Abstract
We sought to investigate changes in choroidal hemodynamics in preeclampsia according to presence of retinal change by quantitatively assessing choroidal vessels using optical coherence tomography (OCT). This retrospective study included 106 eyes (of 53 patients) with preeclampsia, including 70 eyes without retinal change in patients with preeclampsia (Group A), 22 eyes with retinal change in patients with preeclampsia (Group B), and 14 eyes of normal pregnant women (controls). Subfoveal choroidal thickness (SFCT) was measured on OCT B-scan images, and choroidal vessel densities (CVDs) were calculated using binarized OCT B-scan and en face images. Their values were then correlated with clinical parameters associated with preeclampsia. SFCT was greater in Group B compared to Group A or controls (354.32 ± 65.13 vs. 288 ± 55.68 or 277.21 ± 50.08, both P < 0.001). CVD on B-scan images was greater in Group B compared to Group A or controls (76.4 ± 4.9 vs. 73.7 ± 5.3 or 71.5 ± 5.1; both P ≤ 0.046). CVD on en face images was also greater in Group B compared to Group A or controls (64.7 ± 0.8 vs. 63.6 ± 1.5 or 63.3 ± 1.3; both P ≤ 0.001). SFCT and CVD positively correlated with each other (P ≤ 0.009) and were greater in patients with blurred vision and vaginal bleeding (P ≤ 0.020 for blurred vision and P ≤ 0.024 for vaginal bleeding). SFCT and CVDs were higher in preeclampsia patients with retinal change compared to those without retinal change or controls. Both SFCT and CVD showed association with blurred vision and vaginal bleeding.
Similar content being viewed by others
Introduction
Preeclampsia is a pregnancy-specific multisystemic disorder that causes changes in systemic vasculature and hemodynamics, leading to hepatic failure, proteinuria, neurologic symptom, and pulmonary edema1. Approximately 30%–40% of patients with preeclampsia have subjective visual disturbance, and various associated ocular findings are observed2,3.
Retinal vascular abnormalities can be found frequently in preeclampsia pregnancies4,5. Some studies have reported that choroidal ischemia and retinal circulation disorders are present in 30%–100% of preeclampsia pregnancies6. Serous retinal detachment (SRD) is a common cause of visual disturbances in preeclampsia. Alterations in choroidal circulation triggered by elevated blood pressure may cause choroidal thickening and subretinal fluid accumulation in preeclampsia and malignant hypertension, causing SRD7.
This study aimed to investigate changes in choroidal hemodynamics in preeclampsia patients with or without retinal change by assessing choroidal vascularity using optical coherence tomography (OCT) B-scan and en face images. We then analyzed correlations between choroidal and clinical parameters.
Results
Patient demographics and clinical characteristics
In total, 70 eyes (of 35 patients) without retinal change (Group A) and 22 eyes (of 11 patients) with retinal change (Group B) were enrolled. Additionally, the control group consisted of 14 eyes of 7 women with normal pregnancies.
All patients were of Asian descent, and the mean age was 34.62 ± 4.73 (range, 18–46) years. There was no difference in the mean age among groups (P = 0.762). The mean logMAR BCVA was worse in Group A compared to the other groups (P ≤ 0.004). Systolic and diastolic BPs as well as the urine protein level were highest in Group B, followed by Group A and then the control group (P < 0.001, P < 0.001, and P ≤ 0.007). The demographics and clinical characteristics of each group and their comparisons are summarized in Table 1.
In Group B, all eyes showed retinal arteriolar narrowing or tortuosity, 8 eyes (33.3%) showed retinal hemorrhages, 14 eyes (58.3%) showed retinal ischemia visualized as cotton wool spots, and 10 eyes (41.7%) showed serous retinal detachments/exudates.
