Abstract
The effect of light, main zeitgeber of the circadian system, depends on the time of day it is received. A brief trip to the Antarctic summer (ANT) allowed us to explore the impact of a sudden and synchronized increase in light exposure on activity-rest rhythms and sleep patterns of 11 Uruguayan university students, and to assess the significance of light history in determining individual circadian phase shift. Measurements collected in the peri-equinox in Montevideo, Uruguay (baseline situation, MVD) and in ANT, included sleep logs, actigraphy, and salivary melatonin to determine dim-light melatonin onset (DLMO), the most reliable marker of circadian phase. The increase in light exposure in ANT with respect to MVD (affecting both light-sensitive windows with opposite effects on the circadian phase) resulted in no net change in DLMO among participants as some participants advanced their DLMO and some others delayed it. The ultimate cause of each participant’s distinctive circadian phase shift relied on the unique change in light exposure each individual was subjected to between their MVD and ANT. This study shows an association between the individual light history and the circadian phase shift.
Similar content being viewed by others
Introduction
The exposure to the light–dark cycle is the most powerful environmental synchronizing signal for the circadian system across evolution. Light impacts on specific retinal ganglion cells, whose axons reach the mammalian circadian pacemaker, the suprachiasmatic nuclei, located in the hypothalamus1,2. The effect of light on the circadian phase depends on the moment of the day in which light exposure occurs, following a well-documented phase response curve first described in rodents3, but also confirmed in humans4,5,6,7. Light exposure occurring in the evening (early biological night) induces a phase-delay shift of the circadian pacemaker, whereas light exposure occurring early in the morning (late biological night) induces a phase-advance shift. Modern urban life has disrupted the light patterns expected by the biological clock, leading to changes that are difficult to predict8,9,10 and that are highly variable among individuals11. However, it seems clear that all the light and darkness to which humans are exposed throughout the day plays a role in entrainment12. As melatonin is inhibited by light, the detection of the onset of the nocturnal increase of melatonin (i.e., the dim light melatonin onset, DLMO) has emerged as the best predictor of the beginning of the endogenous dark phase13,14, which also accounts for the reported light-dependent changes and individual variability11.
Although distorted by electric light and variable across latitudes and seasons, light exposure still impacts on the human circadian system in normal life, leading to light-dependent phase-shifts and changes in sleep patterns including duration, structure, and sleep disturbances15. For example, around the equator, rubber tappers exposed to electric light at home, and therefore more exposed to evening light, showed later DLMO and sleep than workers without electric light at home16. On the other hand, a seasonal experiment carried out in natural camping conditions in Colorado, USA (~ 40°N, with a seasonal light–dark change of around 10/14 to 14/10) reported the expected longer nocturnal melatonin pulse in winter than in summer, but no significant changes in DLMO across seasons17, maybe because the longer sunlight of summertime impacts in both the phase-advance and phase-delay windows. When seasonal changes in photoperiod are more extreme, for example in Antarctic scientific bases (~ 75°S, with a seasonal light–dark change of around 0/24 to 24/0), circadian rhythms during winter, including melatonin, are usually delayed or even completely desynchronized, adopting free-running patterns18,19. Sleep is also delayed and more disturbed in winter Antarctic18 and Arctic20 populations, reinforcing the importance of the synchronizing power of morning summer light.
The analysis of short-term changes in lighting conditions have revealed the plasticity of the circadian system. The iconic camp experiment21 that evaluated the effect of a sudden change in light exposure (electric light vs natural light) between two successive weeks showed a re-set of the circadian phase in natural light conditions; i.e., after only seven days of natural lighting, DLMO synchronizes with sunset and among individuals. In addition, to this remarkable plasticity of the circadian system to adjust to short-term changes, recent studies have focused on the differences in individual responses to similar light exposure profiles22,23,24. Overall, the effects of light on sleep and circadian phase depend on a wide range of individual traits such as age25,26,27, race/ethnicity28,29,30, sex31,32, chronotype21,33,34, photosensitivity11,27, and photic history35,36,37.
The current understanding of the influence of light history on circadian physiology and behavior, especially in real world settings, is limited and a challenging research gap for chronobiology24. While strong evidence supports that a recent change in light history over days can affect the sensitivity to light of the circadian system21,36, less is known about the influence of long-term photic history over months and seasons22,23,24. Among the few studies that have addressed the effect of long-term light history on circadian system and sleep in real-world settings two of them have taken advantage of Antarctica as a natural laboratory38,39. Seasonal changes from winter to summer imply gradual variations in photoperiod that involve more light exposure on both the phase-advance and the phase-delay windows. This means an ambivalent signal for the circadian system that forces a trade-off between advancing or delaying the circadian phase with no predictable net effect.
