Abstract
Enhancers function with a basal promoter to control the transcription of target genes. Enhancer regulatory activity is often studied using reporter-based transgene assays. However, unmatched results have been reported when selected enhancers are silenced in situ. In this study, using genomic deletion analysis in mice, we investigated the roles of two previously identified enhancers and the promoter of the Rho gene that codes for the visual pigment rhodopsin. The Rho gene is robustly expressed by rod photoreceptors of the retina, and essential for the subcellular structure and visual function of rod photoreceptors. Mutations in RHO cause severe vision loss in humans. We found that each Rho regulatory region can independently mediate local epigenomic changes, but only the promoter is absolutely required for establishing active Rho chromatin configuration and transcription and maintaining the cell integrity and function of rod photoreceptors. To our surprise, two Rho enhancers that enable strong promoter activation in reporter assays are largely dispensable for Rho expression in vivo. Only small and age-dependent impact is detectable when both enhancers are deleted. Our results demonstrate context-dependent roles of enhancers and highlight the importance of studying functions of cis-regulatory regions in the native genomic context.
Similar content being viewed by others
Introduction
Transcriptional regulation is a tenet of cellular development and homeostasis. Each cell type uses a unique set of regulatory sequences, namely promoters and enhancers, to enact expression of specific genes. A gene’s promoter is the DNA region immediately upstream of the transcription start site (TSS). Enhancers are often found distally upstream or downstream of a gene’s coding region and modulate the promoter activity. The cooperative regulation by promoter and enhancers is important for directing gene transcription within a specific cell type at a precise timing and an appropriate level. In particular, promoters and enhancers contain accessible regions of sequence-specific binding sites for cell-type specific and general transcription factors (TFs). Base-content analysis indicates the differences between the two classes: promoters display high-GC content and contain the requisite TATA box, initiator sequences and binding sites for a variety of TFs; enhancers are often AT-rich and contain binding sites for cell-type specific TFs1,2,3,4. Both classes can be defined by the presence of active histone modifications; specifically, promoters with H3K4me3 and H3K27Ac marks5,6, and enhancers with H3K27Ac and H3K4me1 marks7. Studies have further classified the regulatory units based on the landscape and density of the enrichment of these histone modifications. Super enhancers and broad H3K4me3 domains8,9,10,11,12,13 are hence defined as genomic regions regulating the specific gene expression that is imperative to a cell’s state and function.
Rod and cone photoreceptors are the cell types of the retina that mediate the conversion of light photons into electrical signals in a process called phototransduction. In mouse and human retinas, rod photoreceptors reside within the outer nuclear layer (ONL) and comprise 70% of all retinal cells14,15,16. Rod photoreceptors make synaptic connections at the outer plexiform layer (OPL) with horizontal and bipolar cells that reside in the inner nuclear layer (INL) to transfer light-evoked signals further to the brain via ganglion cell axons that comprise the optic nerve. The first step of phototransduction is accomplished within a unique cellular structure called the outer segment (OS) which consists of a stack of membrane discs packed with the light-sensing visual pigment, Rhodopsin17,18,19.
Rhodopsin is a G-protein-coupled receptor consisting of an opsin apo-protein and its chromophore 11-cis-retinal that accounts for the absorption properties. Rhodopsin is also required for OS structural integrity20,21. Mutations in the human Rhodopsin gene (RHO) cause blinding diseases, such as retinitis pigmentosa (RP) and night blindness22,23,24,25. The Rho gene is tightly regulated and highly transcribed, with its mRNA representing the most abundant transcript in rod photoreceptors26,27,28. Irregular Rho expression, either at lower or higher than normal levels, can lead to photoreceptor degeneration in animal models29,30,31. Mechanisms of the precise regulation on Rho expression have been extensively investigated. Initial studies identified the Rho proximal promoter region (PPR), a conserved ~ 200 bp region immediately upstream of Rho TSS, which was sufficient to drive the expression of the LacZ reporter in mouse photoreceptors32,33. Addition of a conserved genomic region, the Rho enhancer region (RER) lying 2 kb upstream of PPR, greatly increased PPR-mediated LacZ expression32,33.
Individual cis-regulatory elements (CREs) within PPR are detailed as sequence motifs bound by photoreceptor-specific TFs, including Cone-Rod Homeobox (CRX)34,35 and Neuroretina Leucine Zipper (NRL)36,37,38. ChIP-seq targetome analysis has discovered that CRX and NRL cooperatively bind not only to PPR and RER, but also to a region 4 kb upstream of Rho TSS, which is designated as CRX-Bound Region 1 (CBR)39,40. Both CBR and RER could further activate a PPR-driven reporter by eight- to tenfold in retinal explant reporter assays39. CRX/NRL synergistically activated PPR activity in transient HEK293 cell transfection assays, and loss of CRX or NRL in mice caused reduction in Rho expression and defects to rod photoreceptor development and maintenance36,41,42. In Crx-knockout mice, photoreceptor cells failed to gain appropriate active chromatin configuration at cis-regulatory regions of CRX-dependent genes, including PPR, RER and CBR of the Rho locus43, implicating the role of these regions in epigenomic remodeling of Rho expression.
In vivo chromosome conformation capture assays (3C) have illustrated CRX/NRL-dependent intrachromosomal loop interactions between RER and Rho PPR/gene body44. These findings collectively suggest that CBR and RER are Rho enhancers, which potentially act by mediating local epigenomic changes to increase PPR activity. However, it is unknown if such enhancer-promoter interactions are necessary for Rho expression in vivo. More specifically, it remains to be determined if these two previously identified Rho enhancers are essential for the epigenomic modulation and transcriptional activation of the Rho gene, and what degree of regulation each contributes.
In order to fill this knowledge gap, we aimed to dissect and determine in vivo functional roles of the Rho enhancers (CBR and RER) and promoter (PPR) using a loss-of-function approach in mice. By delivering CRISPR/Cas9 and specific guide RNAs (gRNAs) to C57BL/6J mouse embryos, we generated mouse lines carrying < 500 bp deletions at each genomic region individually and a line carrying a paired deletion of both CBR and RER. We performed gene expression analyses and cellular phenotype characterization for each mouse line at various ages. Our results indicate that, as expected, the Rho promoter PPR is absolutely required for Rho expression, rod photoreceptor integrity and survival. However, to our surprise, the two enhancers (CBR and RER) collectively play only minor roles in Rho expression, which is only applicable in developing and aging retinas. CBR/RER deficiency did not largely impact rod development and maintenance up to one year of age. Together, our results suggest that Rho proximal promoter is necessary and sufficient for Rho expression in rod photoreceptors, while Rho enhancers are largely dispensable. Our findings highlight the importance of investigating the function of cis-regulatory regions in the native genomic context.
Results
Generation of Rhodopsin (Rho) promoter and enhancer knockout mice
In order to examine the necessity of the Rho promoter and enhancers in Rho transcription, 4 mouse lines carrying a knockout of each genomic region were generated using CRISPR-Cas9/gRNA mediated excision. Based on the locations of CRX ChIP-seq peaks39, a pair of gRNAs targeting each peak at Rho promoter and two enhancer regions were introduced together with Cas9 enzyme into C57BL/6J mouse embryos to generate a 200–400 bp deletion at each region. A mouse line lacking both enhancers (CBR−/−RER−/−) on the same allele was made using a sequential targeting approach, i.e., RER deletion on the CBR−/− allele by introducing RER gRNAs and Cas9 enzymes to the CBR−/− embryos. PCR combined with sanger sequencing confirmed the absence of each region with the indicated length of deletion: 217 for PPR−/− (retaining the TATA box and transcription start site), 234 bp for RER−/− and 491 bp for CBR−/− (Fig. 1A). Mouse lines homozygous for each deletion appeared healthy and reproductive.
