Abstract
The demand for recombinant proteins is rising dramatically, and effective production systems are currently being developed. The production of recombinant proteins in plants is a promising approach due to its low cost and low risk of contamination of the proteins with endotoxins or infectious agents from the culture serum. Plant seeds primarily accumulate seed storage proteins (SSPs), which are transcribed and translated from a few genes; therefore, the mechanism underlying SSP accumulation has been studied to help devise ways to increase recombinant protein production. We found that the 3’UTR of SSP genes are essential for SSP accumulation and can be used in the production of recombinant proteins in Arabidopsis. Fusion of the 3’UTR of SSP genes to the 3’ ends of DNA sequences encoding recombinant proteins enables massive accumulation of recombinant proteins with enzymatic activity in Arabidopsis seeds. This method is also applicable to the production of human Interferon Lambda-3 (IFN-lambda 3), a candidate biopharmaceutical compound against hepatitis C infection. Considering the low cost and ease of protein production in Arabidopsis, as well as the rapid growth of this plant, our method is useful for large-scale preparation of recombinant proteins for both academic research and biopharmaceutical production.
Similar content being viewed by others
Introduction
Plant seeds accumulate large amounts of storage products to supply nutrients for germination and post-germinative growth. Seeds accumulate large amounts of starch and oils as energy sources for proteins as a source of amino acids. In plants, the protein content is much higher in seeds than in roots, leaves, stems, or other organs. Although high levels of proteins are present in seeds, there are only a limited number of protein species, which are referred to as seed storage proteins (SSPs). SSPs comprise the major proportion of proteins in seeds1, thus determining various properties, such as the nutritional value and processing characteristics of seeds2,3. Therefore, breeders have focused on SSP contents in crop seeds. Studies on SSPs have also helped to elucidate the intracellular mechanisms of protein transport and accumulation. Genetic studies in Arabidopsis, maize, rice, and so on have revealed many components of the SSP biosynthetic pathway4,5.
SSP biosynthesis has drawn increasing attention since the demand for biopharmaceutical proteins is rising dramatically6,7,8. Seed-based heterogenous protein production systems have been improved using information about the mechanisms underlying SSP transport and accumulation9,10,11,12,13. In fact, dozens of reports describe the production of biopharmaceutical proteins in seeds of various plant species. Interleukin-7, a cytokine involved in the regulation of lymphoid homeostasis, was produced in rice seeds14. Interleukin-10, a candidate biopharmaceutical for treating inflammatory allergy and autoimmune diseases, was produced in rice and Arabidopsis seeds12,15,16. These studies demonstrate that seed-based biopharmaceutical production systems are promising due to their low cost and high quality. However, these studies also raise the issue of low yields of biopharmaceutical proteins. SSPs are the predominant proteins produced in seeds, whereas the yields of biopharmaceutical proteins in seed-based systems are, in most cases, considerably smaller than those of SSPs. However, the key factors underlying the predominant accumulation of SSPs remain unclear.
In rice seeds, the 3' untranslated region (3’ UTR) has been reported to play a critical role in post-transcriptional regulation17. The SSPs in rice seeds, prolamine and glutelin, are known to be localized to the protein body ER and cisternal ER, respectively, and mRNA is correctly transported to the protein body ER or the cisternal ER. It has been reported that the localization of mRNAs encoding rice prolamine and glutelin is regulated by the 3'UTR18,19. In addition, studies on the synthesis and accumulation of useful recombinant proteins in rice seeds are promising, and the localization of recombinant proteins to the protein body ER has been shown to be an important strategy12,13,20. On the other hand, it has been reported that the 3'UTR itself is not effective in enhancing the accumulation of recombinant proteins in rice seeds21, and the function of the 3'UTR remains unclear.
In this study, we report that the 3’UTRs of SSP genes are a key factor in determining the predominant accumulation of SSPs. We also show that protein yield can be dramatically increased by fusing the 3’UTR of SSPs genes to any genes of interest. This method enables the production of biopharmaceuticals efficiently by changing them as a major proportion of proteins in the seeds.