The ecological experiment provided by a group of Uruguayan University students who travelled to the Antarctic summer emerges as an excellent model system to address this issue. By comparing the baseline situation in the Montevideo fall equinox (MVD) to the Antarctic summer trip (ANT) we previously showed that while the average circadian phase of these students did not change, individual DLMO shifts were chronotype-dependent34. This previous study also reported that all participants, regardless of their chronotype, were significantly more exposed to light in ANT during fixed hours of the morning and evening. However, the relevant question of how light is associated with individual circadian phase shifts remains to be addressed. To do this, we first provide a comprehensive view of the effects of ANT on light exposure, activity-rest rhythms, and sleep patterns using objective measurements and self-reports. Then, we consider the light exposure adjusted to the individual phase-advance and phase-delay windows according to participant’s DLMO; and finally, we evaluate long-term light history (assessed by the difference in individual light exposure between ANT and MVD) and its association with the valence (advance or delay) and the magnitude of the individual circadian phase shift.
Results
In this study we present a longitudinal comparative analysis of the activity-rest and light exposure patterns of 11 healthy University students (8 females, 3 males, 22.45 ± 1.13 years old from Universidad de la República, Uruguay) between the 2016 fall peri-equinox in Montevideo (baseline situation, MVD) and the 2016 summer in Antarctica (Antarctic trip, ANT, Fig. 1), whose chronotype and circadian phases have been previously reported34. In this previous study we also reported a preliminary analysis of the light exposure (based in the same dataset) by comparing the participants with early versus late chronotype.
Experimental design and layout. Participants were studied in two conditions: Montevideo (MVD, baseline situation) and during Antarctica trip (ANT), where they filled sleep logs and wore wrist actigraphs continuously for 10 days. On the last day of both locations hourly samples of saliva were collected to determine the dim light melatonin onset (DLMO). In MVD participants also filled questionnaires to assessed chronotypes.
The impact of the Antarctic summer on the rhythms of activity, light exposure, and sleep
Both conditions, MVD and ANT, represented a similar academic challenge for the participants as MVD corresponded to the start of the university semester in the 2016 austral fall and ANT corresponded to the II Uruguayan Summer Antarctic School held during the 2016 austral summer in the Uruguayan Antarctic Scientific Base Artigas34,40. In contrast, light exposure was longer (more minutes exposed to > 1000 lx, Table 1) and more intense (average light exposure of the brightest 10 h period, MB10, Table 1) in ANT than in MVD in both the morning and the evening (Fig. 2a). In addition, all participants showed circadian rhythms of light exposure (i.e., with significant fit to a cosine function over 24 h period) in both MVD and ANT. The acrophase of this light circadian rhythm was earlier in ANT than in MVD, while MB10c (midpoint of the brightest 10 h period) and LB5c (midpoint of the least bright 5 h period) were not affected (Table 1).
(a) Average light exposure (in lux) plotted on a log scale for both locations: Montevideo (MVD, orange) and Antarctica (ANT, blue). Data are double plotted so that light levels across midnight can be more easily observed. (b) Representative activity actograms for one participant during the 10 consecutive days of recording for both locations: MVD (top, orange) and ANT (below, blue). Data are presented in rows of 48 h, bars at the bottom indicate light–dark cycle, scale on top indicates time in hours.
Representative activity actograms during 10 consecutive days in both MVD (8 workdays and 2 weekend days) and ANT (all considered workdays) illustrate additional impacts of the Antarctic summer (Fig. 2b; Table 1; Supplementary Fig. S1). First, all participants showed circadian rhythms of activity in both recording sites but with a different timing between them, as the acrophase and the midpoint of the most active 10 h period (M10c) were significantly earlier in ANT than in MVD (Table 1). In contrast, the mid-point of the least active 5 h period (L5c) did not change between MVD and ANT (Table 1), though the onset of the rest phase was more regular in ANT than MVD across days (Fig. 2b) and among participants (Supplementary Fig. S1). Second, the activity period was longer (more minutes in moderate-vigorous physical activity, MVPA > 100 mg, Table 1) and more intense (higher average activity of the most active 10 h period, M10). The rest was shorter and quieter (lower average activity of the least active 5 h period, L5) in ANT than in MVD. This resulted in a more robust circadian rhythm of activity (higher relative amplitude, RA in ANT with respect to MVD (Table 1). Third, because of the tight schedule shared by all participants during ANT, all phase variables of both activity and light exposure rhythms were less dispersed in ANT than in MVD (Table 1). In line with actigraphy, daily self-reported sleep data showed that sleep was shorter and earlier in ANT than in MVD (Table 1). Interestingly, despite the reduction in sleep duration in ANT, self-reported sleep quality from a subjective visual analogue scale (VAS) included in sleep logs, was not different between ANT and MVD (Table 1).