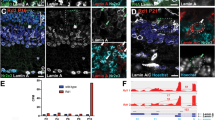
Changes of chromatin accessibility at Rho locus in mutant retinas. (A) Drawing of the Rho cis-regulatory regions (as black boxes) identified by previous studies and the specific knockouts involved in this study. (B) Browser tracks (mm10) of average ATAC-seq reads from duplicate retinal samples of the indicated genotypes at P14. Names of Rho CREs and peak calls are shown at the top of tracks. Scale bar represents 5 kb. (C) Bar plot of average ATAC-seq signals relative to the WT control. One-way ANOVA test is performed. n = 2 for each genotype. CBR, CRX-bound region 1. RER, Rhodopsin enhancer region. PPR, Rhodopsin proximal promoter region. INT, Rhodopsin Intragenic region. 3ʹ UTR, 3ʹ untranslated region. Asterisks (*, **, ***, ****) denote p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001, respectively.
Deletion of Rho promoter, but not enhancers, alters local chromatin accessibility
Since the chromatin of Rho cis-regulatory regions and gene body are activated during postnatal rod photoreceptor differentiation45,46,47, we tested if removal of Rho promoter or enhancers altered the chromatin accessibility of the Rho locus at postnatal day 14 (P14) using Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq). In wildtype (WT) retina, rod photoreceptor specification is completed by P14 and the Rho gene is fully expressed; this timing is consistent with epigenetic remodeling of the Rho locus measured by ATAC-seq and DNAse I hypersensitivity profiling47,48,49. Duplicate ATAC-seq experiments on P14 whole retinas of WT and each of the four knockout mouse lines, PPR−/−, CBR−/−, RER−/−, and CBR−/−RER−/− yielded highly reproducible results (Supplemental Fig. S1). Consistent with the published results, WT samples displayed five significant open chromatin regions within the roughly 12 kb region spanning the Rho locus (Fig. 1B, WT), including the peaks at CBR, RER, PPR, and two intragenic regions (INT and 3ʹUTR). The PPR−/− retina lost ATAC-seq signal not only at Rho promoter as expected, but also at INT and 3ʹUTR (Fig. 1B,C PPR−/−). Furthermore, PPR removal also caused a significant reduction of ATAC-seq signal upstream of the promoter, though the reduction was more profound at the proximal RER than at the more distal CBR (Fig. 1B,C). These results suggest that PPR plays an essential role in achieving appropriate open chromatin configuration within Rho gene body, and is necessary for normal DNA accessibility at upstream enhancers RER and CBR. This role of PPR is specific to the Rho locus, as no significant DNA accessibility changes were detected at other loci in PPR−/− retina (Supplemental Fig. S2A, Supplemental Fig. S3A). Next, we analyzed changes in ATAC-seq signal in retinas of enhancer knockout lines. Interestingly, the removal of RER yielded no significant effect on any other regions, and the removal of CBR only affected the accessibility at the 3ʹUTR (Fig. 1B,C). Thus, neither CBR nor RER is required to establish the normal open chromatin configuration of the Rho locus during photoreceptor differentiation. Surprisingly, the removal of both RER and CBR did not produce an additive effect, but rather showed a similar impact as CBR single knockout (Fig. 1B,C, CBR−/−RER−/−). Similar to the case of PPR−/−, global changes in chromatin accessibility were not observed in single- or double-enhancer knockout samples (Supplemental Figs. S2B–D, S3A). Overall, these results suggest that Rho enhancers have limited functions in establishing Rho chromatin accessibility, but PPR plays an essential and enhancer-independent role.
Histone modifications can also be used as a readout of the local chromatin and transcriptional activation state. Both H3K4me3 and H3K27ac are associated with transcriptionally active chromatin. H3K4me3 usually marks active gene promoters, while H3K27ac is enriched at active enhancers and promoters5,9,50,51. Both marks are detected at the actively transcribed Rho locus in mature mouse retina, which is defined as an active epigenetic state established in a CRX-dependent manner during postnatal development47,49. In order to assess changes in histone marks upon Rho promoter or enhancer knockout, Cleavage Under Targets and Tagmentation (CUT&Tag) analysis was performed to report the high-resolution chromatin profiling on P14 retinal samples. Two validated antibodies specific to these histone marks were used in CUT&Tag experiments. In WT retina, H3K4me3 peaks showed a broad domain encompassing the gene body, starting at the PPR and extending to the final exon of Rho gene (Fig. 2A, WT), but the signal was absent at CBR and RER regions. Analysis of mutants showed strikingly different landscapes: PPR−/− retina lost H3K4me3 deposition at the Rho locus while all enhancer-knockout samples showed comparable H3K4me3 marks to the WT control (Fig. 2A, PPR−/−). Inspection of the 50 kb region surrounding Rho confirmed that no other H3K4me3 sites were affected in the mutants (Supplemental Fig. S3B). The results of H3K4me3 CUT&Tag analysis suggest that Rho enhancers neither contribute to H3K4me3 deposition nor affect PPR activity, while PPR is required for recruiting H3K4me3 to the Rho locus.
Changes of active histone marks, H3K4me3 and H3K27ac, at Rho locus in mutant retinas. Browser tracks display comparative analysis of H3K4me3 (A) and H3K27ac (B) peaks from retinal samples of the indicated genotypes at P14. Top track of each panel shows peaks in the WT control. The difference in H3K4me3 peaks ranges between − 89 and 89. The difference in H3K27ac peaks ranges between − 6.7 and 6.7. The following four tracks of each panel display increased (red) and decreased (blue) signals relative to the WT control in mutant retinas. Scale bar represents 5 kb.
H3K27ac CUT&Tag analysis displayed 5 distinct peaks at Rho locus in WT retina at P14, including CBR, RER, PPR, INT, and 3ʹUTR, respectively (Fig. 2B, WT). In PPR−/− retina, H3K27ac signal was missing or greatly reduced at PPR, INT and 3ʹUTR, but retained WT levels at CBR and RER, implying CBR and RER are capable of loading H3K27ac marks in the absence of PPR (Fig. 2B, PPR−/−). In RER−/− retina, H3K27ac signal was similar to the WT control, besides a drop at the deleted RER region (Fig. 2B, RER−/−). However, in CBR−/− retina, a compensatory increase in H3K27ac signal was observed at RER, INT and 3ʹUTR, as well as at a few regions outside the Rho locus (Fig. 2B, Supplemental Fig. S3C, CBR−/−), possibly suggesting coordination or compensation between enhancers. In CBR−/−RER−/− retina, no change in H3K27ac signal was found at PPR, suggesting that the epigenetic activation state of the Rho promoter is unaltered by CBR and RER knockouts (Fig. 2B, CBR−/−RER−/−). Collectively, H3K27ac CUT&Tag results suggest that Rho promoter and enhancers can independently modify their histone tails to establish an active epigenetic environment and there is little crosstalk between these three regulatory regions in establishing the local epigenetic state. Altogether, these CUT&Tag data indicate that PPR is absolutely required for establishing the chromatin configuration and active histone marks at the accessible regions of the Rho locus, especially at the promoter and intragenic regions, but Rho enhancers are largely dispensable for this remodeling.