Results
Transcripts of SSP genes are abundant but not dominant in seeds
Seeds predominantly accumulate a limited number of SSPs, and 80% of the total protein in Arabidopsis thaliana seeds consists of 12S globulin encoded by the 12S1, 12S3, and 12S4 genes and 2S albumin encoded by the 2S3 gene22,23. However, the mechanisms that regulate the predominant accumulation of SSPs remain unclear. To determine what drives the accumulation of SSPs, we focused on 12S globulins, the most abundant protein in Arabidopsis seeds, comprising approximately 70% of total proteins22. The single knockout mutants of 12S1, 12S2, 12S3 or 12S4 gene were established (Fig. 1A) and double knockout mutants of both 12S1 and 12S3 or both 12S1 and 12S4 were produced by crossing. It confirmed that 12S1, 12S3, and 12S4 are the major proteins that accumulate in the wild-type plant, and that their accumulation was dramatically reduced in each knockout mutant (Fig. 1B). It is reported that the accumulation of 12S2 in seeds was minor22, and the SDS-PAGE analysis of seed protein in established 12S2 T-DNA mutants was similar to that of the wild-type plants (Fig. 1B). To compare the transcript level of SSPs and non-SSPs, we measured the transcript levels in developing seeds. The levels of 12S1, 12S3 and 12S4 transcripts relative to that of UBQ10 were 3.76, 0.30 and 0.97, respectively, in developing wild-type (WT) seeds at 15 days after flowering (DAF) (Table 1). Although the transcripts were relatively abundant in developing seeds, their levels were comparable to those of ISOCITRATE LYASE (ICL) and LATE EMBRYOGENESIS ABUNDANT 1 (LEA1), which encode proteins with lower levels of accumulation in seeds (Table 1). These results demonstrate that there is a low correlation between the levels of transcripts and proteins, suggesting that the predominant accumulation of SSPs may result from post-transcriptional regulation.
Establishment of single and double knockout lines of SSPs. (a) Schematic representation of the T-DNA insertion positions in 12s1, 12s2, 12s3, and 12s4 genes. White and black boxes indicate the untranslated regions and the coding regions, respectively. Triangles show the position of T-DNA insertion in the knockout lines. Arrows show the direction of transcription. (b) SDS-PAGE analysis of seed proteins in the single and double knockout lines. Total protein was extracted using SDS sample buffer containing 10% (v/v) 2-mercaptoethanol. Total protein (10 µg) was loaded onto each well of a 12.5% polyacrylamide gel and protein bands were visualized by CBB staining. Arrows indicate the subunits of 12S globulins, which were separated using a reducing agent in the buffer. 12s1.3 and 12s1.4 are the knockout mutants of both 12S1 and 12S3, and 12S1 and 12S4, respectively.
3’UTRs of SSP genes are essential for their predominant accumulation
During seed development, the central vacuole rapidly disappears and protein storage vacuoles appear. Proteolytic activity is much lower in seeds than in other tissues, suggesting that low protein degradation is a reason for the accumulation of SSPs at a high level. Additionally, we hypothesized that the accumulation of SSPs is post-transcriptionally controlled because the transcripts of SSPs are comparable to those of low abundant proteins in seeds. We focused on the UTRs of SSP transcripts and investigated their role in protein accumulation. We prepared the coding sequence of 12S1 (12S1CDS), the coding sequence fused with the 5’UTR of 12S1 (5’-12S1CDS), the coding sequence fused with the 3’UTR of 12S1 (12S1CDS-3’), and the coding sequence fused with both the 5’UTR and 3’UTR of 12S1 (5’-12S1CDS-3’), and introduced these sequences (under the control of the 12S1 promoter) into 12s1-knockout plants (Fig. 2A). Accumulation of the 12S1α and β subunits was fully recovered in transgenic plants harboring 12S1CDS-3’ and 5’-12S1CDS-3’, but was not recovered in 12S1CDS or 5’-12S1CDS plants (Fig. 2B). Expression of 12S1 was recovered in all transgenic plants (Fig. S1). These results indicate that the 3’UTR of 12S1 is essential for the predominant accumulation of 12S1 protein in Arabidopsis seeds. Additionally, we investigated whether the 3’UTRs of other SSP genes (12S3, 12S4) play a similar role. In addition, we introduced 12S1CDS fused with the 3’UTR of an SSP gene or the 3’UTR of isocitrate lyase (ICL), a non-SSP gene, under the control of the 12S1 promoter into 12s1-knockout plants (Fig. 3A). Introduction of 12S1CDS-3′12S3 or 12S1CDS-3′12S4 resulted in the recovery of 12S1 protein accumulation in 12s1-knockout plants, while introduction of 12S1CDS-3’ICL did not (Fig. 3B). These results demonstrate that the 3’UTRs of SSP genes specifically function as a determinant for the predominant accumulation of SSPs.