L5c was strongly correlated with the self-reported mid-sleep point (estimated by sleep logs; R = 0.87, p = 0.001) in MVD as expected, but this association became weaker in ANT (L5c versus mid-sleep point, R = 0.56, p = 0.072). In contrast, the expected association between self-reported sleep quality and duration was observed in ANT (R = 0.73, p = 0.011) but not in MVD (R = 0.50, p = 0.11); as well as the association between self-reported sleep quality and RA (ANT: R = 0.70, p = 0.017; MVD: R = 0.52, p = 0.1).
The impact of the Antarctic summer on DLMO and its determinants
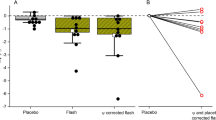
Circadian timing was determined by calculating the dim light melatonin onset (DLMO) as marker of the individual circadian phase and, in particular, as indicator of the beginning of the biological night. As previously reported34 and shown in Fig. 3a, the DLMO was not significantly different between MVD (22:16 ± 1:14) and ANT (22:01 ± 0:43, Wilcoxon signed-rank test, z = 0.27, p = 0.82), although less dispersed in ANT than in MVD. In line with this, as shown in Table 1 and Fig. 3a, L5c, as marker of the individual timing of the rest period was also not significantly different between ANT and MVD (Wilcoxon signed-rank test, z = 1.60, p = 0.11) and less dispersed in ANT. Interestingly, the individual circadian phase shift between ANT and MVD (ΔDLMO), was positively correlated with the individual shift in L5c between both conditions (ΔL5c) (R = 0.69, p = 0.018, Fig. 3b).
(a) Individual changes in the dim light melatonin onset (DLMO) and in the 5 h of less activity (L5c) between both locations Montevideo (MVD, orange) and Antarctica (ANT, blue). Lines connect the values of each participant. Black squares represent the average of all participants (b) Pearson linear correlation for the individual circadian phase shift (∆DLMO) between ANT and MVD with the individual shift in L5c (∆L5c) between ANT and MVD (R = 0.69, p = 0.018). Each participant is represented with a different color (colors are consistent with Fig. 4b).
The Antarctic summer means a significant increase of light exposure. As shown in Fig. 4a and Table S1, when considering the individual 3 h-phase-advance window with respect to individual DLMO (PAW, 3 h before the DLMO antipode) participants were in average significantly more exposed to light in ANT with respect to MVD (MVD 413.98 ± 155.85 lx vs ANT 1250.80 ± 999.98 lx, Wilcoxon signed-rank test z = 2.31, p = 0.019). Likewise, when considering the individual 3 h-phase-delay window (PDW, 3 h before the DLMO), participants were in average significantly more exposed to light in ANT with respect to MVD: (MVD 50.09 ± 39.06 lx vs ANT 346.18 ± 151.27, Wilcoxon signed-rank test z = 2.93, p = 0.001). The individual light exposure in both light-sensitive windows did not predict self-reported mid-sleep point neither in ANT (PAW F1,56 = 0.004, p = 0.952; PDW F1,56 = 0.342, p = 0.561) nor MVD (PAW F1,65 = 0.492, p = 0.486; PDW F1,65 = 0.648, p = 0.424) (Table S2). The great increase in light exposure in both light-sensitive windows had overall opposite effects on the circadian phase, and this made it necessary to analyze individual differences in light exposure between MVD and ANT. The individual circadian phase shift between ANT and MVD (ΔDLMO) was negatively correlated with the individual difference in light exposure in the PAW (R = − 0,80, p = 0.003, Fig. 4b) but was positively correlated with the individual difference in light exposure in the PDW (R = 0.747, p = 0.008, Fig. 4b). In the PAW the greater the light exposure in ANT with respect to MVD (high Δlight exposure in the PAW), the earlier the DLMO (negative ΔDLMO) in ANT with respect to MVD. Moreover, participants who showed very low difference or who were even exposed to less light in ANT than in MVD (Δlight exposure in the PAW around zero) exhibited a circadian phase delay (positive ΔDLMO). In contrast, in the PDW the greater the light exposure in ANT with respect to MVD (high Δlight exposure in the PDW), the later the DLMO (positive ΔDLMO) in ANT with respect to MVD. Accordingly, participants who showed little or no difference in light exposure in ANT than in MVD (low Δlight exposure in the PDW) exhibited a circadian phase advance (negative ΔDLMO).
