Abstract
Numerous studies have reported that circulating cytokines (CCs) are linked to age-related neurodegenerative diseases (ANDDs); however, there is a lack of systematic investigation for the causal association. A two-sample bidirectional Mendelian Randomisation (MR) method was utilized to evaluate the causal effect. We applied genetic variants correlated with concentrations of CCs from a genome-wide association study meta-analysis (n = 8293) as instrumental variables. Summary data of three major ANDDs [Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic lateral sclerosis (ALS)] were identified from the IEU OpenGWAS platform (n = 627, 266). Inverse-variance weighted method is the main approach to analyse causal effect, and MR results are verified by several sensitivity and pleiotropy analyses. In directional MR, it suggested that several CCs were nominally correlated with the risk of ANDDs, with a causal odds ratio (OR) of Interleukin (IL)-5 of 0.909 for AD; OR of IL-2 of 1.169 for PD; and OR of Beta nerve growth factor of 1.142 for ALS). In reverse MR, there were some suggestively causal effects of ANDDs on CCs (AD on increased Basic fibroblast growth factor and IL-12 and decreased Stem cell growth factor beta; PD on decreased Monokine induced by interferon-gamma; ALS on decreased Basic fibroblast growth factor and IL-17). The findings were stable across sensitivity and pleiotropy analyses. However, after Bonferroni correction, there is no statistically significant association between CCs and ANDDs. Through the genetic epidemiological approach, our study assessed the role and presented possible causal associations between CCs and ANDDs. Further studies are warranted to verify the causal associations.
Similar content being viewed by others
Introduction
Age-related neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS), are the leading causes of morbidity, disability, and mortality and impose a considerable social and economic burden worldwide1,2. AD affects approximately 35 million people globally, and it is estimated to triple by 20603,4; PD affects approximately 1% of individuals aged over 65 years, and the incidence is predicted to quadruple by 20405,6; and ALS affects approximately 4.42 per 100,000 individuals worldwide, and a rise in prevalence and incidence is associated with advancing age7,8. As refractory progressive nervous system diseases with various clinical features, they are underlaid by progressive loss of neuronal populations that are susceptible to damage9,10,11,12,13. Numerous studies14,15,16 have been proposed to explain the functional loss of neurons in these age-related neurodegenerative diseases, but the pathophysiologies have not been thoroughly discovered. Owing to the undiscovered pathogenesis, no curable treatments have been developed yet. Thus, there is an imperious need to seek the cause of neuron degeneration.
Nevertheless, the cause is multifactorial, and many crucial components are involved in this process17. Currently, the immune system has been considered a key player linked to the development of age-related neurodegeneration and specifically illuminated for AD, PD, and ALS. Meanwhile, emerging evidence supports a potential role for immunotherapy in the management of disease progression despite the precise mechanisms through which the immune system influences neuron degeneration remaining unclear18. Numerous studies19,20,21 have suggested that circulating cytokines, such as inflammatory-related cytokines, growth factors, and chemokines, as signalling molecules within the immune system, are associated with neuronal degeneration; for instance, overproduction/overusing of circulating pro-inflammatory cytokines [such as Interleukin-1β (IL-1β), IL-6, and Tumour necrosis factor-α]22, anti-inflammatory cytokines (such as IL-1RA, IL-10, and IL-12)23, and several growth factors [such as nerve growth factors and stem cell growth factor (SCGF)]24 could lead a pathophysiology progression. Meanwhile, it could modulate the immune response and may be regarded as a target site for these age-related neurodegenerative diseases prevention and treatments19,20,21. However, the associations between circulating cytokines and age-related neurodegenerative diseases were not explored in depth. Hence, understanding the precise role of circulating cytokines and the risk for age-related neurodegenerative diseases may be beneficial in developing potential prevention, prediction, and treatment targets.
Mendelian Randomization (MR)25, an increasingly widely applied genetic epidemiological tool for a stable and credible deduction of causal relationships, incorporates strong exposure-related genetic instrumental variations (IVs) to assess the causal associations between exposures (e.g., circulating cytokines) and outcomes (e.g., age-related neurodegenerative diseases) to identify inferences about causality for the outcome26. Therefore, this study aimed to analyse the causal associations between 41 circulating cytokines and three age-related neurodegenerative disease types, AD, PD and ALS, by conducting the bidirectional two-sample MR method.