The 3’UTR is essential for 12S1 protein accumulation in Arabidopsis seeds. (a) Schematic representation of constructs of 12S1 with and without the 5’UTR and/or 3’UTR. 12S1CDS indicates the coding sequence of 12S1; 5’ indicates the 5’UTR of 12S1; 3’ indicates the 3’UTR of 12S1; NOSter indicates the nopaline synthase terminator. (b) CBB staining of seed proteins from two independent transgenic lines of 12S1CDS/12s1, 5’-12S1CDS1/12s1, 12S1CDS-3’/12s1, and 5’-12S1CDS-3’/12s1. Total protein was extracted using SDS sample buffer containing 10% (v/v) 2-mercaptoethanol. Total protein (10 µg) was loaded onto each well of a 12.5% polyacrylamide gel and protein bands were visualized by CBB staining. The 12S1 protein was separated into alpha (12S1α, closed arrowhead) and beta (12S1β, open arrowhead) subunits using a reducing agent in the buffer. 12s1 is the knockout mutant of the 12S1 gene.
Fusion with the 3’UTRs of SSPs enables massive accumulation of recombinant pMDH1 protein
To verify whether the control of protein accumulation by the 3’UTRs of SSPs applies to other proteins, we fused the 3’UTR of 12S1 to non-SSPs. In addition, reducing endogenous SSP levels increases the accumulation of exogenous recombinant proteins in rice12 and Arabidopsis11, we generated a 12S1 and 12S4 (12s1.4) double knockout line (Fig. 1B) for use in subsequent experiments. We introduced pMDH1, encoding peroxisomal malate dehydrogenase 1 in Arabidopsis thaliana, with or without the 3’UTR of 12S1 at its 3’ end (pMDH1CDS-3’UTR12S1 and pMDH1CDS, respectively) under the control of the 12S1 promoter into 12S1.4 (Fig. 4A). Gene expression of pMDH1 was confirmed in developing seeds of both pMDH1CDS/12s1.4 and pMDH1-3’UTR12S1/12s1.4 plants (Fig. S2). In the seeds of pMDH1CDS-3’UTR12S1/12s1.4 plants, pMDH1, like SSPs, represented a major protein, whereas this protein was not detected in pMDH1CDS/12s1.4 seeds (Fig. 4B). In the seeds of pMDH1-3’UTR12S1/12s1.4 plants, pMDH1 represented approximately 40% of the total protein content. We measured the enzymatic activity of MDH in the seeds of WT, 12s1.4, pMDH1CDS/12s1.4, and pMDH1-3’UTR12S1/12s1.4 plants. The MDH activity of pMDH1CDS/12s1.4 seeds was eightfold higher than that of WT seeds, while the activity of pMDH1CDS-3’UTR12S1/12s1.4 seeds was approximately 100-fold higher than that of pMDH1CDS/12s1.4 seeds (Fig. 4C). These results demonstrate that fusion of the 3’UTR of 12S1 greatly enhances the accumulation of recombinant proteins. Fig. S3 showed GFP accumulation in another example of recombinant protein accumulation. Significant GFP accumulation and high intensity of fluorescence were observed only when the 3' UTR of 12S1 is fused. These results indicate that the enhancement of recombinant protein accumulation by fusion of the 3' UTR of 12S1 is independent of the origin of the gene encoding the recombinant protein or the protein tag sequence for purification and labeling. Moreover, the MDH activity remained consistent in seeds stored at room temperature for at least 90 days (Fig. 4D). These results demonstrate that fusion of the 3’UTR of 12S1 enables massive accumulation of pMDH1, a non-SSP, in dry seeds, and that this protein maintains its enzymatic activity. Moreover, it is reported that the subcellular localization of pMDH1 was peroxisomal24. Indeed, immunoelectron microscopic analysis also revealed that pMDH1 stained by anti-pMDH1 antibody-conjugated 15 nm gold particles co-localized with catalase, a marker protein for peroxisomes (which were stained by anti-catalase antibody-conjugated 25 nm gold particles; Fig. 4E). These results indicate that pMDH1 localized to the peroxisomes in seeds of pMDH1CDS-3’UTR12S1/12s1.4. The results also suggest that fusion of the 3’UTRs of SSPs is a useful technique for the production of massive amounts of recombinant protein with the correct intracellular localization and activity.
