Abstract
Slow waves are an electrophysiological characteristic of non-rapid eye movement sleep and a marker of the restorative function of sleep. In certain pathological conditions, such as different types of epilepsy, slow-wave sleep is affected by epileptiform discharges forming so-called “spike-waves”. Previous evidence shows that the overnight change in slope of slow waves during sleep is impaired under these conditions. However, these past studies were performed in a small number of patients, considering only short segments of the recording night. Here, we screened a clinical data set of 39′179 pediatric EEG recordings acquired in the past 25 years (1994–2019) at the University Children’s Hospital Zurich and identified 413 recordings of interest. We applied an automated approach based on machine learning to investigate the relationship between sleep and epileptic spikes in this large-scale data set. Our findings show that the overnight change in the slope of slow waves was correlated with the spike-wave index, indicating that the impairment of the net reduction in synaptic strength during sleep is spike dependent.
Similar content being viewed by others
Introduction
Sleep plays a vital role in many physiological functions, such as memory consolidation1, synaptic plasticity2,3,4, and brain metabolic waste clearance5,6. These functions are presumably underlying the fact that sleep is closely related to behavioral performance: insufficient sleep restoration during the night will lead to behavioral deficits during the following day7. Importantly, only sleep can revert these performance deficits. Non-rapid eye-movement (NREM) sleep including deep sleep is essential for the restorative functions of sleep8,9,10,11. Although the mechanisms underlying the restorative function of sleep are still not fully understood, according to the synaptic homeostasis hypothesis (SHY), sleep plays a role in synaptic plasticity: Synaptic strength needs to be reduced during sleep to renormalize the experience-dependent increases that occur during wakefulness12. Human, animal and modelling data shows that the slope of slow-waves is the most direct EEG measure for synaptic strength13,14,15. As expected based on the predictions of SHY, numerous studies have shown an overnight decrease in the slope of slow waves15,16,17, which is reflective of an overnight reduction in synaptic strength. In childhood epilepsies with a spike-wave activation during sleep, epileptic spikes invade NREM sleep. For decades it has been postulated that these spikes are the origin of cognitive and behavioral deficits these patients suffer from18,19,20. These impairments can be as dramatic as loss of language abilities or a global developmental regression20,21. Recent studies provide compelling evidence that the overnight decrease in slope is critically dampened in childhood epilepsies with spike-wave activation during sleep22,23,24,25. Interestingly, these same patient group shows significant deficits in sleep-dependent memory consolidation26. It is tempting to assume that impaired consolidation of newly learned information might lead with time to the patient’s behavioral and cognitive deficits. One of the key limiting factor for this research topic is the fact that so far sleep and epilepsy in pediatric patients has been investigated in only small-size datasets22,23,24,25. Investigating the effect of spikes on sleep in large-scale datasets is only feasible by implementing an automated approach. Furthermore, if cognitive impairment in epilepsy can be linked to both compromised overnight decrease in slow wave slope and spikes in sleep, other disorders may also benefit from this approach. This automated pipeline can be implemented to investigate sleep- and/or spike-related deficits in disorders with increased incidences for epileptic spikes, such as Alzheimer’s disease (memory deficit), or attention-deficit hyperactivity disorder (ADHD, attention deficit)27,28. The identification of specific sleep- and/or spike-related deficits in these disorders can help to disentangle their underlying pathomechanisms. Ultimately, an automated pipeline for sleep and spike detection may be incorporated into clinical diagnostics providing new and easily accessible information to clinicians. The implementation of this novel tool can support developing and establishing new and emerging treatment options, such as the increasingly popular acoustic stimulation during sleep that has shown promising results in patients with epilepsy29.
In this study, we aimed to investigate the relationship between sleep and epilepsy in a large-scale pediatric dataset acquired in the past 25 years at the University Children’s Hospital Zurich. In order to achieve that we developed a fully automated analysis pipeline for spike detection and the assessment of changes in slow wave slope as a marker for the overnight reduction in synaptic strength (SOMNIDEX; Synaptic renOrMalizatioN InDEX).