Rho enhancers make small and age-dependent contributions to Rho transcription levels
In order to analyze the effects of promoter/enhancer deletions on Rho expression, we performed next-generation RNA sequencing (RNA-seq) on P14 retinal samples of mutant and WT mice. Principal component analysis (PCA) showed that PPR samples were transcriptionally distinct from all other samples, evidenced by their distinct clustering (Fig. 3A). CBR−/−RER−/− and CBR−/− samples were also separated from the WT controls (Fig. 3A). Gene expression analysis showed that only Rho was differentially expressed in PPR−/− retina, and all other photoreceptor-enriched genes such as Crx, Nrl, Nr2e3, Gnat1, Pde6a, were not affected (Fig. 3B). Analysis of other mutants showed similar profiles to the WT control, except Rho showed some degree of differential expression in CBR−/−RER−/− retina (Fig. 3C, Supplemental Fig. S4A,B). These results suggest that PPR plays a more dominant role in Rho transcription than either enhancer, and the combinatorial knockout of both enhancers causes only a minor decrease of Rho transcription.
We further investigated the degree by which promoter and enhancer knockouts impacted Rho expression using qRT-PCR with retinal samples of specific allelic arrangements at various ages. P14 retinal samples were collected for qRT-PCR experiments from genotypes illustrated in Fig. 4A. At P14, Rho expression was completely ablated in PPR−/− retina and roughly reduced by half in PPR+/− retina (Fig. 4B, PPR−/−, PPR+/−), suggesting that no compensatory regulation or feedback from the intact allele when one PPR allele is lost. In contrast, RER−/− retina had comparable Rho expression to the WT control, while CBR−/− retina reduced Rho expression to roughly 70–80% of the WT level (Fig. 4B, RER−/−, CBR−/−), suggesting that CBR, not RER, makes a small contribution to Rho expression at P14. CBR−/−RER−/− retina showed reduced Rho expression to approximately 70–80% of the WT level, similar to, or slightly lower than that of CBR−/− retina (Fig. 4B, CBR−/−RER−/−). These results suggest that the regulatory capacity of Rho enhancers, specifically CBR, contributes 20–30% of Rho expression. When a copy of RER was present in Rho enhancer regions (CBR−/−RER+/−), Rho expression generally matched with those in CBR−/− and CBR−/−RER−/− retinas, collectively suggesting that RER is not required for Rho expression (Fig. 4B, CBR−/−RER+/−). When a copy of CBR was present in Rho enhancer regions (CBR+/−RER−/−), Rho expression reached to an indistinguishable level as the WT control (Fig. 4B, CBR+/−RER−/−), suggesting that at least one copy of CBR is needed to achieve maximum-level Rho expression in the presence of two PPR copies. Lastly, Rho expression in a retina heterozygous for PPR and Rho enhancers was comparable to that of PPR+/− retina, i.e., about 50% to the WT control (Fig. 4B, CBR+/−RER+/−, PPR+/−). These results together indicate that PPR is absolutely required for Rho expression and the regulatory functions of Rho enhancers are detectable and secondary to PPR in retinal development.
Quantitative PCR analysis of Rho expression in mutant retinas at various ages. (A) Diagrams of WT and mutant samples measured in this study. (B) qPCR analysis of Rho mRNA expression in P14 WT (n = 8) and mutant retinas (n = 10 for CBR−/−; RER−/−; CBR−/−RER−/−; n = 8 for CBR−/−RER+/−; CBR+/−RER−/−; n = 6 for PPR−/−; PRR+/−; CBR+/−RER+/−, PPR+/−). (C) Relative Rho expression profiles of CBR−/− (□), RER−/− (Δ), and CBR−/−RER−/− (●) samples up to 1YO (n ≥ 6 for each age). Interpolation is performed with MATLAB and Wolfram Mathematica: dashed line represents the CBR−/− profile, dash-dotted line represents the RER−/− profile, solid black line represents the CBR−/−RER−/− profile. All results are plotted as relative expression to WT control. Asterisks (*, **, ***, ****) denote p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001, respectively by one-way ANOVA with Tukey’s multiple comparisons. ns means not significant.
Furthermore, we tested if Rho enhancers functioned to maintain Rho expression in adult retina. qRT-PCR experiments were performed with enhancer-knockout retinas up to 1 year-old (1YO). Rho expression in RER−/− retina always remained indistinguishable from that in the WT control (Fig. 4C, RER−/−). Rho expression in CBR−/− retina initially reached about 70–80% of the WT level at P14 but increased steadily to a comparable level after the completion of retinal development and throughout adulthood (Fig. 4C, CBR−/−). Interestingly, although Rho expression in CBR−/−RER−/− retina was as low as 70–80% to the WT control at P14, the relative Rho expression profile became comparable from P21 to 4-month-old (MO). However, at 6MO, the relative Rho expression dropped back to 70–80% of WT expression and persisted to 1YO (Fig. 4C, CBR−/−RER−/−). Overall, these results suggest that Rho enhancers possess age-dependent regulatory activity to control Rho expression during rod differentiation and aging, but this regulation is only responsible for 20–30% of the overall expression.
In addition, we also assessed Rho expression in P7 retinas prior to eye opening. The immunohistochemistry (IHC) staining (Supplemental Fig. S5A) and qRT-PCR analysis (Supplemental Fig. S5B) suggest reduced Rho expression in PPR+/−, CBR−/−, and CBR−/−RER−/− mutants as compared to WT control, which echoes the findings of P14 samples. We did not observe any mislocalized RHO-positive cells in inner nuclear layer at P7 (Supplemental Fig. S5A) and the ONL thickness was comparable among all tested samples (Supplemental Fig. S5C). Besides changes in Rho expression in the PPR−/−, PPR+/− and CBR−/−RER−/− mutants, other rod photoreceptor-related genes such as Crx and Gnat1 displayed comparable expression to the WT control at P14 (Supplemental Fig. S5E) and 6MO (Supplemental Fig. S5G). The IHC staining with anti-RHO and GNAT1 antibodies showed that both proteins were made in CBR−/−RER−/− and PPR+/− retinas and appeared at normal subcellular locations (Supplemental Fig. S5D,F). Together, these results suggest that the loss of Rho regulatory elements and the resulted decrease in Rho expression in PPR+/− or CBR−/−RER−/− mice does not alter rod cell identity, localization, or differentiation.