Massive accumulation of pMDH1 protein using the 3’UTR of 12S1. (a) Schematic representation of pMDH1CDS fused with or without 3′12S1. pMDH1CDS represents the coding sequence of the pMDH1 gene. (b) CBB staining of seed proteins from four independent transgenic lines of pMDH1CDS/12s1.4 and pMDH1CDS-3′12S1/12s1.4. 12s1.4 is the knockout mutant of both 12S1 and 12S4. The arrowhead indicates pMDH1 bands. (c) MDH activity in seeds of the transgenic lines. The activity of MDH/Total soluble protein (TSP) is expressed relative to the activity of WT. Values are presented as the mean ± SE of four independent experiments. (d) Enzymatic activity of pMDH1 in seeds stored at room temperature. Seeds of 12s1.4 and pMDH1-3′12S1/12s1.4 were collected in 15 ml plastic tubes and stored at room temperature (22–28 °C) under dark conditions. Values are the mean ± SE of four independent experiments. (e) Immunoelectron micrograph of pMDH1CDS-3′12S1/12s1.4 seeds. Localizations of catalase and pMDH1 are indicated by anti-catalase antibody-conjugated 25 nm gold particles (open arrowheads) and anti-pMDH1 antibody-conjugated 15 nm gold particles (closed arrowheads). OB, oil body; PB, protein body. Scale bar, 1.0 µm.
Fusion of the 3’UTRs of SSPs enables massive production of human IFN lambda-3 in Arabidopsis seeds
Biopharmaceuticals against cancer and infectious diseases, such as interferons, are needed worldwide. Plant-based protein production methods represent a promising approach to biopharmaceutical production due to the low risk of endotoxin or infectious agent contamination from serum and the low production costs. To confirm that our method is applicable to the production of interferons, we produced human IFN lambda-3, a candidate biopharmaceutical agent for treating hepatitis C infection25,26, in Arabidopsis seeds by fusing this gene with the 3’UTR of 12S1. Based on previous reports15,16,27, an endoplasmic reticulum (ER)-retention signal (HDEL) was fused to the C-terminal end of IFN lambda-3BCDS (Fig. 5A). IFN lambda-3 accumulated to high levels in seeds of the transgenic plants (Fig. 5B,C), representing approximately 14% of the total protein content in seeds. The activity of IFN lambda-3 was measured in a luciferase assay using HepG2 cells derived from human liver. The HepG2 cells were cultured for 24 h in a medium containing 100 ng/ml of IFN lambda-3 produced with Arabidopsis seeds, IFN lambda-3 produced with human embryonic kidney cells or BSA. IFN lambda-3 purified from Arabidopsis seeds had activity levels similar to that of IFN lambda-3 produced in human cells (Fig. 5D).
Production of IFN lambda-3 in Arabidopsis seeds. (a) Schematic representation of recombinant IFN lambda-3 fused with 3′12S1. IFN lambda-3CDS represents the coding sequence of the IFN lambda-3 gene. 6 × His-HDEL represents the ER-retention signal (HDEL) fused with a 6 × Histidine tag. (b) CBB staining of seed proteins from WT, 12s1.4, and four independent transgenic lines of IFN lambda-3–6 × His-HDEL-3′12S1/12s1.4. The arrowhead indicates IFN lambda-3 bands. (c) Immunoblot analysis of seed protein from WT, 12s1.4, and IFN lambda-3–6 × His-HDEL-3′12S1/12s1.4 plants. (d) Relative activity of purified recombinant IFN lambda-3. IFN lambda-3 activities are relative to the activity of recombinant IFN lambda-3 produced in human cells. Values are presented as the mean ± SE of four independent experiments.
Discussion
SSPs represent the major proteins that accumulate in seeds. Therefore, the mechanisms underlying SSP accumulation have been studied to increase recombinant protein production. In seeds, SSPs are transported from ER to (and accumulate in) protein bodies; the signal peptides of SSPs are responsible for their targeting into ER and subsequent in protein bodies12,23,28. Several recombinant proteins fused to this signal peptide accumulated in protein bodies to higher levels than those not fused to the signal peptide12,15,29,30,31, demonstrating that the intracellular localization of proteins contributes to protein yields. However, the levels of recombinant proteins in these studies were considerably lower than those of SSPs in most case, especially in Arabidopsis seeds, which indicates that a key factor other than intracellular localization is required for the predominant accumulation of SSPs. Indeed, in our study, deletion of the 3’UTR of 12S1 significantly reduced the accumulation of 12S1 (Fig. 2B), and addition of the 3’UTR dramatically increased the levels of pMDH1 and human IFN lambda-3 and GFP in seeds (Figs. 4B, 5B and S3). These results clearly show that the 3’UTR of SSPs is the key factor determining the predominant accumulation of SSPs in Arabidopsis seeds. The pMDH1 protein fused to the 3’UTR correctly localized to peroxisomes (Fig. 4E), suggesting that the 3’UTR enhances the accumulation of target proteins without altering their intracellular localization. In developing seeds of transgenic plants, the transcripts of the transgenes were abundant with or without the 3’UTR (Figs. S1 and S2), suggesting that the 3’UTR is involved in increasing protein accumulation by post-transcriptional regulation. Recent studies have revealed that the 3’UTR is involved in mRNA stability and translation32,33. In rice, prolamine and glutelin mRNAs are specifically localized to the protein body ER and cisternal ER, respectively, and the 3’UTRs of these mRNAs are involved in mRNA localization17,18,19. Although the 3’UTRs of rice SSPs are important for mRNA localization, they have a relatively minor effect on SSP accumulation21. As the addition of the 3’UTR of Arabidopsis SSPs greatly enhanced protein accumulation, the function of the 3’UTRs of SSPs may be fundamentally different between rice and Arabidopsis. Intracellular localization may represent the key factor in the predominant accumulation of SSPs in rice seeds, whereas the presence of the 3’UTR is the key factor in Arabidopsis seeds. To date, the molecular functions of the 3’UTRs remain unclear compared with those of the 5’UTRs. Further studies in various organisms are needed to obtain a better understanding of this important genetic region.