Materials and methods
Study design
In this study, a bidirectional two-sample MR method was implemented to assess the causal effects between concentrations of circulating cytokines and age-related neurodegenerative diseases (AD, PD, and ALS) and improve informing according to Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomisation (STROBE-MR)27,28. The MR design flow chart shown in Fig. 1. To explore the causal effects between circulating cytokines and age-related neurodegenerative diseases, MR analysis was performed to meet the three assumptions as follows: (1) the genetic instruments are strongly correlated with the exposure; (2) the genetic instruments are independent of any potential known confounders; and (3) the genetic instruments-outcome association is mediated only by the exposures. Meanwhile, the reverse MR method was conducted to explore the potential reverse causal effects. All data were retrieved from public and available large-scale genome-wide association studies (GWASs), of which each was an original study approved by the corresponding ethics committees. Informed consent was also obtained in the original studies.
Data sources
To minimize the bias of population, the study only selected the GWAS statistics of European ancestry. The summary statistics for concentrations of 41 circulating cytokines were selected from the largest and latest available GWAS meta-analysis29, which covers 8293 participants from three independent cohort studies (FINRISK 1997, FINRISK 2002, and The Cardiovascular Risk in Young Finns Study). The summary statistics of three age-related neurodegenerative diseases were obtained from GWAS meta-analyses based on the IEU OpenGWAS platform (accessed on 1 October 2022). Summary statistics for AD (ID: ieu-b-2)30, covering 21,982 patients and 41,944 controls, were extracted from the International Genomics of Alzheimer’s Project study; summary statistics for PD (ID: ieu-b-7)31, covering 33,674 patients and 449,056 controls, were derived from the International Parkinson’s Disease Genomics Consortium; summary statistics for ALS (ID: ebi-a-GCST005647)32, covering 20,806 patients and 59,804 controls, were referred from the International Amyotrophic Lateral Sclerosis Genomics Consortium. The details of these GWAS datasets are depicted in Table 1. The details of demographics information regarding datasets about three age-related neurodegenerative diseases in Supplementary Tables 1–3.
Selection of instruments
To assure the validity of the results, the MR analysis was performed following the three steps for quality control to identify instrument variables (IVs): First, single nucleotide polymorphisms (SNPs) remarkably associated with circulating cytokines / age-related neurodegenerative diseases were identified and selected as IVs. In general, the GWAS p-value threshold was set at 5 × 10−8. However, in order to maintain the genetic variance, the number of SNPs and statistical power, in the MR, we relaxed the threshold to 5 × 10−6, which is commonly used in numerous MR studies33,34,35,36 regarding age-related neurodegenerative diseases. Next, the linkage disequilibrium in the selected IVs with R2 threshold of < 0.001 in the distance of ≥ 1000 kilobases was clumped and eliminated using the PLINK algorithm. Third, the F-statistic was estimated to ensure the strength of the genetic instruments. SNPs would be eliminated from MR analysis if F-statistics < 1037. Finally, for circulating cytokine instruments, a total of 354 SNPs were identified; for age-related neurodegenerative diseases, 108 SNPs were included. Detailed summary statistics of these included SNPs are shown in Supplementary Tables 1–6.
Statistical analysis
The inverse-variance weighted (IVW) method38 was considered the primary analysis with the random-effects model to evaluate the causal relationship between the circulating cytokines and age-related neurodegenerative diseases. Additional complementary MR approaches, such as MR-Egger regression and weighted median, were performed to test the robustness of the findings. In addition, Cochran’s Q test and leave-one-out analyses were applied to probe the consistency of the findings. Moreover, a funnel graph was employed to measure the horizontal pleiotropy. Moreover, MR pleiotropy residual sum and outlier (MR-PRESSO) were used to probe and correct for horizontal pleiotropic outliers. All variables were processed with a 95% confidence interval (CI). The causal effects of circulating cytokines on the risk of age-related neurodegenerative diseases were performed using odds ratios (ORs). Meanwhile, the effects of age-related neurodegenerative diseases on the circulating cytokines are displayed as beta. All statistical analyses were carried out using the TwoSample MR and MR-PRESSO packages in R version 4.1.3 software26. In addition, a priori statistical powers of circulating cytokines on age-related neurodegenerative diseases were calculated with type I error rate of 0.05 using https://shiny.cnsgenomics.com/mRnd/39, and the powers of age-related neurodegenerative diseases on circulating cytokines were calculated with significance of 0.05 level using https://sb452.shinyapps.io/power/40. There is suggestive evidence of potential causal effect when the p-value is ≤ 0.05. Moreover, statistically compelling evidence of causality was determined with a p-value of ≤ 0.0004 (0.05/(numbers of circulating cytokines (41) * numbers of age-related neurodegenerative diseases (3)) by multiple testing using the Bonferroni-corrected method41,42.