Redundant roles of Rho enhancers contribute to the maintenance of rod morphology and function in old adults
We next investigated the retinal phenotypes in Rho enhancer and promoter mutants. PPR−/− retina failed to elaborate OS during development and showed only one remaining layer of cells within ONL at 6MO by H&E staining of retinal cross-sections (Fig. 5A, PPR−/−), while CBR−/−RER−/− and PPR+/− retinas maintained well-laminated structures (Fig. 5A, CBR−/−RER−/−, PPR+/−). No mislocalized cells were observed in the mutants. OS length of CBR−/−RER−/− and PPR+/− retinas appeared shorter than that of the WT control (Fig. 4B, OS Length, Supplemental Fig. S5F). ONL thickness in the PPR+/− retina was reduced as compared to WT control (Fig. 5B, ONL Thickness), while CBR−/−RER−/− retina had a comparable ONL thickness. Since rod photoreceptors represent the most abundant cell type within ONL, these morphological measurements suggest that CBR−/−RER−/− retina very likely has indistinguishable cell numbers of rod photoreceptors to the WT control without apparent cell death (data not shown). Therefore, the slightly shorter OS of CBR−/−RER−/− retina is associated with reduced Rho expression. On the other hand, the decreased ONL thickness of PPR+/− retina indicates loss of rod photoreceptors. Furthermore, visual function of mutant retinas was assayed by dark-adapted (rod response) electroretinography (ERG). As expected, PPR−/− mice had no ERG responses, while PPR+/− mice had decreased dark-adapted A-wave and B-wave amplitudes at high-light intensities as compared to the WT control (Fig. 5C). CBR−/−RER−/− mice also showed lower A-wave and B-wave amplitudes at the high light intensities, but less severe than PPR+/− (Fig. 5C). This is consistent with the reduction of Rho expression and OS length in the CBR−/−RER−/− retina that was less severe than observed in the PPR+/− retina. In addition, 6MO CBR−/− and RER−/− single mutants were indistinguishable from the WT control in retinal morphology (Supplemental Fig. S6A), OS length (Supplemental Fig. S6B) and ERG responses (Supplemental Fig. S6C,D), echoing their comparable Rho expression as the WT control. Thus, CBR and RER act redundantly to constitute the Rho enhancer landscape for transcriptional regulation in adult retina, and their combined loss leads to measurable functional deficits.
Changes of retinal morphology and function in mutants at 6MO. (A) Hematoxylin and Eosin (H&E) cross-section staining of 6MO WT and mutant retinas of the indicated genotypes. OS: outer segment; ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer. Scale bar represents 100 µm for all image panels. (B) OS thickness (left panel, in µm) and ONL thickness (right panel, in µm) in 6MO WT and mutant retinas of the indicated genotypes at various positions from the optic nerve head (ONH). Error bars represent mean (SD) (n ≥ 5). Dashed black line indicates the statistical difference between WT and CBR−/−RER−/− samples, solid black line indicates the statistical difference between WT and PPR+/− samples. (C) Electroretinogram (ERG) analysis of 6MO WT and mutant mice of the indicated genotypes, showing amplitude changes of dark-adapted A-waves (left panel) and B-waves (right panel). Mean amplitudes (µV) are plotted against stimulus light intensity. Error bars represent SEM (n ≥ 7). All statistics is done by comparing to WT control. Asterisks (*, **, ***, ****) denote p ≤ 0.05, p ≤ 0.01, p ≤ 0.001, p ≤ 0.0001, respectively by two-way ANOVA with Tukey’s multiple comparisons. ns means not significant.
Rod morphological and functional changes in mutant retinas were also assessed at early adult ages. At 6 weeks (6WK) and 3MO, OS length of the PPR+/− retina appeared shorter (Supplemental Fig. S7A,B). This morphological defect in the PPR+/− retina echoed the lower amplitudes of dark-adapted ERG A-waves at the highest light intensity as compared to that of the WT control (Supplemental Fig. S7E,F). OS length and the amplitudes of dark-adapted ERG A-wave of the CBR−/−RER−/− retina were comparable to WT controls (Supplemental Fig. S7A,B,E,F). Importantly, ONL thickness of all mutants remained comparable to that of the WT control at 6WK and 3MO (Supplemental Fig. S7C,D). Compared to the ONL reduction in 6MO PPR+/− retina, these results suggest that PPR+/− retinal degeneration occurs after 3MO. As a reference, OS and ONL measurements in heterozygous and homozygous Rho-knockout mice31 (Rho−/− and Rho+/−) showed similar results as PPR mutants: ONL thickness of PPR−/− retina degenerated dramatically after 6WK (Supplemental Fig. S8A, PPR−/−). PPR+/− retina began to show ONL degeneration at 6MO but reduced OS length at 3MO, similar to that of Rho+/− retina (Supplemental Fig. S8A,B, PPR+/− vs. Rho+/−).
Discussion
Using CRISPR-Cas9/gRNA mediated excision, this study examined the specificity and strength of two Rho enhancers, namely CBR and RER, and of Rho promoter in regulating Rho expression. Previous reporter-based assays showed that both the RER and CBR were capable of enhancing Rho promoter activity by eight- to tenfold in postnatal mouse retina33,39. However, this study shows that knockout of each or both enhancers surprisingly produce null or only moderate impact (20–30% loss) on endogenous Rho expression. Notably, RER, that was previously identified by a study with transgenic mice33 and known to interact with PPR by 3C assay44, fails to display any necessity for endogenous Rho expression in developmental and adult ages. Consistent with their minor roles, single Rho enhancer knockout also shows limited impact on rod photoreceptor identity, health, and function. These results are in contrast to the essential function of the Locus Control Region (LCR) in its regulation of red/green (R/G) cone opsin expression52,53,54. The conserved LCR is absolutely required for mutually exclusive expression of red vs green opsin in the respective cone subtype in humans, mouse, and zebrafish models. RER and R/G LCR share a conserved core region of similar TF binding motifs, including CRX binding sites33. Several models might explain the minimal relevance of Rho enhancers in situ, but high activity in reporter assays: firstly, the strength of a promoter might determine its cooperation with enhancers. Rho promoter (PPR) is highly robust and sufficient to drive cell type-specific expression of target genes both in episomal vectors39 and at ectopic genomic locations33. Future studies are needed to determine the relative strength of Rho promoter vs other photoreceptor gene promoters and how the promoter strength influences functional interactions with specific enhancers. Secondly, dynamic expression and precision might also be a determinant. Genes coding for cell fate-determining transcription factors with complex spatial and temporal expression patterns often harbor strong distal enhancers or enhancer clusters, such as those found in Pax655, Otx256,57,58, Vsx259, Atoh760 and Blimp161. Rho expression, despite being one of the highest expressed genes in the retina, follows a simple induction curve during rod photoreceptor development62, which may not require a prominent enhancer but a strong self-functioning promoter. Finally, results of reporter assays may have exaggerated Rho enhancer activities, as reporter gene constructs do not contain the correct genomic context, including native gene structure and epigenomic landscape. Overall, the findings of this study highlight the importance to validate functions of reporter-identified enhancers in a native genomic context.
This study shows age-dependent activities of Rho enhancers. During postnatal development, CBR clearly plays a dominant role, as it is responsible for 20–30% Rho expression, while RER’s activity was dispensable. Thus, CBR, but not RER, is required for appropriate Rho transcription during development. However, this minor contribution of CBR does not affect the development of rod subcellular structure and function. In adult retinas, deletion of Rho enhancers results in no detectable effects in young and mid ages, but after 6 MO, RER/CBR knockout causes a loss of 20–30% of Rho transcription. These suggest that CBR and RER play a redundant role in maintaining high-level Rho transcription in aged retinas. Similar “shadow” or redundant enhancers have been reported elsewhere, such as individual enhancers in β-globin LCR, Pax6 lens enhancers and Atoh7 distal enhancer55,60,63,64,65,66. These enhancers mostly act during tissue/organ genesis and are required for regulating the expression timing of specific genes. Our discovery of redundant enhancers in aged retina provides a new insight into enhancers’ roles in aging and tissue-specific maintenance. Consistently, CBR/RER knockout mutants affected Rho expression as well as morphological and functional integrity at 6MO. Since PPR+/− (50% Rho reduction) produced a more severe phenotype than CBR/RER knockout mutants (20–30% Rho reduction) in aged adults, our results confirm that rod homeostasis requires precise control of Rho expression. Aged rod photoreceptors with disrupted Rho enhancer activity may be more susceptible to genetic and/or environmental insults. Since RHO coding mutations are linked to congenital stationary night blindness and retinitis pigmentosa24, sequence variants in the non-coding regions could potentially affect the expression of the mutant (or WT) allele and thus contribute to phenotype severity or age of onset. Future studies in mouse models and phenotype-genotype correlations in humans are required to determine if RHO enhancer deficiency complicates (or modifies) defective phenotypes under disease/stress conditions, particularly in progressive rod photoreceptor degeneration.