Seed-based production systems for recombinant proteins have recently been reported7,8,34. In those systems, they employed ER and protein bodies to enhance recombinant protein yield; the recombinant proteins are fused with SSP signal peptides and ER-retention signals to their localization, yet the recombinant protein levels were lower than those of SSPs. By contrast, in the current study, the fusion of the 3’UTR of SSPs enabled massive accumulation of pMDH1, despite the absence of SSP signal peptides and ER-retention signals (Fig. 4A,B). These results indicate that fusion of the 3’UTR is a promising, novel method to produce recombinant proteins. This method, which does not alter the intracellular localization of recombinant proteins, leads to the production of foreign proteins with the correct intracellular localization under near-native cellular conditions. In fact, pMDH1 produced in the Arabidopsis seeds was correctly localized to the peroxisome and had MDH activity (Fig. 4C,E). These results indicate that this method is useful for producing recombinant proteins for life science research, providing high yields and activity. In addition, this method is promising to produce biopharmaceuticals due to the low risk of endotoxin or infectious agent contamination from serum and the low production costs. High levels of IFN lambda-3, a biopharmaceutical candidate for treating hepatitis C infection, were produced in seeds, with adequate activity (Fig. 5B,E). Previously, the yield of human interleukin-10 produced in Arabidopsis seeds was approximately 0.02% of total soluble protein levels in seeds16 and that of murine interleukin-10 was 0.7%15. The content of IFN lambda-3 produced by our method was 14% of total soluble protein in seeds, indicating that fusion of the 3’UTR of 12S1 greatly increases interferon production in Arabidopsis seeds. Yang et al. (2012) increased the yield of IL-10 in rice to 220 ng/grain, corresponding to approximately 1% of seed weight12. Since the protein content of Arabidopsis seeds is approximately 30%, the yield of IFN lambda-3 produced by our method was approximately 4.2% of seed weight. Compared with rice, Arabidopsis is easy to grow indoors, requiring less soil and fewer fluorescent lamps. In addition, it takes only 2 months to germinate plants and harvest new seeds from Arabidopsis, and the transformation process of Arabidopsis (Agrobacterium floral dip method) is simpler than that of other plants (transformation via callus and regeneration of plants)35,36. Considering the low cost, ease, and rapid growth of Arabidopsis, our method is applicable to interferon production, and it represents one of the most effective methods for biopharmaceutical manufacturing using plant species.
Material and methods
Plant materials
Arabidopsis thaliana Col-0 strain was used as the WT. All seeds were surface-sterilized in 2% (w/v) NaClO (sodium hypochlorite) and 0.02% (v/v) Triton X-100. Seeds were sown on growth medium containing half-strength Murashige and Skoog salts, Gamborg’s B5 vitamins, 0.5 mg/ml MES, 0.8% (w/v) agar, and 1% (w/v) sucrose. The pH of the medium was adjusted to 5.8 with KOH. Two-week-old plants were transferred to soil under long-day conditions (16 h light/8 h dark) at 22 °C. The Arabidopsis T-DNA insertion mutant collections are useful resources for genetic studies and are used by many researchers. The T-DNA insertion line of 12s1 was obtained from the European Arabidopsis Stock Centre at the University of Nottingham, Sutton Bonington Campus, UK (GK-283D09.01). The Col-0 and the T-DNA insertion lines of 12s2, 12s3, and 12s4 were obtained from the Arabidopsis Biological Resource Center based at Ohio State University, USA (SALK_049575, SALK_085866, and SALK_002668, respectively). The 12s1.3 and 12s1.4 double knockout lines were established by crossing 12s1 with 12s3, and 12s1 with 12s4, respectively. Growth of the 12s1.4 was comparable to the WT, at least, under the laboratory conditions. It has been reported that a number of mutants with T-DNA insertions in intron sequences show phenotypes similar to those of knockouts37. 12s1.3.4 triple knockout line could not be established. The names of the 12S1, 12S2, 12S3 and 12S4 genes encoding 12S seed storage proteins by UniProt are CRC, CRD, CRB and CRA1, respectively. Experimental studies on plants, including the collection of plant material, were conducted in accordance with relevant institutional, national and international guidelines and legislations.