Methods
In a first step, the automated methods for sleep and epileptic spike detection were developed based on a small-scale dataset, carefully scored by clinical neurophysiology experts. In a next step, these automated methods were applied to a large-scale dataset, not scored by experts. This was a retrospective study, approved by Zurich Cantonal Ethics Committee BASEC-Nr. 2019–00,165. Informed consent was present for all subjects from their legal guardians. All methods were carried out in accordance with relevant Swiss guidelines and regulations.
Small-scale dataset
A dataset pooled from several previous studies22,23,24,30 included 39 pediatric patients with epilepsies with a spike-wave activation during sleep was used for the development and testing of the algorithms for automatic sleep and spike detection. Sleep was scored by an expert in 20-s epochs during the entire recording night according to AASM criteria31. Spikes were marked by an expert (SS) in a 10-min EEG segment for each patient, starting after first sleep stage 2. Further, in order to estimate inter-rater variability, 1-min EEG segments were marked by 2 experts (SS and BS) in 20 recordings. Automated methods for spike and sleep detection were developed in this dataset and then applied to the large-scale dataset.
Large-scale dataset
A data set containing 39′179 EEG recordings performed at the University Children’s Hospital Zurich in 1994–2019 was available for analysis (Fig. 1, available recordings). These EEG recordings did not contain systematic annotations of sleep or spikes. In the first step, we considered recordings with duration ≥ 4 h (candidate recordings), since these were more likely to contain sleep episodes. Of 1′553 candidate recordings, 695 recordings included sleep recordings of ≥ 4 h. Other than sleep duration, no other exclusion criteria were applied. Random exclusion of repeat recordings from the same patient, resulted in 413 recordings (each from a single patient) included for slope of slow waves analysis. Exclusion of patients with age and diagnosis which were not electronically accessible in an automated fashion resulted in 357 patients included for age analysis and 296 for diagnosis analysis.
EEG recordings and data pre-processing
EEG electrodes were placed according to the international 10–20 system. Recordings were performed at a 128 or 256 sampling rate by the Deltamed® (Paris, France) EEG system. Data were exported to the European Data Format (EDF) and further processed in MATLAB R2020a (The Math Works Inc., Natick, MA). The following filters were applied: a bandpass 0.3–40 Hz, and a notch filter at 50 Hz. EEG data were re-referenced to the contralateral mastoids, and downsampled to 128 Hz, where applicable.
Classifiers
We developed two classifiers: (1) an automatic epileptic spike detector, and (2) an automatic sleep detector. For both classifiers we applied a long short-term memory (LSTM) recurrent neural network. We used recurrent neural networks as they are taking the temporal structure into account and therefore have a good performance for time series data32. The small-scale dataset, used for classifier development, was divided into a training (27 patients), validation (6 patients), and testing dataset (6 patients). Models were optimized by manual tuning of (1) the number of neurons in the hidden layer, (2) the number of hidden layers, and (3) the inclusion and probability of dropout layers. The best performing model was then used for the test set applied to the large-scale dataset.
Epileptic spike detector
The epileptic spike detector was developed based on expert scoring and raw data. The structure of our LSTM was as follows: an input layer (1 neuron), two LSTM layers (128 neurons each) each followed by a dropout layer (dropout probability 0.1), followed by a fully connected layer (2 neurons), a softmax layer, and a classification output layer. Six training epochs were applied, i.e., the entire training data were passed through the neural network six times. The adaptive moment estimation optimization algorithm (Adam) was used to update network weights during training33. The input of the LSTM consisted of a moving time window of 1/16 s (16 samples; step 125 ms).
