4032 (2023).
Dong, Y., Li, W.-H. & Dong, Y.-B. Dual-metal N-Heterocyclic carbene complex (M = Au and Pd)-functionalized UiO-67 MOF for alkyne hydration-suzuki coupling tandem reaction. J. Org. Chem. 86, 1818–1826 (2021).
Sarkar, F. K. et al. A green and sustainable approach for the synthesis of 1,5-benzodiazepines and spirooxindoles in one-pot using a MIL-101(Cr) metal–organic framework as a reusable catalyst. New J. Chem. 45, 19553–19564 (2021).
Li, X., Pi, Y., Xia, Q., Li, Z. & Xiao, J. TiO2 encapsulated in Salicylaldehyde-NH2-MIL-101(Cr) for enhanced visible light-driven photodegradation of MB. Appl. Catal. B Environ. 191, 192–201 (2016).
Kardanpour, R. et al. Anchoring of Cu(II) onto surface of porous metal-organic framework through post-synthesis modification for the synthesis of benzimidazoles and benzothiazoles. J. Solid State Chem. 235, 145–153 (2016).
Liu, H., Ramella, D., Yu, P. & Luan, Y. A novel modified MIL-101-NH2 ligand for CuI-catalyzed and air promoted oxidation of secondary alcohols. RSC Adv. 7, 22353–22359 (2017).
Lin, Y., Kong, C. & Chen, L. Direct synthesis of amine functionalized MIL-101(Cr) nanoparticles and application for CO2 capture. RSC Adv. 2, 6417–6419 (2012).
Shaabani, A., Mohammadian, R., Farhid, H., Alavijeh, M. K. & Amini, M. M. Iron-decorated, guanidine functionalized metal-organic framework as a non-heme iron-based enzyme mimic system for catalytic oxidation of organic substrates. Catal. Lett. 149, 1237–1249 (2019).
Cai, Y. et al. Efficient capture of ReO4− on magnetic amine-functionalized MIL-101(Cr): Revealing from selectivity to mechanism. Sci. Total Environ. 771, 144840 (2021).
Zhang, P. et al. Mesoporous nitrogen-doped carbon for copper mediated Ullmann-type C–O/–N/–S cross-coupling reactions. RSC Adv. 3, 1890–1895 (2013).
Nuri, A. et al. Synthesis and characterization of palladium supported amino functionalized magnetic-MOF-MIL-101 as an efficient and recoverable catalyst for Mizoroki-heck cross-coupling. Catal. Lett. 150, 2617–2629 (2020).
Acknowledgements
The authors thank the UGC NEHU-NON NET fellowship and SERB (EMR/2016/005089 Dated 30.06.2017) for financial assistance. The authors also acknowledge SAIF NEHU, IIC-IIT Roorkee, and KIIT Bhubaneswar. The authors specially thank Dr. Anasuya Bandyopadhyay and Mr. Jagannath Majhi, Department of Polymer & Process Engineering, IIT Roorkee.
Author information
Authors and Affiliations
Contributions
F.K.S.: conceptualization, methodology, investigation, experimental studies, data analysis, writing – original draft preparation. L.K.: data analysis, methodology. S.G.: data analysis, software. R.S.: experimental studies, data analysis. S.J.: sample analysis, data collection. A.K.P.: supervision, conceptualization, writing – reviewing, editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sarkar, F.K., Kyndiah, L., Gajurel, S. et al. A sustainable avenue for the synthesis of propargylamines and benzofurans using a Cu-functionalized MIL-101(Cr) as a reusable heterogeneous catalyst. Sci Rep 13, 12908 (2023). https://doi.org/10.1038/s41598-023-40154-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40154-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.