Choroidal parameters
There were significant differences in CTs and CVDs among groups (all P ≤ 0.005). The mean subfoveal, nasal, and temporal CTs were higher in Group B compared to the other groups (all P ≤ 0.004). Meanwhile, there was no significant difference in CT values between Group A and the control group (all P ≥ 0.438). The mean CVD on OCT B-scan images was higher in Group B compared to the other groups (all P ≤ 0.005); moreover, the mean CVD on en face OCT images was also higher in Group B (all P ≤ 0.001). Conversely, OCT B-scan and en face images did not reveal a significant difference in mean CVD between Group A and the control group (all P ≥ 0.270). The choroidal parameters of each group and their comparisons are summarized in Table 2.
When the choroidal parameters were compared according to the presence of retinal change types, eyes with serous retinal detachments/exudate showed significantly thicker subfoveal CT compared to eyes without (P = 0.002). Presence of hemorrhage nor ischemia did not affect either CT or CVD (all P ≥ 0.070; Table 3).
Correlations among choroidal parameters
Overall, foveal, nasal, and temporal CTs showed strong positive correlations with each other (all P < 0.001). The CVDs on OCT B-scan images showed positive correlations with the foveal, nasal, and temporal CTs and the CVD on en face images (all P ≤ 0.007). Meanwhile, the CVD on en face OCT images showed positive correlations with foveal and temporal CTs and the CVD on B-scan images (all P ≤ 0.044). Correlations among choroidal parameters are summarized in Table 4 and Fig. 1.
Correlations between choroidal thickness (CT) and choroidal vessel density (CVD). (a) A plot of foveal, nasal, and temporal CTs and CVDs on optical coherence tomography B-scan images shows positive associations. (b) A plot of foveal and temporal CTs and CVDs on en face optical coherence tomography images shows positive correlations.
Correlations among choroidal parameters and clinical parameters
The logMAR BCVA positively correlated with foveal and temporal CTs and the CVD on en face OCT images (all P ≤ 0.037). Systolic BP was positively correlated with foveal and temporal CTs and CVDs on OCT B-scan and en face images (all P ≤ 0.024). Blurred vision was associated with foveal, nasal, and temporal CTs and CVDs on OCT B-scan and en face images (all P ≤ 0.020). Vaginal bleeding was associated with foveal and nasal CTs and CVDs on OCT B-scan and en face images (all P ≤ 0.030). Choroidal parameters correlating with blurred vision and vaginal bleeding are shown in Fig. 2. Correlations among choroidal and clinical parameters are summarized in Table 5.
Subfoveal choroidal thickness (CT) and choroidal vessel density (CVD) from optical coherence tomography B-scan and en face images in relation to blurred vision and vaginal bleeding. (A) CT and CVD values were higher in subjects with blurred vision. (B) CT and CVD values were higher in subjects with vaginal bleeding.
Discussion
Changes in the choroid with preeclamptic pregnancies have been studied previously8,9,10,11. However, most prior studies compared CT values on OCT images, and their results seem controversial. In this study, we analyzed CT values in preeclampsia patients according to the presence of retinal changes and compared patients with one another and with women with normal pregnancies to see whether retinal change is associated with choroidal change. Additionally, the CVD, along with the CT, was measured vertically and horizontally on OCT images to obtain more detailed information about choroidal hemodynamic change in these patients.
Diastolic and systolic BPs as well as the urine protein level were higher in preeclampsia patients with retinal changes compared to those without retinal changes. The BP and urine protein level indicate the severity of preeclampsia; in other words, more severe preeclampsia can be associated with changes in these values. Our results are consistent with those of Kaliaperuma et al., who reported that diastolic BP positively correlated with retinopathy in severe preeclampsia12. In addition, Garg et al. documented subclinical retinal and choroidal thickening in the setting of severe preeclampsia8.