Typical “super enhancer” characteristics were detected in wildtype retina within 5 kb of the Rho upstream regulatory sequence. Changes of these characteristics in mutants, in general, agreed with the impact of each region on Rho transcription. In particular, loss of ATAC signals at Rho gene body was only seen in PPR−/−, but not in enhancer knockout mutants, highlighting the interdependency and necessity of PPR in epigenomic modulation during Rho transcription activation. It is notable that only loss of PPR caused dramatic depletion of H3K4me3 from the locus, suggesting a specific reliance on the PPR and/or transcription for this histone modification. Deleting CBR and/or RER enhancers had limited impact on the epigenomic landscape of the Rho locus, despite the 20–30% contribution to transcription, suggesting that the two enhancers do not act in a “trans” manner on DNA accessibility, histone modifications, or chromatin configuration. Most likely, they promote RNA polymerase II activity at the promoter and gene body via CRX/NRL-dependent looping interactions44. A similar interactive pattern of predominant promoter versus subsidiary enhancers has been previously reported67. Although CBR and RER deletions are insufficient to elicit large changes in Rho expression and DNA accessibility of Rho PPR, this study cannot exclude the existence or importance of more distal Rho-specific CREs in regulating chromatin dynamics at the Rho PPR region. One potential candidate is a region(s) located in the distal intron of the neighboring lft122 gene (Supplemental Fig. S3). This region displays an open chromatin configuration in WT retina and the H3K27Ac enhancer mark that has compensatory changes in the PPR, CBR or RER knockouts. There is a potential loop interaction between this distal region with Rho CBR/RER/PPR proximal regulatory region in published Hi-C data68,69,70. Future mouse models will be required to determine the in vivo role of this distal region in regulating Rho expression.
The mouse Rho gene shares about 95% sequence homology with the human gene33 (Mouse Genome Informatics). Importantly, Rho enhancers at the Rho locus also share remarkably conserved topography between the two species in terms of CRE number and relative locations33,39,71. The profile of transcription factors bound to Rho enhancers and promoter is also conserved39,72. While numerous non-synonymous and nonsense variants within the Rho coding region have been found to cause human retinal disease, no regulatory variants have yet been discovered. This study suggests that future efforts should be primarily focused on the proximal promoter region, particularly at critical TF binding motifs. Distal regulatory variants within the RER and CBR are unlikely to cause pathogenic transcriptional deficits for severe developmental defects. These variants however could be genetic modifiers of other pathogenic mechanisms or independently yield minor but detectable effects on visual function and alter long-term photoreceptor health.
In conclusion, this study has described unexpectedly minor roles of two previously identified Rho enhancers on in vivo Rho transcription, and their redundant activity in rod functional maintenance in aging retinas. These findings contribute to our understanding of enhancer functions and mechanism of action in vivo and highlight the importance of dissecting individual enhancer functions and interactions in the native genomic context at various ages.
Materials and methods
Animals
All mice in this study were on the C57BL/6J background. Both male and female mice were used in experiments. All animal procedures were conducted according to the ARRIVE guidelines (https://arriveguidelines.org), and were approved by the Washington University in St. Louis Institutional Animal Care and Use Committee (Animal Welfare Assurance # D1600245 and Protocol approval # 21-0414).
Generation of mutant mice
CRISPR/Cas9 gene editing was performed using two single-guide RNA (sgRNAs) flanking each element of CBR, RER, PPR in order to delete these elements (see Fig. 1). The gRNA sequences are: For CBR, 5ʹ:TAGCTCCGTTTCCACATTGA and 3ʹ:TCAAGATACACTGTCCCCAC. For RER, 5ʹ: GCTTCATCGTGGTCTCCGCG and 3ʹ: TCCATGCAGGTGTCTTGTTT. For PPR, 5ʹ: GAAGTGAATTTAGGGCCCAA and 3ʹ: GCGGATGCTGAATCAGCCTC. Fertilized mouse oocytes injected with sgRNAs were allowed to proceed to birth to generate mouse lines. In order to generate the mutant carrying deletions of both CBR and RER on the same chromosome, a mouse line homozygous for CBR−/− was used to prepare fertilized oocytes that were subsequently injected with sgRNAs for RER deletion. F0 mutants were genotyped by DNA sequencing of PCR products overlapping the mutations.
Histology and immunohistochemistry
Eyes were enucleated at tested ages and fixed in 4% paraformaldehyde at 4 °C overnight for paraffin embedded sections. Each retinal cross-section was cut 5 microns thick on a microtome. Hematoxylin and Eosin (H&E) staining was used to examine retinal morphology.
For IHC staining, sections firstly went through antigen retrieval with citrate buffer, and blocked with a blocking buffer of 5% donkey serum, 1% BSA, 0.1% Triton-x-100 in 1× PBS (pH-7.4) for 2 h. Sections were then incubated with primary antibodies at 4 °C overnight, then washed, followed by 2-h incubation of specific secondary antibodies. Primary antibody to Rho (MilliporeSigma, O4886) or Gnat1 (MilliporeSigma, GT40562) and secondary antibodies were applied with optimal dilution ratios. All slides were mounted with hard-set mounting medium with DAPI (Vectashield, Vector Laboratories, Inc., CA).
Electroretinogram
ERGs were performed on adult mice at different ages using UTAS-E3000 Visual Electrodiagnostic System (LKC Technologies Inc., MD). Mice were dark-adapted overnight prior to the tests. The experimental procedures were previously established in our lab. ERG responses of biological replicates were recorded, averaged, and analyzed using Graphpad Prism 8 (GraphPad Software, CA). The mean peak amplitudes of dark-adapted A and B waves and light-adapted B waves were plotted against log values of light intensities (cd*s/m2). The statistical analysis was done by two-way ANOVA with multiple pairwise comparisons (Tukey’s).
Quantitative PCR
Each RNA sample was extracted from 2 retinae of a mouse using the NucleoSpin RNA Plus kit (Macherey–Nagel, PA). RNA concentrations were measured using a NanoDrop One spectrophotometer (ThermoFisher Scientific). 1 μg of RNA was used to synthesize cDNA with First Strand cDNA Synthesis kit (Roche, IN). The reaction master mix contained EvaGreen polymerase (Bio-Rad Laboratories, CA), 1 μM primer mix, and diluted cDNA samples. Samples were run using a two-step 40-cycle protocol on a Bio-Rad CFX96 Thermal Cycler (Bio-Rad Laboratories, CA). At least 3 technical triplicates were run for each gene. Primers (5ʹ–3ʹ) used in this study were Rho (F: GCTTCCCTACGCCAGTGTG, R: CAGTGGATTCTTGCCGCAG), Crx (F: GTCCCATACTCAAGTGCCC, R: TGCTGTTTCTGCTGCTGTCG), Gnat1 (F: ACGATGGACCTAACACTTACGAGG, R: TGGAAAGGACGGTATTTGAGG). Data were analyzed with QBase software (Biogazelle, Belgium). The statistical analysis was done by Student’s t-test with p < 0.05, CI 95%.