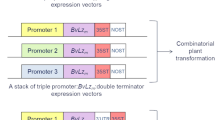
Plasmid construction and plant transformation
A series of 12S1 fragments, coding sequences of pMDH1 (NCBI Reference Sequence: NM_127843.5), and were amplified from Arabidopsis cDNA by PCR. The coding sequence of IFN lambda-3 (GenBank: AY129149.1) was amplified from cDNA from Raji, a human T cell leukemia cell line, by PCR. The 3’UTR of SSPs and 6 × His-HDEL were fused by fusion PCR. These DNA fragments were cloned into pDONR221 with Gateway® cloning technology (Thermo Fisher Scientific, Massachusetts, USA). The promoter sequence 1671 bp upstream of the 12S1 gene was amplified from the Arabidopsis genome and cloned into pDONRP4P1R. The primer sets for the plasmid construction are shown in Table S1. The expression vectors were constructed to combine the 12S1 promoter from pDONR P4-P1R and DNA fragments from pDONR221 into R4pGWB50138 with Multisite Gateway® technology (Thermo Fisher Scientific, Massachusetts, USA). These expression vectors were transformed into Agrobacterium tumefaciens strain C58C1rif and introduced into WT, 12s1, or 12s1.4 lines using the floral dip method. Transformants were selected on medium containing 25 μg mL−1 hygromycin B. The accumulation of recombinant protein was evaluated in at least ten independent T3 progeny.
SDS-PAGE and immunoblot analysis
To extract total proteins, 100 seeds of each line were ground using a stainless-steel pestle in 100 µl of SDS buffer containing 125 mM Tris–HCl (pH 7.5), 10% (v/v) 2-mercaptoethanol, 4% (w/v) SDS, and 10% (w/v) sucrose. The samples were boiled for 5 min and centrifuged at 10,000 g for 5 min. The supernatants were collected and the concentrations of total protein were measured by Bradford ULTRA™. The protein contents of the supernatants were adjusted to 0.5 μg/μl by adding SDS buffer and used as protein samples. Protein samples extracted from seeds were separated in 12.5% or 15.0% SDS–polyacrylamide gels. Protein bands were detected with CBB-R250 staining or transferred to Immobilon-P PVDF membranes (Merck Millipore, Massachusetts, USA) using semidry electroblotting for immunoblot analysis. Monoclonal anti- IFN lambda-3 antibody (MAB5259, R&D Systems, Minneapolis, USA) was used in the immunoblot analysis. The PVDF membrane was cut out to include the region of interest and immuno-reactive protein bands were detected using the SNAP i.d. (Merck Millipore, Massachusetts, USA) and ECL systems (
Cytiva, Tokyo, Japan). The recombinant protein levels were estimated based on the intensities of the protein bands in SDS–polyacrylamide gels using MultiGauge Ver3.0 (Fujifilm, Tokyo, Japan). The uncropped blots are shown in Fig. S4.
Electron microscopy
Ultrathin sections were prepared from dry seeds of WT and transgenic lines. Sectioning, immunogold labeling, and microscopic observation were performed as described previously39.
MDH activity
One hundred seeds of WT or transgenic lines were ground using a stainless-steel pestle in 200 µl of buffer containing 100 mM KH2PO4 (pH 8.0) on ice. The samples were centrifuged at 10,000 g for 5 min at 4 °C. After removing the oil layer, the supernatants were carefully collected and used as crude extracts. The contents of total soluble proteins (TSP) of the crude extracts were measured by Bradford ULTRA™. MDH activities were measured according to the previous protocol40. A standard curve was generated using MDH from porcine heart (M-7383, Sigma-Aldrich, Missouri, USA).