Foveal, nasal, and temporal CTs were higher in preeclampsia patients with retinal change compared to the other groups. And there was no difference in CTs between preeclampsia patients without retinal change and women in the control group. This suggests that the presence of retinal changes may have affected SFCT results among preeclampsia patients. The discrepancies between previous studies may be explained by differences in disease severity or the presence of retinal changes among participants. It was not possible to analyze the severity of preeclampsia or the presence of retinal changes in previous studies since most did not provide these data. Only Garg et al. reported that differences in CT values between patients with and without visual changes trended toward significance, which was similar to our result8.
CVDs on OCT B-scan and en face images were also higher in preeclampsia patients with retinal change compared to the other groups, and they did also differ between preeclampsia patients without retinal change and the control group. CVD values also showed a positive correlation with CT values. From this, it can be inferred that increased vascular area in the choroid might be a major factor for choroidal thickening during pregnancy. Previously, Azuma et al. reported that a larger choroidal luminal area was associated with a higher systolic BP in pregnancy13. Choroidal overperfusion and increased vascular resistance in a pregnancy-induced hypertension patients with SRD had been demonstrated using laser speckle flowgraphy14. Also, SRD was associated with thicker CT in the current study. Regarding a significant positive correlation among ocular blood pressure, choroidal blood flow, and CT, an increase in choroidal vessel luminal area might be caused by the increased volume of circulating blood during pregnancy15. In addition, en face OCT images revealed a broader macular area compared to OCT B-scan images, suggesting that an increased diameter of Haller vessels is responsible for the choroidal thickening.
Among the clinical parameters associated with preeclampsia, BCVA, systolic BP, and blurred vision and vaginal bleeding correlated with both CTs and CVDs. Again, the correlation between visual disturbances and choroidal parameters is consistent with the previous results of Garg et al.8 A high systolic BP suggests a more severe state of preeclampsia; therefore, the severity of the disease seems to be associated with choroidal parameters. Meanwhile, there is a study showing an inverse correlation between vaginal bleeding and preeclampsia earlier in pregnancy and a positive correlation with post-partum bleeding, but there are limited data on the association between vaginal bleeding and preeclampsia severity during late pregnancy16, necessitating further investigation.
There are several limitations to this study. First, our sample size was relatively small and only consisted of Asian patients. Also, it has been suggested that CT changes can differ according to the pregnancy trimester17. Since this study included pregnant women in their third trimester only, we could not analyze differences in choroidal parameters according to gestation week. Nonetheless, this study evaluated choroidal changes in preeclampsia according to retinal change, which we do not believe any previous study has clearly demonstrated. In addition, not only CT values, but also CVD values on both OCT B-scan and en face images were analyzed, proving correlations between CT and CVD values and showing broader areas of choroidal change.
In conclusion, CTs and CVDs were higher in preeclampsia patients with retinal change compared to those without retinal changes or controls. CTs and CVDs correlated with each other and showed an association with blurred vision and vaginal bleeding. We believe that the results of this study have value in explaining discrepancies between previous studies.
Methods
Study population
This study was conducted in the Ophthalmology Department at Buchen St. Mary Hospital of The Catholic University of Korea in Gyeonggi-do, Republic of Korea (HC22RASI0091). In this retrospective chart review study, consecutive patients seen between June 2017 and July 2021 who were diagnosed with preeclampsia, hospitalized during their pregnancy, and referred to the ophthalmology department during gestation weeks 32–36 were enrolled. Women with normal pregnancies were age- and gestational age–matched and enrolled as a control group.
Diagnosis of preeclampsia followed the criteria agreed upon by the National High Blood Pressure Education Program Working Group of the U.S. National Institutes of Health, which were blood pressure (BP) ≥ 140/90 mmHg after 20 weeks of gestation on ≥ 2 occasions 6 h apart except for gestational trophoblastic disease or multiple pregnancies, with proteinuria > 0.3 g per 24 h in previously normotensive patients18. Preeclampsia patients were divided into two groups according to presence of retinal changes. Retinal changes included focal or generalized arteriolar narrowing, scattered intraretinal hemorrhages and/or ischemia, arterial and venous occlusion, serous retinal detachments, and exudations observed during fundus OCT examination.