RNA-seq data generation and analysis
RNA was extracted from retinal samples using the NucleoSpin® RNA kit (Macherey–Nagel) using the manufacturer’s protocol. Total RNA integrity was determined using Agilent Bioanalyzer or 4200 Tapestation. Library preparation and sequencing experiments were performed at Genome Technology Access Center, Washington University in St. Louis. A brief description of the experimental procedures was outline as follows. Library preparation was performed with 5–10 μg of total RNA with a RIN score greater than 8.0. Ribosomal RNA was removed by poly-A selection using Oligo-dT beads (mRNA Direct kit, Life Technologies). mRNA was then fragmented in reverse transcriptase buffer and heating to 94° for 8 min. mRNA was reverse transcribed to yield cDNA using SuperScript III RT enzyme (Life Technologies, per manufacturer’s instructions) and random hexamers. A second strand reaction was performed to yield ds-cDNA. cDNA was blunt ended, had an A base added to the 3ʹ ends, and then had Illumina sequencing adapters ligated to the ends. Ligated fragments were then amplified for 12–15 cycles using primers incorporating unique dual index tags. Fragments were sequenced on an Illumina NovaSeq-6000 using paired end reads extending 150 bases. Reads were trimmed using Trim-Galore! (v0.6.7) a wrapper for Cutadapt (v3.4) and FastQC (v0.11.9). Trimmed reads were mapped to mm10 using STAR (v2.7.0)73. Mapped reads were cleaned using Samtools (v1.9.4), gene counts generated by HTseq (v0.11.2)74, and visualizations generated in R (v3.6.1).
ATAC-seq data generation
ATAC-seq libraries were generated as published in Ruzycki et al.49, a slightly modified protocol to that published by Buenrostro et al.75. Briefly, retinas were dissected from P14 mice of each genotype and washed in PBS. Retinal cells were dissociated using 2% collagenase in TESCA buffer for 13 min at 37C. DNase I (0.5 Units; Roche, Basel, Switzerland) was added for the final 3 min to minimize clumping. DMEM + 10% FBS was added to stop the reaction. Nuclei were stained with SYBR Gold (ThermoFisher Scientific) diluted 1:100 in trypan blue and counted using a fluorescent microscope. 50,000 cells were resuspended in TD buffer for a 1 h incubation at 37 °C with TDE1 (Nexetra DNA Library Prep Kit; Illumina, San Diego, CA). Remaining steps of library prep were performed as published by Buenrostro et al. Final libraries were pooled and sequenced using the Illumina 2500.
CUT&Tag data generation
Retinal cells were dissociated and counted in same manner as described above for ATAC-seq. After quantification, cells were diluted, aliquoted and cryopreserved by adding DMSO to a final volume of 10% and slowly freezing to – 80 °C in a Mr. Frosty container. CUT&Tag reactions were performed using Epicypher reagents (protocol v1.5; Epicypher, USA). Briefly, cells were thawed on ice and 100,000 bound to ConA beads for each sample. Primary antibody incubation was left at 4 °C overnight on a nutator. 0.5 μg of antibody was used for each reaction; H3K4me3 (MABE647; EMD Millipore Corporation, USA) and H3K27Ac (ab177178; Abcam Inc, USA). 0.5ug secondary antibody (CUTANA anti-rabbit secondary antibody; 13-0047; Epicypher) was incubated for 0.5 h at room temperature, and Tn5 binding and transposition done exactly as specified in CUTANA protocol. After transposition, custom 10 bp unique-dual-indexed primers were used to amplify transposed fragments (P7-CAAGCAGAAGACGGCATACGAGAT-10bpBC-GTCTCGTGGGCTCGGAGATGTG, P5-AATGATACGGCGACCACCGAGATCTACAC-10bpBC-TCGTCGGCAGCGTCAGATGTGTAT) for 20 cycles. Libraries were cleaned and size-selected using Ampure XP beads. Libraries were pooled and sequenced using the Illumina NovaSeq 6000.
ATAC-seq and CUT&Tag data analysis
Reads were trimmed using Trim-Galore! (v0.6.7) a wrapper for Cutadapt (v3.4) and FastQC (v0.11.9). Trimmed reads were mapped to the mm10 genome build using bowtie2 (v2.4.1)76,77. Mapped reads were cleaned using Samtools (v1.9.4), Picard (v2.25.7), and Bedtools (v2.27.1). For visualization, reads were converted to bigwig format and compared using Deeptools (v3.5.1). ATAC-seq peak calling was accomplished using MACS2 (v2.2.7.1).
Ethics statement
All animal procedures were conducted according to the Guide for the Care and Use of Laboratory Animals of the National Institute of Health and were approved by the Washington University in St. Louis Institutional Animal Care and Use Committee.
Data availability
All raw and processed data has been uploaded to the NCBI SRA database (BioProject ID: RJNA909209). The data is accessible via the link https://dataview.ncbi.nlm.nih.gov/object/PRJNA909209?reviewer=harmjbholgq1lk0c0cgrkel0o1.
References
Field, A. & Adelman, K. Evaluating enhancer function and transcription. Annu. Rev. Biochem. 89, 213–234. https://doi.org/10.1146/annurev-biochem-011420-095916 (2020).
Higgs, D. R. Enhancer–promoter interactions and transcription. Nat. Genet. 52, 470–471. https://doi.org/10.1038/s41588-020-0620-7 (2020).
Andersson, R. Promoter or enhancer, what’s the difference? Deconstruction of established distinctions and presentation of a unifying model. BioEssays 37, 314–323. https://doi.org/10.1002/bies.201400162 (2015).
Lim, B. & Levine, M. S. Enhancer-promoter communication: Hubs or loops?. Curr. Opin. Genet. Dev. 67, 5–9. https://doi.org/10.1016/j.gde.2020.10.001 (2021).
Howe, F. S., Fischl, H., Murray, S. C. & Mellor, J. Is H3K4me3 instructive for transcription activation? BioEssays 39, e201600095. https://doi.org/10.1002/bies.201600095 (2017).
Zhang, T., Cooper, S. & Brockdorff, N. The interplay of histone modifications—writers that read. EMBO Rep. 16, 1467–1481. https://doi.org/10.15252/embr.201540945 (2015).
Spicuglia, S. & Vanhille, L. Chromatin signatures of active enhancers. Nucleus 3, 126–131. https://doi.org/10.4161/nucl.19232 (2012).
Cao, F. et al. Super-enhancers and broad H3K4me3 domains form complex gene regulatory circuits involving chromatin interactions. Sci. Rep. 7, 2186. https://doi.org/10.1038/s41598-017-02257-3 (2017).
Beacon, T. H. et al. The dynamic broad epigenetic (H3K4me3, H3K27ac) domain as a mark of essential genes. Clin. Epigenet. 13, 138. https://doi.org/10.1186/s13148-021-01126-1 (2021).