Purification of recombinant IFN lambda-3 from seeds
One hundred seeds of IFN lambda-3–3′12S1 were ground using a stainless-steel pestle in 500 µl of lysis buffer containing 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, and 10 mM imidazole on ice. The samples were centrifuged at 10,000 g for 10 min at 4 °C. After removing the oil layer, the supernatants were carefully collected and loaded onto a Ni–NTA spin column (QIAGEN, Venlo, the Netherlands) and centrifuged at 300 g for 10 min at 4 °C, and the flow-through was discarded. The spin column was washed four times with 600 µl of wash buffer containing 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, and 20 mM imidazole, followed by centrifugation at 800 g for 2 min at 4 °C. The recombinant IFN lambda-3 protein was eluted with 100 µl of elution buffer containing 50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, and 500 mM imidazole, followed by centrifugation at 800 g for 5 min at 4 °C (shown in Fig. S5).
Evaluation of IFN lambda-3 activity using luciferase assays
Luciferase assays of recombinant protein were performed using a Dual-Glo Luciferase reporter assay system (Promega, Wisconsin, USA). To assess recombinant protein activity, HepG2 cells were transfected with pISRE-Luc and pGL4.74, and harvested 24 h after treatment with purified recombinant interferon IL28B from seeds. Chemiluminescence was measured by SpectraMax L (Molecular Devices, California, USA). Firefly luciferase activity was normalized to Renilla activity to adjust for transfection efficiency. Bovine serum albumin (100 ng/mL) and recombinant human IFN lambda-3 produced by 293F cells, derived from human embryonic kidney cells25, were used as the negative and positive controls, respectively.
Data availability
All data and materials appearing in this study are available from the corresponding author (M.K.) upon reasonable request.
References
Shewry, P. R., Napier, J. A. & Tatham, A. S. Seed storage proteins: Structures and biosynthesis. Plant Cell 7, 945–956 (1995).
Shewry, P. R. & Halford, N. G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 53, 947–958 (2002).
Wang, D., Li, F., Cao, S. & Zhang, K. Genomic and functional genomics analyses of gluten proteins and prospect for simultaneous improvement of end-use and health-related traits in wheat. Theor. Appl. Genet. 133, 1521–1539 (2020).
Zheng, P., Zheng, C., Otegui, M. S. & Li, F. Endomembrane mediated-trafficking of seed storage proteins: From Arabidopsis to cereal crops. J. Exp. Bot. 73, 1312–1326 (2022).
Verdier, J. & Thompson, R. D. Transcriptional regulation of storage protein synthesis during dicotyledon seed filling. Plant Cell Physiol. 49, 1263–1271 (2008).
Vianna, G. R., Cunha, N. B. & Rech, E. L. Soybean seed protein storage vacuoles for expression of recombinant molecules. Curr. Opin. Plant Biol. 71, 102331 (2023).
Paradia, P. K., Bhavale, R., Agnihotri, T. & Jain, A. A review on edible vaccines and biopharmaceutical products from plants. Curr. Pharm. Biotechnol. 24, 495–509 (2023).
Zhu, Q., Tan, J. & Liu, Y.-G. Molecular farming using transgenic rice endosperm. Trends Biotechnol. 40, 1248–1260 (2022).
Boothe, J. et al. Seed-based expression systems for plant molecular farming. Plant Biotechnol. J. 8, 588–606 (2010).
De Jaeger, G. et al. Boosting heterologous protein production in transgenic dicotyledonous seeds using Phaseolus vulgaris regulatory sequences. Nat. Biotechnol. 20, 1265–1268 (2002).
Lin, Y., Pajak, A., Marsolais, F., McCourt, P. & Riggs, C. D. Characterization of a cruciferin deficient mutant of Arabidopsis and its utility for overexpression of foreign proteins in plants. PLoS ONE 8, e64980 (2013).
Yang, L., Hirose, S., Takahashi, H., Kawakatsu, T. & Takaiwa, F. Recombinant protein yield in rice seed is enhanced by specific suppression of endogenous seed proteins at the same deposit site. Plant Biotechnol. J. 10, 1035–1045 (2012).
Takaiwa, F., Hirose, S., Takagi, H., Yang, L. & Wakasa, Y. Deposition of a recombinant peptide in ER-derived protein bodies by retention with cysteine-rich prolamins in transgenic rice seed. Planta 229, 1147–1158 (2009).
Kudo, K. et al. ER stress response induced by the production of human IL-7 in rice endosperm cells. Plant Mol. Biol. 81, 461–475 (2013).
Morandini, F. et al. Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnol. J. 9, 911–921 (2011).
Chen, L. et al. Identification of the factors that control synthesis and accumulation of a therapeutic protein, human immune-regulatory interleukin-10, in Arabidopsis thaliana. Plant Biotechnol. J. 11, 546–554 (2013).
Tian, L., Chou, H.-L., Fukuda, M., Kumamaru, T. & Okita, T. W. mRNA localization in plant cells. Plant Physiol. 182, 97–109 (2020).