Ethical approval
This study used the published articles or publicly available GWAS summary data. We did not collect additional raw data, and therefore approval from medical ethical committee is not required. Each study included has been approved by their institutional ethics review committees.
Results
Causal effect of genetically predicted circulating cytokines on age-related neurodegenerative diseases
MR analysis was conducted to investigate the potential causal effects of circulating cytokines on age-related neurodegenerative diseases. Based on the Bonferroni-corrected threshold, there was no statistically significant causal effect of circulating cytokines on age-related neurodegenerative diseases (all p-values > 0.0004). Nevertheless, the results indicated that several circulating cytokines were nominally correlated with age-related neurodegenerative diseases. Using the IVW method, the genetically predicted IL-5 was associated with a lower risk of AD (OR, 0.909; 95% CI 0.832–0.993; p-value = 0.035); IL-2 was associated with a higher risk of PD (OR, 1.169, 95% CI, 1.000–1.368; p-value = 0.05); and beta nerve growth factor (BNGF) was associated with a higher risk of ALS (OR, 1.142, 95% CI 1.017–1.283; p-value = 0.025). Meanwhile, there is suggestive evidence of circulating BNGF levels on ALS risk, as observed by the weighted median method. Detailed results are shown in Fig. 2 and Supplementary Materials (Table 7).
Causal effect of genetically predicted age-related neurodegenerative diseases on circulating cytokines
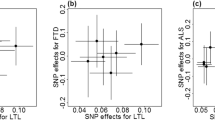
Possible causal effects of age-related neurodegenerative diseases on circulating cytokines were analysed using the reverse MR method. The reverse MR method revealed that age-related neurodegenerative diseases have no significant causal effect on circulating cytokines. Nevertheless, using the IVW method, genetically predicted AD demonstrated a nominally causal effect on basic fibroblast growth factor (bFGF) (β, 0.05; 95% CI 0.021–0.079; p-value = 0.017), in line with findings using the weighted median method and MR-Egger method; and genetically predicted AD demonstrated a nominally causal effect on IL-12 (β = 0.040; 95% CI 0.020 to 0.060; p-value = 0.046). Genetically predicted AD demonstrated a nominally causal effect on SCGFβ (β, − 0.069; 95% CI − 0.100 to − 0.038; p-value = 0.027), in line with the finding using the MR-Egger method; and genetically predicted AD demonstrated a nominally causal effect on IL-12 (β, 0.040; 95% CI 0.020–0.060; p-value = 0.046); genetically predicted PD showed a potential causal effect on Monokine induced by interferon-gamma (MIG: β, − 0.067; 95% CI − 0.098 to − 0.036; p-value = 0.03), in line with the finding using the weighted median method; genetically predicted ALS showed a potential causal effect on bFGF (β, − 0.110; 95% CI − 0.156 to − 0.064; p-value = 0.016), in line with findings using the weighted median method and MR-Egger method; and IL-17 (β, − 0.097; 95% CI − 0.142 to − 0.052; p-value = 0.03), in line with finding using the MR-Egger method. The detailed results are illustrated in Fig. 3 and Supplementary Materials (Table 8).
Associations between genetically predicted age-related neurodegenerative diseases on circulating cytokines; AD, Alzheimer’s disease; PD, Parkinson’s disease; ALS, Amyotrophic lateral sclerosis; bFGF, Basic fibroblast growth factor; IL-12, Interleukin-12; SCGFβ, Stem cell growth factor beta; MIG, Monokine induced by interferon-gamma; IL-17, Interleukin-17.
Sensitivity and pleiotropy analysis
To measure the robustness of these findings, the following sensitivity and pleiotropy analyses were carried out and shown in Tables 2 and 3. For the heterogeneity analysis, little evidence was found using Cochran’s Q test and the leave-one-out method. For the pleiotropy analysis, the funnel graph showed no evidence to hold up directional pleiotropy. Meanwhile, the MR-PRESSO analysis did not show that there was pleiotropy (all p-values > 0.05). Detailed results are shown in Tables 2 and 3 and Supplementary Materials (Tables 7–8, eFigures 1–246).
Discussion
Numerous observational studies have illustrated the association between circulating cytokines and age-related neurodegenerative diseases; however, there are only a few MR studies in this regard. These MR studies usually focused on single age-related neurodegenerative disease43,44,45,46 or implemented unidirectional MR studies47,48. Thus, this is the first study to systematically evaluate the potential causal effects between 41 circulating cytokines and the risk of three major age-related neurodegenerative diseases using a bidirectional two-sample MR approach.