Medical records, including systolic and diastolic BPs and symptoms associated with preeclampsia (e.g., headache, blurred vision, dizziness, vaginal bleeding, dyspnea, edema) were reviewed. Laboratory test values for urine protein, serum hemoglobin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), D-dimer, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were also recorded. Finally, collected ophthalmologic examination data included best-corrected visual acuity (BCVA) and OCT images (Cirrus 4000 or Cirrus 6000; Carl Zeiss Meditec, Jena, Germany).
Image acquisition and analysis
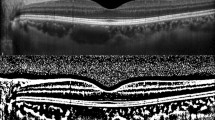
Choroidal thickness (CT) was measured on HD line scan OCT B-scan imager that crossed the fovea. CT was measured at three points, i.e., the center and nasally and temporally 1500 µm from the center. CT was defined using the vertical distance between Bruch’s membrane and the chorio-scleral junction. Figure. 3A Choroidal vessel densities (CVDs) were measured on OCT B-scan images (Fig. 3B) and en face image slabs of Haller’s layer (Fig. 3C). A Haller vessel slab was obtained by moving the reference line to a point that was 50% of the total CT. To measure the CVD, binarization of the OCT image was completed using a modified Niblack method with Fiji (available at fiji.sc, free of charge)19. The CVD was calculated by dividing the number of pixels in the vascular area (dark pixels) by that of the total choroidal area on the B-scan image and that of the total area on the en face image, respectively (Fig. 1C). CTs and CVDs were compared between groups and correlated with other clinical parameters.
Analysis of optical coherence tomography (OCT) images. (A) Choroidal thickness (CT) was measured at three points, i.e., the center and 1500 µm nasally and temporally from the center on HD line scan OCT B-scan images that crossed the fovea. (B) Choroidal vascularity (CV) was calculated by dividing the number of pixels in the vascular area (dark pixels) within the choroid by that of the total choroidal area on the OCT B-scan image after binarization. (C) A Haller vessel slab was obtained at 50% of the total CT. CV was calculated by dividing the number of pixels in the vascular area (dark pixels) by that of the total area after binarization.
Statistical analysis
Statistical analysis was performed using SPSS for Windows (version 23.0.1; IBM Corporation, Armonk, NY, USA). Mann–Whitney U and Kruskal–Wallis tests were used to compare continuous variables among and between groups. Fisher’s least significant difference was used as the post-hoc test after analysis of variance. Categorical variables between groups were compared using the chi-square test, and standardized adjustment was completed as the post-hoc test after the chi-square test. Correlations between variables were accessed using Spearman’s correlation coefficient. P < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was conducted in accordance with the tenets of the Declaration of Helsinki. The study was approved by the Institutional Review Board of Bucheon St. Mary’s Hospital, which waived the need for written informed consent because of the study’s retrospective design (HC22RASI0091).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 122, 1122–1131. https://doi.org/10.1097/01.Aog.0000437382.03963.88 (2013).
Dinn, R. B., Harris, A. & Marcus, P. S. Ocular changes in pregnancy. Obstet. Gynecol. Surv. 58, 137–144. https://doi.org/10.1097/01.Ogx.0000047741.79433.52 (2003).
Royburt, M., Seidman, D. S., Serr, D. M. & Mashiach, S. Neurologic involvement in hypertensive disease of pregnancy. Obstet. Gynecol. Surv. 46, 656–664. https://doi.org/10.1097/00006254-199110000-00002 (1991).
Lupton, S. J. et al. Changes in retinal microvascular caliber precede the clinical onset of preeclampsia. Hypertension 62, 899–904. https://doi.org/10.1161/hypertensionaha.113.01890 (2013).
Capoor, S., Goble, R. R., Wheatley, T. & Casswell, A. G. White-centered retinal hemorrhages as an early sign of preeclampsia. Am. J. Ophthalmol. 119, 804–806. https://doi.org/10.1016/s0002-9394(14)72793-x (1995).