Choi, S. B. et al. Messenger RNA targeting of rice seed storage proteins to specific ER subdomains. Nature 407, 765–767 (2000).
Tian, L. & Okita, T. W. mRNA-based protein targeting to the endoplasmic reticulum and chloroplasts in plant cells. Curr. Opin. Plant Biol. 22, 77–85 (2014).
Kawakatsu, T. & Takaiwa, F. Cereal seed storage protein synthesis: Fundamental processes for recombinant protein production in cereal grains. Plant Biotechnol. J. 8, 939–953 (2010).
Yang, L., Wakasa, Y., Kawakatsu, T. & Takaiwa, F. The 3’-untranslated region of rice glutelin GluB-1 affects accumulation of heterologous protein in transgenic rice. Biotechnol. Lett. 31, 1625–1631 (2009).
Higashi, Y. et al. Proteomic and transcriptomic analysis of Arabidopsis seeds: Molecular evidence for successive processing of seed proteins and its implication in the stress response to sulfur nutrition. Plant J. 48, 557–571 (2006).
Shimada, T. et al. Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 100, 16095–16100 (2003).
Pracharoenwattana, I., Cornah, J. E. & Smith, S. M. Arabidopsis peroxisomal malate dehydrogenase functions in beta-oxidation but not in the glyoxylate cycle. Plant J. 50, 381–390 (2007).
Sugiyama, M., Tanaka, Y., Wakita, T., Nakanishi, M. & Mizokami, M. Genetic variation of the IL-28B promoter affecting gene expression. PLoS ONE 6, e26620 (2011).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41, 1105–1109 (2009).
Wandelt, C. I. et al. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 2, 181–192 (1992).
Matsuoka, K. & Nakamura, K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc. Natl. Acad. Sci. U. S. A. 88, 834–838 (1991).
Giddings, G., Allison, G., Brooks, D. & Carter, A. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 18, 1151–1155 (2000).
Eskelin, K. et al. Production of a recombinant full-length collagen type I alpha-1 and of a 45-kDa collagen type I alpha-1 fragment in barley seeds. Plant Biotechnol. J. 7, 657–672 (2009).
Takagi, H. et al. Oral immunotherapy against a pollen allergy using a seed-based peptide vaccine. Plant Biotechnol. J. 3, 521–533 (2005).
Mayr, C. Regulation by 3’-untranslated regions. Annu. Rev. Genet. 51, 171–194 (2017).
Mayr, C. What are 3’ UTRs doing?. Cold Spring Harb. Perspect. Biol. 11, a034728 (2019).
Mirzaee, M., Osmani, Z., Frébortová, J. & Frébort, I. Recent advances in molecular farming using monocot plants. Biotechnol. Adv. 58, 107913 (2022).
Bent, A. Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 343, 87–103 (2006).
Gelvin, S. B. Integration of Agrobacterium T-DNA into the plant genome. Annu. Rev. Genet. 51, 195–217 (2017).
Osabe, K., Harukawa, Y., Miura, S. & Saze, H. Epigenetic regulation of intronic transgenes in Arabidopsis. Sci. Rep. 7, 45166 (2017).
Nakagawa, T. et al. Development of R4 gateway binary vectors (R4pGWB) enabling high-throughput promoter swapping for plant research. Biosci. Biotechnol. Biochem. 72, 624–629 (2008).
Hayashi, M., Toriyama, K., Kondo, M. & Nishimura, M. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. Plant Cell 10, 183–195 (1998).
Hayes, M. K., Luethy, M. H. & Elthon, T. E. Mitochondrial malate dehydrogenase from corn: Purification of multiple forms. Plant Physiol. 97, 1381–1387 (1991).
Acknowledgements
We thank Prof. Tsuyoshi Nakagawa (Shimane University) for providing experimental materials. We thank the staff at the Model Organisms Facility, and Trans-Omics Facility, at the NIBB Trans-Scale Biology Center for technical support.
Funding
This work was supported by AMED under Grant Number JP22fk0310520.
Author information
Authors and Affiliations
Contributions
M.K., K.Y., M.N. and S.M. devised and designed the experiments. M.K. and M.S. performed the experiments. M.K. and S.M. wrote the manuscript. M.S. measured the activity of recombinant IL-28B. M. Kondo performed the electron microscopic analyses.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanai, M., Sugiyama, M., Kondo, M. et al. Fusing the 3’UTR of seed storage protein genes leads to massive recombinant protein accumulation in seeds. Sci Rep 13, 12217 (2023). https://doi.org/10.1038/s41598-023-39356-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39356-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.