Summary of main findings
In directional MR, findings showed that IL-5 was nominally associated with an decreased risk of AD, IL-2 was nominally associated with an increased risk of PD and BNGF was nominally associated with an increased risk of ALS. In reverse MR, the nominally causal associations of age-related neurodegenerative diseases on circulating cytokines were also detected. AD was nominally associated with increased bFGF and IL-12 and decreased SCGFβ, PD was nominally associated with decreased MIG, and ALS was nominally associated with decreased bFGF and IL-17. The results were stable in sensitivity and pleiotropy analyses.
The associations between age-related neurodegenerative diseases and circulating cytokines
As for age-related neurodegenerative diseases, an impaired immune response was regarded as a relevant pathological factor49. Interleukins, as immune circulating cytokines, play a vital role in neurodegeneration.
The association between AD and IL-5
IL-5, a neuroprotective cytokine50, is produced by Th2 cells and ILC2s51. An observational study illustrated that AD brains showing IL-5 changes were associated with the severity of pathology52. Moreover, IL-5 has been shown to promote neurogenesis, reduce neuroinflammation, and protect neurons from Aβ-induced cell death in aged mice53,54,55. However, few studies have explored the effects of IL-5 as a therapeutic target in AD56. In our study, IL-5 was associated with a decreased risk of AD and was identified to have a nominally causal effect on AD, which is comparable to previous research and suggested it as a potential therapeutic target for AD.
The association between AD and IL-12
IL-12, a heterodimeric pro-inflammatory cytokine, is produced by activated monocytes, glial cells, and macrophages57. Evidence showed that IL-12 might be associated with AD and cognitive ageing58. Several observational studies49,58,59 and a meta-analysis60 found that the level of IL-12 in cerebrospinal fluid/serum was elevated in AD. Meanwhile, in a neuroimaging study, IL-12 was found to be correlated with default mode network functional connectivity of the brain61. In the present study, AD was nominally associated with increased IL-12, which was similar to the above studies.
The association between PD and IL-2
IL-2, an immunoregulatory cytokine, is produced by CD4 + helper T cells62. IL-2 has been considered a regulator of brain neuronal function in PD63. Several studies64,65,66 found that the level of IL-2 in blood was elevated in PD. In addition, it is a modulator of dopamine activity in the brain of PD67. In our study, IL-2 was found to be nominally associated with an increased risk of PD.
The association between ALS and IL-17
IL-17, a pro-inflammatory cytokine, is produced by T helper 17 cells, CD8 + T cells, innate lymphoid cells, and the like68. Numerous studies69,70,71,72 found that the level of IL-17 in cerebrospinal fluid/blood was significantly increased in ALS. In the present study, ALS was nominally associated with decreased IL-17. Thus, the level of IL-17 could act as a potential marker in ALS.
The associations between age-related neurodegenerative diseases and circulating growth factors
Meanwhile, growth factors, chemokines and the like, as circulating cytokines, are also a crucial part of regulation of the immune system.
The association between ALS and BNGF
BNGF is a necessary growth factor for the survival and maintenance of neurons73. The abnormal level of nerve growth factor was considered a possible cause of ALS74. An observational study showed that the expression of plasma BNGF was associated with disease duration75. Moreover, a clinical study found that NGF plus riluzole treatment is a possible treatment76. In addition, a study found that NGF could induce the death of motor neurons77. In our study, BNGF was found to be associated with an increased risk of ALS and may be a potential therapeutic target for ALS.
The association between AD and SCGFβ
SCGFβ, a secreted sulfated glycoprotein, is produced by primitive haematopoietic progenitor cells78. Current evidence suggests that SCGFβ is associated with amyloid deposition in AD79. In addition, SCGFβ was considered a biomarker in the diagnosis and prognosis80. In the present study, AD was nominally associated with decreased SCGFβ, which was in accordance with the above studies.
The association between AD and bFGF
bFGF, a heparin-binding growth factor, is produced by bone marrow stromal cells81. It is characterized by neuroprotective and neurite growth activity82. An observational study83 found that the level of bFGF was increased in the brains of AD patients. Moreover, it is associated with neurotic plaques and neurofibrillary tangles83,84. In the present study, AD was nominally associated with increased bFGF, which was in accordance with the above studies.
The association between ALS and bFGF
Meanwhile, the level of these circulating cytokines was suggested as a potential biomarker. In addition, bFGF was also correlated with ALS. Some observational studies85,86 found that the level of bFGF was changed in the cerebrospinal fluid/blood of ALS patients. Meanwhile, a cross-sectional study87 illustrated that bFGF protein levels had a significant negative correlation with ALS function. In the current study, ALS was found to be nominally associated with decreased bFGF. Therefore, the level of bFGF was considered a helpful biomarker that could predict disease progression in ALS.