(a) Light exposure (lux) plotted on a log scale for the phase advance window (PAW) and phase delay window (PDW) in both locations: Montevideo (MVD, orange) and Antarctica (ANT, blue). Light exposure was different in both the PAW (Wilcoxon singed-rank test, p = 0.019) and the PDW (Wilcoxon singed-tank test, p = 0.001). (b) Pearson linear correlation for the individual circadian phase shift (∆DLMO) between ANT and MVD with the difference in light exposure in the PAW (on top, R = -0.8, p = 0.003), and in the PDW (below, R = 0.75, p = 0.008) between ANT and MVD. Each participant is represented with a different color (colors are consistent with Fig. 3b).
Discussion
This study builds upon prior research using the same dataset34, to show that the great amount of light participants were exposed to in the Antarctic summer impacted on the activity-rest rhythms, sleep patterns, and circadian phase of Uruguayan university students. During their normal lives around the equinox in Montevideo, Uruguay, the light exposure participants were used to was different among them and these differences were key to evaluate the effects of the Antarctic summer. Overall, the activity-rest rhythm was earlier, more robust, and less variable among participants in ANT with respect to MVD, while self-reported sleep was also earlier and shorter. More importantly, the shifts of the circadian phase and sleep patterns observed in ANT were linked to participants’ individual habits in MVD: (a) participants who advanced their sleep timing in ANT respect to MVD, also advanced their DLMO in ANT, while participants who delayed their sleep timing in ANT respect to MVD, also delayed their DLMO in ANT; and (b) shifts of the circadian phase depended differed on the individual long-term light history; i.e., DLMO was advanced in participants exposed to more light in the phase-advance window in ANT, and delayed in participants exposed to more light in the phase-delay window in ANT.
The participants of this study, who were used to a normal individual lifestyle in MVD, including personal specific profiles of light exposure and social demands, were subjected to a 10 days-summer trip to an Antarctic scientific base, in which they shared a daily routine of classes, field work, meals, social activities, and conditioned sleep schedules34,40. Unlike previous studies that mainly focused on the impact of the huge increase of light in the Antarctic summer34,41,42, this new data processing aimed to evaluate the effect of individual differences in light exposure between the two conditions. We previously showed that when considering the fixed time windows for the morning and the evening, participants were significantly more exposed to light in ANT with respect to MVD except for the latest time window from 22:00 to 0:59 h34. Interestingly, the baseline situation (MVD) was not a fully controlled condition, because participants in MVD were not indicated to follow any protocol of either scheduled activity or exposure to light. Rather, they just displayed their normal lives and thus had variable patterns of activity and light exposure. In contrast, the Antarctic summer was a homogeneous situation among participants, who shared the same strict schedule of activities imposed by the Antarctic scientific base agenda. The social synchronization generated by the schedule of scientific bases in Polar regions is indisputable as shown by previous self-reported and objective data from short summer trips34,40,42,43. In the case of this study, the social synchronization imposed by ANT was crucial for the emergence of striking differences in individual light exposure between the two conditions; and consequently, it enabled us to assess the effect of individual habits, specially of long-term light history.
Objective reported parameters of activity patterns (Table 1) were consistently earlier in ANT than in MVD (except for L5c that did not change), but in contrast to an Arctic summer trip study that showed a delay in the acrophase43. In addition, the comparable indicators provided by different instruments (L5c from actimetry, self-reported midsleep point, and DLMO) were significantly associated in MVD. The loss of these expected associations in ANT suggests that the Antarctic summer induced a misalignment of the circadian system. This vision of the suffering of the circadian system in ANT is in line with previous studies that have argued that the extreme light condition of the Antarctic summer acts as an apparent stressor to the circadian clock, decreasing the quality of sleep, delaying melatonin phase, and causing desynchronization39,41,42,44.