Sleep detector
Since overnight change in slope of slow waves depends on NREM sleep stages 2 and 38,9,10,11, we limited our automated scoring to the detection of NREM sleep stages 2 and 3. Hence, we identified only two classes: NREM sleep stages 2 or 3, and all other stages (REM sleep, wake and NREM sleep stage 1). The classifier was developed based on the expert sleep scoring and engineered features. We used feature engineering to extract quantifiable properties of the sleep EEG, calculated from power spectral analysis of three EEG channels: F3-M2, C3-M2, and O1-M2 as: power in alpha (8–12 Hz), sigma (i.e., spindle range; 12–16 Hz), delta (i.e. slow wave activity, SWA, 0.75–4.5 Hz), and artifact range (30–40 Hz). Spectral analysis of EEG channels was performed on consecutive 20 s epochs (FFT, Tukey window [r = 0.5], average of five 4-s epochs; matched with sleep stages), resulting in a 0.25 Hz frequency resolution. As epileptic spikes may distort the power spectra, spectral analysis was performed after removing epileptic spikes from the data, as detected by our spike detector. The structure of the LSTM classifier was as follows: an input layer (12 neurons), two LSTM layers (64 neurons each), each followed by a dropout layer (dropout probability 0.1), followed by a fully connected layer (two neurons), a softmax layer, and a classification output layer. Twenty training epochs were applied. The input of the LSTM consisted of a moving time window of 11 sleep epochs (220 s; step: one sleep epoch or 20 s).
Assessment of classifier performance
We assessed the performance of the classifiers by determining specificity, sensitivity, precision, accuracy, and the Cohen’s kappa coefficient34,35,36,37. We focused on Cohen’s kappa coefficient, as it is a robust measure, accounting for the possibility of the agreement occurring by chance36. We interpreted the performance results for Cohen’s kappa using Landis and Koch levels [60]: < 0.00—poor; 0.00–0.20—slight; 0.21–0.40—fair; 0.41–0.60—moderate; 0.61–0.80—substantial; 0.81–1.00—almost perfect identification38. Overall performance measures across all patients (pooled data) and mean values across patients were reported.
Assessment of inter-scorer variability for spike detection
Out of 39 patient recordings, 20 were scored independently by 2 different experts. These records were randomly selected. One minute of EEG was scored for epileptic spikes. Performance measures were calculated in the same way as for the classification algorithms.
Estimation of SOMNIDEX
SOMNIDEX was estimated by calculating overnight change in the slope of slow waves during NREM sleep. Numerous studies have shown that the slope of slow waves best reflects sleep-dependent changes in neuronal network activity, presumably underlying the reduction in synaptic strength during sleep13,14,15,39.
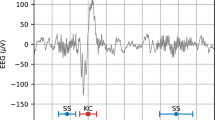
Slow-wave detection was performed in line with Riedner et al.15 First, the signal was band-pass filtered (0.5–4.0 Hz, stopband 0.1 and 10 Hz, Chebyshev Type II filter). Negative deflections between two zero-crossings were identified as slow-waves if they were separated by 0.25–1.0 s. The ascending slope of slow waves was determined by calculating the amplitude divided by the time from the most negative peak to the second zero-crossing (Figure 3A). As it is unknown if slow waves directly associated with epileptic spikes, so called spike-wave complexes, contribute to sleep homeostasis, all slow waves occurring in a window of 0.5 s after the epileptic spike were excluded from further analyses, in line with previous studies22,23,24,30. The slope of slow waves was calculated in the first hour (FH) and last hour (LH) of sleep. Instead of taking into account a fixed time period, in order to achieve a fair comparison across patients and between first and last hour of sleep, fixed amounts of NREM sleep stages 2 and 3 epochs was taken into account for this calculation, i.e. the first and last 180 20-s epochs. To account for differences in slopes due to the overnight difference in amplitude, slow waves were matched by amplitude in the first and last hour of sleep, as proposed by Jaramillo et al.17 In case there were less than 250 matched waves remaining after the amplitude matching procedure, this patient was excluded from the analysis (13 patients). The overnight change in the slope of slow waves reflecting SOMNIDEX was calculated as (LH-FH)/FH. Therefore, a negative overnight change indicates the expected slope decline across sleep, while no negative overnight change or a positive change indicates a pathological condition.
All analyses were performed on one EEG channel which was automatically selected as the focus of the epilepsy. The focus channel was determined as the channel with the highest spike wave index (SWI; number of spikes per 10-s time interval).
Variables are described as mean and standard deviation of the mean, and median with interquartile range.