The associations between age-related neurodegenerative diseases and circulating chemokine
MIG, a CXC chemokine, is positive for activated T cells88. Only one study89 found that the level of MIG in the substantia nigra was significantly changed in PD. In the present study, PD was nominally associated with decreased MIG. Thus, the level of MIG may be a potential marker in PD.
Strengths and weaknesses
There are some strengths in the present study. First, for the MR study, the utilised statistical data were accessed from relatively up-to-date largest GWASs, which could improve the stability and accuracy of effect estimates. Second, the bidirectional MR design is aimed at reducing confounding by potential influencing elements and avoiding any reverse causality. Third, the three major age-related neurodegenerative diseases and 41 circulating cytokines were presented in the current study, which made it the most comprehensive MR study of age-related neurodegenerative diseases and circulating cytokines. Finally, discovering potential causality may influence public health policies about the diagnosis, prevention, prediction, and potential medical targets for age-related neurodegenerative diseases. The GWAS statistic included all with European ancestry, minimising the probability of bias by region and increasing the credibility and rationality of MR assumptions.
Despite the advantages of the MR design, this study has several limitations. First, we used the GWAS summary statistics in the present study with European ancestry to reduce the population bias, which may be a barrier in the application of these findings to other ethnicities. Second, to support adequate statistical power in MR, we relaxed the p-value threshold, which means the variance ratio introduced by the correlations between exposures and IVs might be relatively small. Even though F-statistics showed that weak IVs do not exist, data from more studies with large and universal samples could supply a more credible estimation of genetic impacts on exposure. In addition, the GWAS method is a significant contributor to the genetic risk factor, however, the detail was not given for the original dataset. Next, the statistical power may be deficient of circulating cytokines with a limited sample size, and therefore the MR may have overlooked potential weak associations. Finally, all p-values ranged as nominal levels (0.008 to 0.05), although the findings failed validation in the clinical and basic research. Thus, the potential causal associations should be interpreted cautiously and still need to be investigated for potential mechanisms.
Conclusion
This MR research thoroughly examines, supports, and provides new findings regarding the potential causal relationship evidence between circulating cytokines and age-related neurodegenerative diseases. Nevertheless, there is no statistically compelling evidence regarding causal associations between them. Further studies are supposed to ensure the causal associations.
Data availability
All data used in the study were obtained from published articles or publicly available GWAS platform, and all data can be obtained for free.
References
Heemels, M. T. Neurodegenerative diseases. Nature 539(7628), 179 (2016).
Berson, A., Nativio, R., Berger, S. L. & Bonini, N. M. Epigenetic regulation in neurodegenerative diseases. Trends Neurosci. 41(9), 587–598 (2018).
Rabbito, A., Dulewicz, M., Kulczyńska-Przybik, A. & Mroczko, B. Biochemical markers in Alzheimer’s disease. Int. J. Mol. Sci. 21(6), 1989 (2020).
Gaugler, J. et al. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18(4), 700–789 (2022).
Bastide, M. F. et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 132, 96–168 (2015).
Melo, A. et al. Oxidative stress in neurodegenerative diseases: Mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longev. 2011, 467180 (2011).
Xu, L. et al. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. 267(4), 944–953 (2020).
Xu, L. et al. Incidence and prevalence of amyotrophic lateral sclerosis in urban China: A national population-based study. J. Neurol. Neurosurg. Psychiatry 91(5), 520–525 (2020).
Li, D. & Liu, C. Conformational strains of pathogenic amyloid proteins in neurodegenerative diseases. Nat. Rev. Neurosci. 23(9), 523–534 (2022).
Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. https://doi.org/10.1038/s41591-021-01382-x (2021).
Dugger, B. N. & Dickson, D. W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 9(7), a028035 (2017).
Stephenson, J., Nutma, E., van der Valk, P. & Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 154(2), 204–219 (2018).
Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 19(1), 206 (2022).
Rhinn, H., Tatton, N., McCaughey, S., Kurnellas, M. & Rosenthal, A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol. Sci. 43(8), 641–652 (2022).
Sivandzade, F. & Cucullo, L. Regenerative stem cell therapy for neurodegenerative diseases: An overview. Int. J. Mol. Sci. 22(4), 2153 (2021).
Subhramanyam, C. S., Wang, C., Hu, Q. & Dheen, S. T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 94, 112–120 (2019).
Baltazar, M. T. et al. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases: A mechanistic approach. Toxicol. Lett. 230(2), 85–103 (2014).
Mortada, I. et al. Immunotherapies for neurodegenerative diseases. Front. Neurol. 12, 654739 (2021).