Park, S., Kim, G. W., Kwon, S. H. & Lee, J.-S. Broad domains of histone H3 lysine 4 trimethylation in transcriptional regulation and disease. FEBS J. 287, 2891–2902. https://doi.org/10.1111/febs.15219 (2020).
Pott, S. & Lieb, J. D. What are super-enhancers?. Nat. Genet. 47, 8–12. https://doi.org/10.1038/ng.3167 (2015).
Benayoun, B. A. et al. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 158, 673–688. https://doi.org/10.1016/j.cell.2014.06.027 (2014).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947. https://doi.org/10.1016/j.cell.2013.09.053 (2013).
Molday, R. S. & Moritz, O. L. Photoreceptors at a glance. J. Cell Sci. 128, 4039–4045. https://doi.org/10.1242/jcs.175687 (2015).
Hussey, K. A., Hadyniak, S. E. & Johnston, R. J. Patterning and development of photoreceptors in the human retina. Front. Cell Dev. Biol. 10. https://doi.org/10.3389/fcell.2022.878350 (2022).
Hendrickson, A. et al. Rod photoreceptor differentiation in fetal and infant human retina. Exp. Eye Res. 87, 415–426. https://doi.org/10.1016/j.exer.2008.07.016 (2008).
Kraft, T. W., Schneeweis, D. M. & Schnapf, J. L. Visual transduction in human rod photoreceptors. J. Physiol. 464, 747–765. https://doi.org/10.1113/jphysiol.1993.sp019661 (1993).
Fain, G. L., Hardie, R. & Laughlin, S. B. Phototransduction and the evolution of photoreceptors. Curr. Biol. 20, R114-124. https://doi.org/10.1016/j.cub.2009.12.006 (2010).
Arshavsky, V. Y., Lamb, T. D. & Pugh, E. N. G Proteins and Phototransduction. Annu. Rev. Physiol. 64, 153–187. https://doi.org/10.1146/annurev.physiol.64.082701.102229 (2002).
Goldberg, A. F., Moritz, O. L. & Williams, D. S. Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res. 55, 52–81. https://doi.org/10.1016/j.preteyeres.2016.05.003 (2016).
Makino, C. L. et al. Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS One 7, e37832 (2012). https://doi.org/10.1371/journal.pone.0037832
Dryja, T. P. et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N. Engl. J. Med. 323, 1302–1307. https://doi.org/10.1056/NEJM199011083231903 (1990).
Iannaccone, A. et al. Retinitis pigmentosa associated with rhodopsin mutations: Correlation between phenotypic variability and molecular effects. Vis. Res. 46, 4556–4567. https://doi.org/10.1016/j.visres.2006.08.018 (2006).
Athanasiou, D. et al. The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy. Prog. Retin. Eye Res. 62, 1–23. https://doi.org/10.1016/j.preteyeres.2017.10.002 (2018).
Wilson, J. H. & Wensel, T. G. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol. Neurobiol. 28, 149–158. https://doi.org/10.1385/MN:28:2:149 (2003).
Hargrave, P. A. Rhodopsin structure, function, and topography the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 42, 3–9 (2001).
Filipek, S., Stenkamp, R. E., Teller, D. C. & Palczewski, K. G protein-coupled receptor rhodopsin: A prospectus. Annu. Rev. Physiol. 65, 851–879. https://doi.org/10.1146/annurev.physiol.65.092101.142611 (2003).
Palczewski, K. G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767. https://doi.org/10.1146/annurev.biochem.75.103004.142743 (2006).
Jaissle, G. B. et al. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest. Ophthalmol. Vis. Sci. 42, 506–513 (2001).
Tan, E. et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 42, 589–600 (2001).
Liang, Y. et al. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J. Biol. Chem. 279, 48189–48196. https://doi.org/10.1074/jbc.M408362200 (2004).
Zack, D. J. et al. Unusual topography of bovine rhodopsin promoter-IacZ fusion gene expression in transgenic mouse retinas. Neuron 6, 187–199. https://doi.org/10.1016/0896-6273(91)90355-4 (1991).
Nie, Z., Chen, S., Kumar, R. & Zack, D. J. RER, an evolutionarily conserved sequence upstream of the rhodopsin gene, has enhancer activity (*). J. Biol. Chem. 271, 2667–2675. https://doi.org/10.1074/jbc.271.5.2667 (1996).
Chen, S. et al. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19, 1017–1030. https://doi.org/10.1016/S0896-6273(00)80394-3 (1997).
Hennig, A. K., Peng, G.-H. & Chen, S. Regulation of photoreceptor gene expression by Crx-associated transcription factor network. Brain Res. 1192, 114–133. https://doi.org/10.1016/j.brainres.2007.06.036 (2008).
Mears, A. J. et al. Nrl is required for rod photoreceptor development. Nat. Genet. 29. https://doi.org/10.1038/ng774 (2001).
Rehemtulla, A. et al. The basic motif-leucine zipper transcription factor Nrl can positively regulate rhodopsin gene expression. Proc. Natl. Acad. Sci. USA 93, 191–195. https://doi.org/10.1073/pnas.93.1.191 (1996).
Kumar, R. et al. The bZIP transcription factor Nrl stimulates rhodopsin promoter activity in primary retinal cell cultures. J. Biol. Chem. 271, 29612–29618. https://doi.org/10.1074/jbc.271.47.29612 (1996).
Corbo, J. C. et al. CRX ChIP-seq reveals the cis-regulatory architecture of mouse photoreceptors. Genome Res. 20, 1512–1525. https://doi.org/10.1101/gr.109405.110 (2010).
Hao, H. et al. Transcriptional regulation of rod photoreceptor homeostasis revealed by in vivo NRL targetome analysis. PLoS Genet. 8, e1002649–e1002649. https://doi.org/10.1371/journal.pgen.1002649 (2012).
Mitton, K. P. et al. The leucine zipper of NRL interacts with the CRX homeodomain: A possible mechanism of transcriptional synergy in rhodopsin regulation. J. Biol. Chem. 275, 29794–29799. https://doi.org/10.1074/jbc.M003658200 (2000).
Furukawa, T., Morrow, E. M., Li, T., Davis, F. C. & Cepko, C. L. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat. Genet. 23, 466. https://doi.org/10.1038/70591 (1999).
Ruzycki, P. A., Tran, N. M., Kefalov, V. J., Kolesnikov, A. V. & Chen, S. Graded gene expression changes determine phenotype severity in mouse models of CRX-associated retinopathies. Genome Biol. 16, 171–171. https://doi.org/10.1186/s13059-015-0732-z (2015).
Peng, G. H. & Chen, S. Active opsin loci adopt intrachromosomal loops that depend on the photoreceptor transcription factor network. Proc. Natl. Acad. Sci. U S A 108, 17821–17826. https://doi.org/10.1073/pnas.1109209108 (2011).
Mo, A. et al. Epigenomic landscapes of retinal rods and cones. eLife 5, e11613. https://doi.org/10.7554/eLife.11613 (2016).
Hughes, A. E. O., Enright, J. M., Myers, C. A., Shen, S. Q. & Corbo, J. C. Cell type-specific epigenomic analysis reveals a uniquely closed chromatin architecture in mouse rod photoreceptors. Sci. Rep. 7, 43184. https://doi.org/10.1038/srep43184 (2017).