Correlation analysis
Correlations were performed using Spearman’s correlation coefficient.
Results
We created an automated pipeline for data processing, where all calculations, including pre-processing, spike detection, sleep detection, and SOMNIDEX calculation, were performed in a computerized manner, without any user dependencies.
Spike and sleep classifiers performance
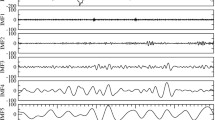
Two classifiers were developed: an automatic detector of epileptic spikes, and an automatic detector of sleep (NREM sleep stages 2 and 3). As exemplified in the EEG trace including epileptic activity (Fig. 2A) there was a good overlap between expert and automated spike detection. The time–frequency plot of an exemplary night (Fig. 2B) shows a similarly good overlap between the expert and automated NREM sleep stages 2 and 3 classification. When quantifying these overlaps (Supplementary Table), both the automatic detection of epileptic spikes and the automatic detection of sleep showed good performance, with a Cohen’s kappa coefficient of 0.72 and 0.80, and an accuracy of 92.3% and 90.3% respectively. On a validation dataset, Cohen’s kappa coefficient was 0.73 for spike detection and 0.71 for sleep detection, while accuracy was 93.7% and 86.4%. Cohen’s kappa of inter-scorer reliability calculated in 1-min EEG segments of 20 patients was 0.71 for the spike detection, indicating similar agreement between two experts as between one expert and the algorithm.
Spike detection (top, ten-second EEG trace with epileptic activity) and sleep detection (bottom, spectrogram of a 10-h night EEG recording with frequency at the y-axis and time at x-axis, and warmer colors representing higher power values) of a patient with spike-wave epilepsy. Expert scoring is depicted in blue and automatic detection (LSTM) in red, below the respective graph.
Slope of slow waves decreases across age in children
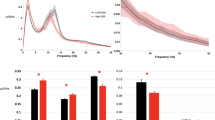
The slope of slow waves was calculated for the first and last hour of NREM sleep, as illustrated in Fig. 3. As an indirect validation of our methods, we investigated whether we could replicate previous findings in healthy children17. We assessed the correlation between the age of our patients (6.94 ± 5.11; 6.61[1.81–11.13] years) and the slow wave slopes in the first (446.08 ± 193.11; 414.78[301.53–546.54] µV/s), the last hour of NREM sleep (429.61 ± 203.46; 393.62[281.69–518.80] µV/s), and the overnight change in slope (− 3.72 ± 14.95; − 5.84[− 10.88–1.07] %). The slopes both in the first and last hour of sleep were negatively correlated with age (R = − 0.39, p = 3.3 × 10−14 and R = − 0.37, p = 4.5 × 10−13 respectively), and the overnight change in slope did not show a correlation with age (R = − 0.05, p = 0.34).
Slope of slow waves. (A) 10-s EEG trace with epileptic spikes in shaded gray, and slow waves in dark red, as identified by our automated methods. Note that slow waves occurring 0.5 s after the spike were not considered for analysis. Also, only slow waves that were matched in the amplitude matching procedure between the first and last hour of NREM sleep were further analyzed. The slope was calculated as the ascending slope of slow waves from the negative peak to the zero crossing. (B) Correlation between age and slope of slow waves in the first hour (FH; R = − 0.39, p < 0.001), in the last hour of sleep (LH; R = − 0.37, p < 0.001), and overnight change in slope (not significant). N = 357.
SOMNIDEX is impaired in children with epilepsy
Finally, we investigated sleep aspects in our patient cohort (Fig. 4) by assessing SOMNIDEX: changes in the slope of slow waves from the first (mean ± standard deviation: 441.96 ± 190.92 µV/s; median and interquartile range: 413.01 [297.85–538.01] µV/s) to the last hour of NREM sleep (424.29 ± 199.53; 391.87 [281.47–510.38] µV/s). In the healthy population a decline in slope from the first to the last hour is expected17,22. We found no significant change of the slope of slow waves (− 4.04 ± 14.16; − 5.86 [− 11.29–0.78]%) in our cohort (Wilcoxon rank sum test, U = 176,791, z = 1.75, p = 0.08, CI = [− 0.06, 0.22], Cohen’s d = 0.09), indicating impaired synaptic renormalization during sleep. Specifically, 54% of children showed the expected overnight decrease in the slope of slow waves, but one-third (33%) of children showed no change, and 13% of children showed an overnight increase (Figs. 4A and 4B). The patients showing an overnight increase in the slope may represent a clinically relevant population, as their overnight change in slope is severely impaired.