Khosravi, N., Stoner, L., Farajivafa, V. & Hanson, E. D. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain Behav. Immun. 81, 92–104 (2019).
Lim, S. Y. et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin. Cancer Res. 25(5), 1557–1563 (2019).
Bendorius, M., Po, C., Muller, S. & Jeltsch-David, H. From systemic inflammation to neuroinflammation: The case of neurolupus. Int. J. Mol. Sci. 19(11), 3588 (2018).
Van Eldik, L. J., Thompson, W. L., Ralay, R. H., Behanna, H. A. & Martin, W. D. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: Function-based and target-based discovery approaches. Int. Rev. Neurobiol. 82, 277–296 (2007).
Sabatino, J. J., Pröbstel, A. K. & Zamvil, S. S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 20(12), 728–745 (2019).
Lindsay, R. M. Neurotrophic growth factors and neurodegenerative diseases: Therapeutic potential of the neurotrophins and ciliary neurotrophic factor. Neurobiol. Aging 15(2), 249–251 (1994).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. JAMA 318(19), 1925–1926 (2017).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey, S. G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27(8), 1133–1163 (2008).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement. JAMA 326(16), 1614–1621 (2021).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 375, n2233 (2021).
Ahola-Olli, A. V. et al. Genome-wide association study identifies 27 loci influencing concentrations of circulating cytokines and growth factors. Am. J. Hum. Genet. 100(1), 40–50 (2017).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51(3), 414–430 (2019).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 18(12), 1091–1102 (2019).
Nicolas, A. et al. Genome-wide analyses identify KIF5A as a novel ALS gene. Neuron 97(6), 1268–1283 (2018).
Li, R. et al. Genetically predicted circulating levels of glycine, glutamate, and serotonin in relation to the risks of three major neurodegenerative diseases: A Mendelian randomization analysis. Front. Aging Neurosci. 14, 938408 (2022).
Cui, G. et al. Are neurodegenerative diseases associated with an increased risk of inflammatory bowel disease? A two-sample Mendelian randomization study. Front. Immunol. 13, 956005 (2022).
Cullell, N. et al. Sleep/wake cycle alterations as a cause of neurodegenerative diseases: A Mendelian randomization study. Neurobiol. Aging 106, 320–321 (2021).
Yi, M. et al. Causal analysis between altered levels of interleukins and obstructive sleep apnea. Front Immunol. 13, 888644 (2022).
Pierce, B. L., Ahsan, H. & Vanderweele, T. J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40(3), 740–752 (2011).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37(7), 658–665 (2013).
Deng, L., Zhang, H. & Yu, K. Power calculation for the general two-sample Mendelian randomization analysis. Genet. Epidemiol. 44(3), 290–299 (2020).
Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 43(3), 922–929 (2014).
Zhang, Z., Fang, T. & Lv, Y. Causal associations between thyroid dysfunction and COVID-19 susceptibility and severity: A bidirectional Mendelian randomization study. Front. Endocrinol. (Lausanne) 13, 961717 (2022).
Wu, F., Huang, Y., Hu, J. & Shao, Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 18(1), 312 (2020).
Bottigliengo, D. et al. A Mendelian randomization study investigating the causal role of inflammation on Parkinson’s disease. Brain 145(10), 3444–3453 (2022).
Pagoni, P. et al. Causal effects of circulating cytokine concentrations on risk of Alzheimer’s disease and cognitive function. Brain Behav. Immun. 104, 54–64 (2022).
Yeung, C. & Schooling, C. M. Systemic inflammatory regulators and risk of Alzheimer’s disease: A bidirectional Mendelian-randomization study. Int. J. Epidemiol. 50(3), 829–840 (2021).
Yuan, S., Roos, P. M. & Larsson, S. C. Interleukin-1 receptor antagonist, interleukin-2 receptor alpha subunit and amyotrophic lateral sclerosis. Eur. J. Neurol. 27(10), 1913–1917 (2020).
Zhao, Y. et al. Genetically predicted levels of circulating inflammatory cytokines and the risk and age at onset of Parkinson’s disease: A two-sample Mendelian randomization study. Front. Aging Neurosci. 14, 811059 (2022).
Tsui, A. & Davis, D. Systemic inflammation and causal risk for Alzheimer’s dementia: Possibilities and limitations of a Mendelian randomization approach. Aging Med. (Milton) 1(3), 249–253 (2018).
Richartz, E. et al. Decline of immune responsiveness: A pathogenetic factor in Alzheimer’s disease?. J. Psychiatr. Res. 39(5), 535–543 (2005).