Aldiri, I. et al. The dynamic epigenetic landscape of the retina during development, reprogramming, and tumorigenesis. Neuron 94, 550-568.e510. https://doi.org/10.1016/j.neuron.2017.04.022 (2017).
Wilken, M. S. et al. DNase I hypersensitivity analysis of the mouse brain and retina identifies region-specific regulatory elements. Epigenet. Chromatin 8, 8. https://doi.org/10.1186/1756-8935-8-8 (2015).
Ruzycki, P. A., Zhang, X. & Chen, S. CRX directs photoreceptor differentiation by accelerating chromatin remodeling at specific target sites. Epigenetics Chromatin 11, 42. https://doi.org/10.1186/s13072-018-0212-2 (2018).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. 107, 21931–21936. https://doi.org/10.1073/pnas.1016071107 (2010).
Haberle, V. & Stark, A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 19, 621–637. https://doi.org/10.1038/s41580-018-0028-8 (2018).
Winderickx, J., Battisti, L., Motulsky, A. G. & Deeb, S. S. Selective expression of human X chromosome-linked green opsin genes. Proc. Natl. Acad. Sci. U.S.A. 89, 9710–9714 (1992).
Tsujimura, T., Hosoya, T. & Kawamura, S. A single enhancer regulating the differential expression of duplicated red-sensitive opsin genes in Zebrafish. PLoS Genet. 6, e1001245. https://doi.org/10.1371/journal.pgen.1001245 (2010).
Wang, Y. et al. Mutually exclusive expression of human red and green visual pigment-reporter transgenes occurs at high frequency in murine cone photoreceptors. Proc. Natl. Acad. Sci. 96, 5251–5256. https://doi.org/10.1073/pnas.96.9.5251 (1999).
Antosova, B. et al. The gene regulatory network of lens induction is wired through Meis-dependent shadow enhancers of Pax6. PLOS Genet. 12, e1006441. https://doi.org/10.1371/journal.pgen.1006441 (2016).
Emerson, M. M. & Cepko, C. L. Identification of a retina-specific Otx2 enhancer element active in immature developing photoreceptors. Dev. Biol. 360, 241–255. https://doi.org/10.1016/j.ydbio.2011.09.012 (2011).
Bhansali, P., Cvekl, A. & Liu, W. A distal enhancer that directs Otx2 expression in the retinal pigment epithelium and neuroretina. Dev. Dyn. 249, 209–221. https://doi.org/10.1002/dvdy.127 (2020).
Wang, S., Sengel, C., Emerson, M. M. & Cepko, C. L. A gene regulatory network controls the binary fate decision of rod and bipolar cells in the vertebrate retina. Dev. Cell 30, 513–527. https://doi.org/10.1016/j.devcel.2014.07.018 (2014).
Honnell, V. et al. Identification of a modular super-enhancer in murine retinal development. Nat. Commun. 13, 253. https://doi.org/10.1038/s41467-021-27924-y (2022).
Miesfeld, J. B. et al. The Atoh7 remote enhancer provides transcriptional robustness during retinal ganglion cell development. Proc. Natl. Acad. Sci. U S A 117, 21690–21700. https://doi.org/10.1073/pnas.2006888117 (2020).
Goodson, N. B., Kaufman, M. A., Park, K. U. & Brzezinski, J. A. I. V. Simultaneous deletion of Prdm1 and Vsx2 enhancers in the retina alters photoreceptor and bipolar cell fate specification, yet differs from deleting both genes. Development 147, dev190272. https://doi.org/10.1242/dev.190272 (2020).
Treisman, J. E., Morabito, M. A. & Barnstable, C. J. Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Mol. Cell Biol. 8, 1570–1579. https://doi.org/10.1128/mcb.8.4.1570-1579.1988 (1988).
Bender, M. A., Bulger, M., Close, J. & Groudine, M. Beta-globin gene switching and DNase I sensitivity of the endogenous beta-globin locus in mice do not require the locus control region. Mol. Cell 5, 387–393. https://doi.org/10.1016/s1097-2765(00)80433-5 (2000).
Cimbora, D. M. et al. Long-distance control of origin choice and replication timing in the human beta-globin locus are independent of the locus control region. Mol. Cell Biol. 20, 5581–5591. https://doi.org/10.1128/mcb.20.15.5581-5591.2000 (2000).
Osterwalder, M. et al. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554, 239–243. https://doi.org/10.1038/nature25461 (2018).
Zibetti, C. Deciphering the retinal epigenome during development, disease and reprogramming: Advancements, challenges and perspectives. Cells 11 (2022).
Li, K. et al. Interrogation of enhancer function by enhancer-targeting CRISPR epigenetic editing. Nat. Commun. 11, 485. https://doi.org/10.1038/s41467-020-14362-5 (2020).
Norrie, J. L. et al. Nucleome dynamics during retinal development. Neuron 104, 512-528.e511. https://doi.org/10.1016/j.neuron.2019.08.002 (2019).
Falk, M. et al. Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature 570, 395–399. https://doi.org/10.1038/s41586-019-1275-3 (2019).
Issam Al, D. et al. The nucleome of developing murine rod photoreceptors. bioRxiv, 369702. https://doi.org/10.1101/369702 (2018).
Cherry, T. J. et al. Mapping the cis-regulatory architecture of the human retina reveals noncoding genetic variation in disease. Proc. Natl. Acad. Sci. 117, 9001–9012. https://doi.org/10.1073/pnas.1922501117 (2020).
Reks, S. E., McIlvain, V., Zhuo, X. & Knox, B. E. Cooperative activation of Xenopus rhodopsin transcription by paired-like transcription factors. BMC Mol. Biol. 15, 4. https://doi.org/10.1186/1471-2199-15-4 (2014).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. https://doi.org/10.1093/bioinformatics/bts635 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. https://doi.org/10.1093/bioinformatics/btu638 (2015).
Buenrostro, J. D., Wu, B., Chang, H. Y. & Greenleaf, W. J. ATAC-seq: A method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109, 21–29. https://doi.org/10.1002/0471142727.mb2129s109 (2015).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. https://doi.org/10.1038/nmeth.1923 (2012).
Langmead, B., Wilks, C., Antonescu, V. & Charles, R. Scaling read aligners to hundreds of threads on general-purpose processors. Bioinformatics 35, 421–432. https://doi.org/10.1093/bioinformatics/bty648 (2019).
Acknowledgements
We thank Courtney Linne, Mingyan Yang, Guangyi Ling, Belinda Dana and Dr. Xiaodong Zhang for technical assistance; We also thank Susan Penrose and Mike Casey with the Molecular Genetics Service Core for generating Rho promoter/enhancer knockout mice. This work was supported by NIH grants EY012543 (to SC) and EY002687 (to WU-DOVS), and unrestricted funds from Research to Prevent Blindness (to WU-DOVS).
Author information
Authors and Affiliations
Contributions
S.C. conceived of the study, C.S., P.R., and S.C. designed the experiments, C.S. and P.R. performed experiments and analyzed the results. C.S., P.R., and S.C. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, C., Ruzycki, P.A. & Chen, S. Rho enhancers play unexpectedly minor roles in Rhodopsin transcription and rod cell integrity. Sci Rep 13, 12899 (2023). https://doi.org/10.1038/s41598-023-39979-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39979-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.