(A) Slope of slow waves in the first and last hour of NREM sleep (N = 413). Black line represents the average value. There was no significant difference between the slope in the first and in the last hour of sleep (two-sided Wilcoxon rank sum test, p > 0.05). (B) Overnight change in slope (higher values represent higher impairment in synaptic renormalization during sleep, i.e., positive values represent overnight increase in slope). (C) Spike wave index, a clinical marker of severity of epilepsy. (D) Correlation between the overnight change in slope and spike wave index (R = 0.17, p < 0.001). (E) Correlation between overnight change in slope and spike wave index for Lennox Gastaut patients (N = 13).
Further, we calculated the spike wave index (SWI; 17.60 ± 20.34; 9.43[2.26–26.82]%), a clinically relevant marker of the severity of epilepsy (Fig. 4C). Similar to our observation regarding overnight slope changes, 52% of children showed low rates of spike activity, while 42% showed high, and 6% very high rates of spike activity. Interestingly, the change in the slope of slow waves was positively correlated with the SWI (R = 0.17, p < 0.001), i.e., the more spikes, the stronger the impairment in synaptic renormalization during sleep. Finally, we identified Lennox Gastaut patients as the patient group showing the highest correlation between these two parameters (R = 0.65, p = 0.018).
Discussion
Both classifiers showed good performance, indicating that reliable computerized detection of epileptic spikes and sleep is feasible. Similar performance in the validation and testing datasets indicate that there was no overfitting of the training model. Importantly, the spike detection model achieved the same performance as the inter-rater variability, indicating that our spike detector achieved expert level of performance. Inter-scorer agreement for sleep scoring has been previously reported in the literature, and it was not assessed in the scope of the current study. Our classification performance is in the range of previously reported classifiers in healthy volunteers and patients40,41, and in line with the observed inter-rater agreement for the AASM standard (0.76 as overall Cohen's kappa coefficient)42,43.
The entire data analysis pipeline in this study was performed in an automated manner. We analyzed approximately 50 years of EEG data (1553 candidate recordings, 19 EEG derivations), which would take approximately 90 years for an expert to annotate. To the best of our knowledge, this is the first study to investigate the relationship between sleep and epilepsy with automated methods in such a large-scale pediatric dataset. The fully automated approach has the obvious advantage of not requiring intermediate checking steps, manual tuning of parameters, or similar. This automation facilitates fast and efficient data analysis and enables the otherwise unfeasible processing of large-scale datasets. Moreover, as spike detection algorithm derives spike counts for each individual EEG channel, this approach may also permit the topographical analysis of epileptic spikes, i.e., the comparison of the SWI within various epileptic foci against the rest of the brain. This topographical mapping may also allow for an automated detection of the epileptic focus. Further, this automatic approach together with our SOMNIDEX findings are of importance for the clinical diagnostics as the entire night recording can be considered, and there may be some variability across a sleep episode. Finally, our approach allows an assessment of sleep aspects, which are currently not considered in clinical practice, and may provide important additional information about the pathophysiology of the disorder. Of course, the applicability to medical diagnostic still needs to be proven with the potential benefit of faster and more standardized diagnostics. Furthermore, automated methods in conjunction with mobile EEG devices open doors for hospital-at-home applications.