Merriwether, E. N. et al. IL-5 mediates monocyte phenotype and pain outcomes in fibromyalgia. Pain 162(5), 1468–1482 (2021).
Nagase, H., Ueki, S. & Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 69(2), 178–186 (2020).
Wood, L. B. et al. Identification of neurotoxic cytokines by profiling Alzheimer’s disease tissues and neuron culture viability screening. Sci. Rep. 5, 16622 (2015).
Lopez, A. F. et al. Molecular basis of cytokine receptor activation. IUBMB Life 62(7), 509–518 (2010).
Fung, I. et al. Activation of group 2 innate lymphoid cells alleviates aging-associated cognitive decline. J Exp. Med. https://doi.org/10.1084/jem.20190915 (2020).
Tennakoon, A. et al. Normal aging, motor neurone disease, and Alzheimer’s disease are characterized by cortical changes in inflammatory cytokines. J. Neurosci. Res. 100(2), 653–669 (2022).
Fung, I. et al. Group 2 innate lymphoid cells are numerically and functionally deficient in the triple transgenic mouse model of Alzheimer’s disease. J. Neuroinflamm. 18(1), 152 (2021).
Rentzos, M. et al. Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J. Neurol. Sci. 249(2), 110–114 (2006).
Tan, M. S. et al. IL12/23 p40 inhibition ameliorates Alzheimer’s disease-associated neuropathology and spatial memory in SAMP8 mice. J. Alzheimers Dis. 38(3), 633–646 (2014).
Vom, B. J. et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 18(12), 1812–1819 (2012).
Su, C., Zhao, K., Xia, H. & Xu, Y. Peripheral inflammatory biomarkers in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Psychogeriatrics 19(4), 300–309 (2019).
Magalhães, T. et al. Systemic inflammation and multimodal biomarkers in amnestic mild cognitive impairment and Alzheimer’s disease. Mol. Neurobiol. 55(7), 5689–5697 (2018).
Orozco, V. A., Camargo, K. M., Suavinho, F. E. & Antonio, S. M. Interleukin-2 as immunotherapeutic in the autoimmune diseases. Int. Immunopharmacol. 81, 106296 (2020).
Petitto, J. M., McCarthy, D. B., Rinker, C. M., Huang, Z. & Getty, T. Modulation of behavioral and neurochemical measures of forebrain dopamine function in mice by species-specific interleukin-2. J. Neuroimmunol. 73(1–2), 183–190 (1997).
Stypuła, G., Kunert-Radek, J., Stepień, H., Zylińska, K. & Pawlikowski, M. Evaluation of interleukins, ACTH, cortisol and prolactin concentrations in the blood of patients with Parkinson’s disease. NeuroImmunoModulation 3(2–3), 131–134 (1996).
Qin, X. Y., Zhang, S. P., Cao, C., Loh, Y. P. & Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 73(11), 1316–1324 (2016).
Brodacki, B. et al. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 441(2), 158–162 (2008).
Zalcman, S. S. Interleukin-2-induced increases in climbing behavior: Inhibition by dopamine D-1 and D-2 receptor antagonists. Brain Res. 944(1–2), 157–164 (2002).
Berry, S. et al. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int. Immunopharmacol. 102, 108402 (2022).
Jin, M. et al. Interleukin-17 and Th17 lymphocytes directly impair motoneuron survival of wildtype and FUS-ALS mutant human iPSCs. Int. J. Mol. Sci. 22(15), 8042 (2021).
Chen, X., Hu, Y., Cao, Z., Liu, Q. & Cheng, Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front. Immunol. 9, 2122 (2018).
Furukawa, T. et al. CSF cytokine profile distinguishes multifocal motor neuropathy from progressive muscular atrophy. Neurol. Neuroimmunol. Neuroinflamm. 2(5), e138 (2015).
Rentzos, M. et al. Interleukin-17 and interleukin-23 are elevated in serum and cerebrospinal fluid of patients with ALS: A reflection of Th17 cells activation?. Acta Neurol. Scand. 122(6), 425–429 (2010).
Shelton, D. L. & Reichardt, L. F. Studies on the regulation of beta-nerve growth factor gene expression in the rat iris: The level of mRNA-encoding nerve growth factor is increased in irises placed in explant cultures in vitro, but not in irises deprived of sensory or sympathetic innervation in vivo. J. Cell Biol. 102(5), 1940–1948 (1986).
Milonas, I. Amyotrophic lateral sclerosis: An introduction. J. Neurol. 245(Suppl 2), S1–S3 (1998).
Ngo, S. T. et al. Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 357(1–2), 22–27 (2015).