Sheth, B. P. & Mieler, W. F. Ocular complications of pregnancy. Curr. Opin. Ophthalmol. 12, 455–463. https://doi.org/10.1097/00055735-200112000-00011 (2001).
Lee, C. S., Choi, E. Y., Lee, M., Kim, H. & Chung, H. Serous retinal detachment in preeclampsia and malignant hypertension. Eye Lond. 33, 1707–1714. https://doi.org/10.1038/s41433-019-0461-8 (2019).
Garg, A. et al. Choroidal and retinal thickening in severe preeclampsia. Invest. Ophthalmol. Vis. Sci. 55, 5723–5729. https://doi.org/10.1167/iovs.14-14143 (2014).
Kim, J. W., Park, M. H., Kim, Y. J. & Kim, Y. T. Comparison of subfoveal choroidal thickness in healthy pregnancy and pre-eclampsia. Eye Lond. 30, 349–354. https://doi.org/10.1038/eye.2015.215 (2016).
Ataş, M. et al. Evaluation of the macula, retinal nerve fiber layer and choroid in preeclampsia, healthy pregnant and healthy non-pregnant women using spectral-domain optical coherence tomography. Hypertens. Pregnancy 33, 299–310. https://doi.org/10.3109/10641955.2013.877924 (2014).
Sayin, N. et al. Subfoveal choroidal thickness in preeclampsia: Comparison with normal pregnant and nonpregnant women. Semin. Ophthalmol. 29, 11–17. https://doi.org/10.3109/08820538.2013.839813 (2014).
Kaliaperumal, S., Setia, S., Gupta, A. & Rao, V. Fetal birthweight and diastolic blood pressure: Association with retinopathy in severe preeclampsia. Eur. J. Ophthalmol. 18, 809–812 (2008).
Azuma, K. et al. Assessment of the choroidal structure in pregnant women in the first trimester. Sci. Rep. 11, 4629. https://doi.org/10.1038/s41598-021-84204-x (2021).
Yata, K., Hashimoto, R., Masahara, H., Oyamada, M. & Maeno, T. Changes in choroidal circulation and pulse waveform in a case of pregnancy-induced hypertension with serous retinal detachment. Am. J. Ophthalmol. Case Rep. 20, 100911. https://doi.org/10.1016/j.ajoc.2020.100911 (2020).
Akahori, T., Iwase, T., Yamamoto, K., Ra, E. & Terasaki, H. Changes in choroidal blood flow and morphology in response to increase in intraocular pressure. Invest. Ophthalmol. Vis. Sci. 58, 5076–5085. https://doi.org/10.1167/iovs.17-21745 (2017).
Eskild, A. & Vatten, L. J. Abnormal bleeding associated with preeclampsia: A population study of 315,085 pregnancies. Acta Obstet. Gynecol. Scand. 88, 154–158. https://doi.org/10.1080/00016340802613242 (2009).
Temel, E., Kocamış, Ö., Aşıkgarip, N., Örnek, K. & Arıöz, O. Evaluation of choroidal thickness and choroidal vascularity index during pregnancy. Can. J. Ophthalmol. 56, 237–243. https://doi.org/10.1016/j.jcjo.2020.12.002 (2021).
Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 183, S1-s22 (2000).
Sonoda, S. et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest. Ophthalmol. Vis. Sci. 55, 3893–3899. https://doi.org/10.1167/iovs.14-14447 (2014).
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (no. NRF001663041G0003101).
Author information
Authors and Affiliations
Contributions
I.K.K and J.B. wrote the main manuscript text and prepared figures. J.E.S, M.J.K., and H.R. provided materials. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, I.K., Shin, J.E., Kim, M.J. et al. Quantitative analysis of choroidal morphology in preeclampsia during pregnancy according to retinal change. Sci Rep 13, 13171 (2023). https://doi.org/10.1038/s41598-023-40144-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40144-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.