We performed several analyses to support the validity and significance of our approach:
First, we found a negative correlation between slow wave slopes and age, in line with previous studies in healthy children and adults aged 8–26 years17, but found no correlation between age and overnight change in slow wave slope, in contrast to these studies17,44. The lack of correlation between overnight changes in slow wave slope and age in our study may be attributed to the impaired overnight change in slope we observed in our cohort, since higher variability in impairment may have masked the effect of age in our dataset. Several hypotheses about the function of sleep associate sleep restoration to processes critical for learning and memory, i.e., memory consolidation1, synaptic plasticity2,3,4, and the brain metabolic waste clearance5,6. In the scope of this paper, an overnight change in the slope of slow waves was calculated. The slope of slow waves is hypothesized to represent an electrophysiological marker of synaptic strength, and an overnight decline in slope indicates synaptic down-selection, a critical process for sleep dependent learning and memory consolidation processes45,46. Hence, impaired overnight change in slope may reflect these fundamental processes of sleep restoration. It is thus no surprise that patients suffering from severe forms of sleep-associated spike wave epilepsies show impaired memory consolidation30,44. Moreover, it is speculated that the cognitive deficits of these patients may be a consequence of impaired sleep functions20. When we applied arbitrary cutoffs to SOMNIDEX, the overnight change in the slope of slow waves, 13% of patients had severely impaired overnight change in slope, 33% of patients showed moderately impaired overnight change in slope, and 54% of patients had normal overnight change in slope. This variability in our patient group is in line with a previous study in a small-scale dataset (N = 9)22 where an increased overnight slope change (severe impairment) was observed in two patients, a reduced overnight slope decline (moderate impairment) was observed in four, and an overnight slope decrease in three patients. In contrast, healthy children exhibit on average a 15% overnight decrease in the slope of slow waves17. Although healthy populations show some variability in the overnight change in slope, none of the included study participants showed an overnight increase in the slope of slow waves17,22. Therefore, we can consider an overnight increase in slope pathological, and we propose this sleep restoration marker as an additional parameter in clinical diagnostics.
Second, we analyzed an important clinical parameter—the SWI. When applying arbitrary cutoffs to our clinical marker, 6% of patients had a very high SWI (> 60%), 43% showed a SWI over 10% (but under 60%), and half (52%) of the patients had a low SWI (< 10%). The variability in SWI is in line with previous studies30 observing SWI in the range of 9 to 98% in 14 patients with self-limited focal epilepsies of childhood45. In a retrospective review47 of 102 children with continuous spike waves during sleep, those with SWI > 50% were more likely to present with global developmental problems, while those with SWI < 50% were more likely to manifest specific forms of neurological impairment. However46, the latest position paper of the International league against epilepsy (ILAE) Task Force on Nosology and Definitions48 proposes the term developmental and epileptic encephalopathy with continuous spike waves during sleep without any specification regarding a minimum SWI but rather the causal relation between epileptic activity and developmental regression. Nevertheless, the SWI is an objective marker for the risk to develop cognitive deficits, and we propose that the SOMNIDEX linked to the restorative function of sleep might be an additional marker more tightly linked to the pathomechanisms behind these cognitive impairments.
Third, we assessed the relationship between net reduction in synaptic strength during sleep and SWI. In line with previous results24 we observed a correlation between these two markers: The higher the SWI, the higher the impairment in the SOMNIDEX during sleep. This correlation was highest for Lennox Gastaut patients. Although no studies have investigated the association between SWI and overnight slope of slow waves in Lennox Gastaut patients, it should be noted that this patient group is characterized by a strong increase of spike waves during sleep, multiple seizure types, and cognitive impairment49. In the scope of the current study, SOMNIDEX was correlated to SWI only, however, it would be important to test the relevance of SOMNIDEX by correlating it to other clinical markers in future studies.
In conclusion, investigating sleep restoration parameters in clinical populations may provide new information about the underlying mechanisms of the disease. In this study, our methodology has been applied to patients with childhood epilepsy, but the proposed methods are applicable to any disorder related to sleep or spike alterations (e.g., Alzheimer’s, Parkinson’s, autism, ADHD). A better understanding of the underlying pathology has become increasingly important in recent years, due to the rise of new technologies enabling us to manipulate sleep, such as acoustic stimulation50, which may lead to new treatment options for these disorders.
Data availability
Patient data used in the scope of this study cannot be made publicly available to protect patients ‘ privacy. Data can be accessed by investigators upon reasonable request and proof of adequate ethical approval to Prof. Reto Huber.