Li, J. T., Dong, S. Q., Qian, T., Yang, W. B. & Chen, X. J. Mouse nerve growth factor injection and progression rate in patients with amyotrophic lateral sclerosis: An observational study. Front. Neurol. 13, 829569 (2022).
Pehar, M. et al. Astrocytic production of nerve growth factor in motor neuron apoptosis: Implications for amyotrophic lateral sclerosis. J. Neurochem. 89(2), 464–473 (2004).
Ito, C. et al. Serum stem cell growth factor for monitoring hematopoietic recovery following stem cell transplantation. Bone Marrow Transplant. 32(4), 391–398 (2003).
Abe, Y. et al. Relationship between cytokine levels in the cerebrospinal fluid and 11C-Pittsburgh compound B retention in patients with mild cognitive impairment. Geriatr. Gerontol. Int. 17(11), 1907–1913 (2017).
Sukowati, C. et al. Serum stem cell growth factor beta for the prediction of therapy response in hepatocellular carcinoma. Biomed. Res. Int. 2018, 6435482 (2018).
Bruno, E., Cooper, R. J., Wilson, E. L., Gabrilove, J. L. & Hoffman, R. Basic fibroblast growth factor promotes the proliferation of human megakaryocyte progenitor cells. Blood 82(2), 430–435 (1993).
Ogino, R. et al. SUN11602 has basic fibroblast growth factor-like activity and attenuates neuronal damage and cognitive deficits in a rat model of Alzheimer’s disease induced by amyloid β and excitatory amino acids. Brain Res. 1585, 159–166 (2014).
Stopa, E. G. et al. Basic fibroblast growth factor in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 171(2), 690–696 (1990).
Siedlak, S. L., Cras, P., Kawai, M., Richey, P. & Perry, G. Basic fibroblast growth factor binding is a marker for extracellular neurofibrillary tangles in Alzheimer disease. J. Histochem. Cytochem. 39(7), 899–904 (1991).
Guo, J., Yang, X., Gao, L. & Zang, D. Evaluating the levels of CSF and serum factors in ALS. Brain Behav. 7(3), e637 (2017).
Gong, Z., Gao, L., Guo, J., Lu, Y. & Zang, D. bFGF in the CSF and serum of sALS patients. Acta Neurol. Scand. 132(3), 171–178 (2015).
Koh, S. H. et al. The functional deficiency of bone marrow mesenchymal stromal cells in ALS patients is proportional to disease progression rate. Exp. Neurol. 233(1), 472–480 (2012).
Sgadari, C. et al. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood 89(8), 2635–2643 (1997).
Walker, D. G. et al. Altered expression patterns of inflammation-associated and trophic molecules in substantia nigra and striatum brain samples from Parkinson’s disease, incidental Lewy body disease and normal control cases. Front. Neurosci. 9, 507 (2015).
Acknowledgements
We are grateful to the Ahola-Olli’s GWAS meta-analysis, FINRISK 1997, FINRISK 2002, and the Cardiovascular Risk in Young Finns Study. And we thank the International Genomics of Alzheimer’s Project study, International Parkinson's Disease Genomics Consortium, International Amyotrophic Lateral Sclerosis Genomics Consortium for providing summary data of these analyses. In addition, thanks to Xu Zhang of Youhe AI and Medicine.
Funding
This work was financially supported by the State Administration of Traditional Chinese Medicine, National key research and development program of China (No. 2019YFC1709700), the National Natural Science Foundation of China (Nos. 81590951, 81722050, 81973961) and the Project of Science and Technology Department of Sichuan Province (Nos. 20ZDYF1199 and 2019YFS0081).
Author information
Authors and Affiliations
Contributions
Z.Y.: Conceptualization, Data curation, Formal analysis, Writing—original draft. J.C.: Supervision, Writing—original draft. M.X.: Supervision, Formal analysis. X.Z.: Data curation, Investigation. Y.L.: Formal analysis. Z.C.: Investigation. Q.B.: methodology. W.Z.: methodology. J.Y.: methodology. K.W.: methodology. L.Z.: Conceptualization, Writing—review and editing, Funding acquisition. F.L.: Conceptualization, Writing—review and editing, Funding acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, Z., Chen, J., Xia, M. et al. Assessing causal relationship between circulating cytokines and age-related neurodegenerative diseases: a bidirectional two-sample Mendelian randomization analysis. Sci Rep 13, 12325 (2023). https://doi.org/10.1038/s41598-023-39520-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39520-9
This article is cited by
-
Association between sugar-sweetened beverages and pure fruit juice with risk of six cardiovascular diseases: a Mendelian randomization study
European Journal of Clinical Nutrition (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.