Code availability
The code can be accessed by investigators upon a reasonable request to the authors.
References
Rasch, B. & Born, J. About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013).
Cirelli, C. & Tononi, G. Sleep and synaptic homeostasis. Sleep 38, 161–162 (2015).
Tononi, G. & Cirelli, C. Sleep and synaptic homeostasis: A hypothesis. Brain Res. Bull. 62, 143–150 (2003).
Tononi, G. & Cirelli, C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62 (2006).
Larsen, S. M. U. et al. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol. 18, 5984 (2020).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 1979(342), 373–377 (2013).
van Dongen, H. P. A., Maislin, G., Mullington, J. M. & Dinges, D. F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26, 117–126 (2003).
Dijk, D. J., Groeger, J. A., Stanley, N. & Deacon, S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep 33, 211–223 (2010).
Fattinger, S. et al. Deep sleep maintains learning efficiency of the human brain. Nat. Commun. 8, 15405 (2017).
Rasch, B. & Born, J. About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013).
Borbély, A. A. A two process model of sleep regulation. Hum. Neurobiol. 1, 195–204 (1982).
Cirelli, C. & Tononi, G. The why and how of sleep-dependent synaptic down-selection. Semin. Cell Dev. Biol. 125, 91–100 (2022).
Esser, S. K., Hill, S. L. & Tononi, G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep 30, 1617–1630 (2007).
Vyazovskiy, V. V., Riedner, B. A., Cirelli, C. & Tononi, G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep 30, 1631–1642 (2007).
Riedner, B. A. et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep 30, 1643–1657 (2007).
Kurth, S. et al. Characteristics of sleep slow waves in children and adolescents. Sleep 33, 475–480 (2010).
Jaramillo, V. et al. Characterization of overnight slow-wave slope changes across development in an age-, amplitude-, and region-dependent manner. Sleep 43, 1–10 (2020).
Roulet Perez, E., Davidoff, V., Despland, P. A. & Deonna, T. Mental and behavioural deterioration of children with epilepsy and CSWS: Acquired epileptic frontal syndrome. Dev. Med. Child Neurol. 35, 661–674 (1993).
Holmes, G. L. & Lenck-Santini, P. P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 8, 504–515 (2006).
Tassinari, C. A. & Rubboli, G. Cognition and paroxysmal EEG activities: From a single spike to electrical status epilepticus during sleep. Epilepsia 47, 40–43 (2006).
Kramer, U. et al. Clinical spectrum and medical treatment of children with electrical status epilepticus in sleep (ESES). Epilepsia 50, 1517–1524 (2009).
Bölsterli, B. K. et al. Impaired slow wave sleep downscaling in encephalopathy with status epilepticus during sleep (ESES). Clin. Neurophysiol. 122, 1779–1787 (2011).
Bölsterli, B. K. et al. Remission of encephalopathy with status epilepticus (ESES) during sleep renormalizes regulation of slow wave sleep. Epilepsia 58, 1892–1901 (2017).
Bölsterli Heinzle, B. K. et al. Spike wave location and density disturb sleep slow waves in patients with CSWS (continuous spike waves during sleep). Epilepsia 55, 584–591 (2014).
Fattinger, S. et al. Impaired slow wave sleep downscaling in patients with infantile spasms. Eur. J. Paediatr. Neurol. 19, 134–142 (2015).
Latreille, V., Schiller, K., Peter-Derex, L. & Frauscher, B. Does epileptic activity impair sleep-related memory consolidation in epilepsy? A critical and systematic review. J. Clin. Sleep Med. https://doi.org/10.5664/JCSM.10166 (2022).
Lee, E. H., Choi, Y. S., Yoon, H. S. & Bahn, G. H. Clinical impact of epileptiform discharge in children with attention-deficit/hyperactivity disorder (ADHD). J. Child Neurol. 31, 584–588. https://doi.org/10.1177/0883073815604223 (2015).
Lam, A. D. et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat. Med. 23, 678–680 (2017).
Klinzing, J. G. et al. Auditory stimulation during sleep suppresses spike activity in benign epilepsy with centrotemporal spikes. Cell Rep. Med. 2, 100432 (2021).
Storz, S. et al. Sleep-dependent memory consolidation in children with self-limited focal epilepsies. Epilepsy Behav. 113, 107513 (2020).
Iber, C., Ancoli-Israel, S., Chesson, A. & Quan, S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchest. Am. Acad. Sleep Med. (2007).
Graves, A. et al. A novel connectionist system for unconstrained handwriting recognition. IEEE Trans. Pattern Anal. Mach. Intell. 31, 855–868 (2009).
Kingma, D. P. & Ba, J. Adam: A method for stochastic optimization. arXiv preprint arXiv:1412.6980 (2014).
Altman, D. G. & Bland, J. M. Diagnostic tests. 1: Sensitivity and specificity. BMJ 308, 1552 (1994).
Smeeton, N. C. Early history of the kappa statistic. Biometrics 41, 795 (1985).
Sim, J. & Wright, C. C. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 85, 257–268 (2005).
Powers, D. M. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness and correlation. J. Mach. Learn. Technol. 2, 36–37 (2011).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 59, 159–174 (1977).
Tononi, G. & Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014).
Stephansen, J. B. et al. Neural network analysis of sleep stages enables efficient diagnosis of narcolepsy. Nat. Commun. 9, 596 (2018).
Malafeev, A. et al. Automatic human sleep stage scoring using deep neural networks. Front. Neurosci. 12, 781 (2018).
Danker-Hopfe, H. et al. Interrater reliability for sleep scoring according to the Rechtschaffen & Kales and the new AASM standard. J. Sleep Res. 18, 74–84 (2009).
Danker-Hopfe, H. et al. Interrater reliability between scorers from eight European sleep laboratories in subjects with different sleep disorders. J. Sleep Res. 13, 63–69 (2004).
Galer, S. et al. Impaired sleep-related consolidation of declarative memories in idiopathic focal epilepsies of childhood. Epilepsy Behav. 43, 16–23 (2015).
Diekelmann, S. & Born, J. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126 (2010).
Tononi, G. & Cirelli, C. Sleep and synaptic down-selection. Eur. J. Neurosci. https://doi.org/10.1111/ejn.14335 (2020).
van Hirtum-Das, M. et al. Children with ESES: Variability in the syndrome. Epilepsy Res. 70, 248–258 (2006).
Specchio, N. et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE task force on nosology and definitions. Epilepsia 63, 1398–1442 (2022).
Ramanathan, R. S., Ahluwalia, T. & Sharma, A. Lennox-Gastaut syndrome: An overview. J. Pediatr. Neurosci. 5, 86 (2010).
Zeller, C. J., Züst, M. A., Wunderlin, M., Nissen, C. & Klöppel, S. The promise of portable remote auditory stimulation tools to enhance slow-wave sleep and prevent cognitive decline. J. Sleep Res. 32, e13818. https://doi.org/10.1111/JSR.13818 (2023).
Acknowledgements
This study was funded by the University of Zurich (Postdoc Grant FK-21-049) and the Swiss National Science Foundation (320030_179443) and conducted as part of the SleepLoop Flagship project of Hochschulmedizin Zürich.
Author information
Authors and Affiliations
Contributions
R.H. and B.S designed the study. S.L. and J.S. participated in the digital acquisition of data. S.S. and B.S. marked the data for epileptic spikes. S.S., B.S., B.B., and G.R. contributed with valuable clinical inputs. J.S. created automated methods and analyzed the data. J.S. and R.H. wrote the manuscript and co-authors reviewed and approved the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
R.H. and J.S. are inventors of a technology filed for patent. Other authors have no relevant competing financial or non-financial interests as defined by Nature Portfolio.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Skorucak, J., Bölsterli, B.K., Storz, S. et al. Automated analysis of a large-scale paediatric dataset illustrates the interdependent relationship between epilepsy and sleep. Sci Rep 13, 12882 (2023). https://doi.org/10.1038/s41598-023-39984-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39984-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.