Sleep quality is vital to individual health and wellbeing and involves individual's self‐satisfaction with all aspects of the sleep experience45. Sleep quality is therefore associated with longer sleep duration, proper timing, high efficiency, and less fragmented sleep46,47,48,49,50,51. Participants’ sleep quality, estimated by VAS, did not change between MVD and ANT in line with one of the few studies reporting sleep changes in a summer Antarctic expedition52. This lack of change in sleep quality probably depended on the interaction of multiple causes acting in opposite directions. On one hand, self-reported sleep duration in ANT was less than the minimum of 7 h recommended for young adults (Table 1)40, with potential detrimental effects on sleep quality. On the other hand, actimetry recordings showed that sleep was less fragmented in ANT as the activity-rest rhythm was more robust in ANT than in MVD (RA in Table 1).
DLMO is an accurate, non-invasive, and reliable measure of the endogenous circadian rhythm13,14, whose shifts are also used to show variations of the circadian phase to adapt to acute transitions53 even in real-life situations17,21,34. Although it is well known that bright light is the main cue that modulates the body clock timing24, it has been reported that age25,54,55, chronotype56,57, and social demands (e.g., school or work schedules)58,59,60, among others, interact with light in the modulation of human circadian phase inducing a wide individual variation. Interestingly the lack of an average DLMO shift between MVD and ANT that can be interpreted at first glance as the absence of effect, is indeed the demonstration of how much individual attributes matter34. Although neither DLMO nor L5c changed in average between MVD and ANT, participants’ individual circadian phase shifted between both conditions in a comprehensive way associated to both their circadian preferences34 and their sleep patterns (Fig. 3). Moreover, the circadian phase and sleep timing changed in concert: (a) both DLMO and L5c were more dispersed in MVD than in ANT as an additional evidence of the social synchronization the Antarctic summer imposed; (b) both shifts in DLMO and L5c were associated showing a relationship between the change of sleep timing on the shift of the circadian phase, and (c) the magnitude of both shifts was comparable; i.e., when sleep was advanced by 2 h, the DLMO accompanied this change with a 2-h advance. On the other hand, the strong association between these two different objective phase parameters (DLMO and L5c) proves the reliability of our data by showing that each individual reacts in a holistic way.
As the light–dark cycle is the most important entrainer of the circadian system across evolution61,62, the way that light influences the DLMO has been extensively documented in controlled experimental conditions; namely DLMO can either be advanced by bright light exposure in the morning5,63,64,65,66, or delayed by light exposure in the evening25,67. The precise moment these light sensitive PAW and PDW occur is, of course, dependent on the individual DLMO and should thus be calculated considering the individual circadian time5,68. In this study, light exposure was significantly higher in average in ANT with respect to MVD in both the PAW and the PDW, as expected (Fig. 4a). However, the difference in light exposure between ANT and MVD in these individual light-sensitive windows was less pronounced than the difference observed during the morning and the evening (Fig. 2a). Indeed, individual changes in light exposure between ANT and MVD were highly variable in both the PAW and the PDW, and even negative for three participants in the PAW (Fig. 4b). Interestingly, the two participants who showed the greatest difference in light exposure in the PAW were the ones who showed the least change in the PDW (Fig. 4b). These results confirm the importance of the individual assessment not only of the light-sensitive windows, but also of the evaluation of individual differences between conditions in line with the current debate of the importance of individual long-term photic history at various time scales, including seasonal changes in the light environment22,23,24.
Photic history may influence the individual physiological response to light and underlies plastic changes of the circadian phase across seasons (long-term light history)69, and less gradual transitions such as trans-latitudinal trips or between urban and natural environments (short-term light history)17,21,24,70. Although it is clear that photic history will affect subsequent response to light, only a few studies have focused on light exposure differences to assess phase shifts in real-life conditions15,39,71. Transitions between locations with artificial to natural lighting induce acute phase-advance shifts attributable to a concerted change in light exposure, which is simultaneously increased in the PAW and decreased in the PDW17,21. In contrast, as any seasonal change towards the summer, the Antarctic trip means an increase of light exposure in both light sensitive windows with opposing effects on the circadian phase: when light exposure increased at the same time in both the PAW and the PDW, the average DLMO did not change between ANT and MVD. However, our study also highlights the need to focus on individual long-term light history as the higher the difference in light exposure in the PAW between ANT and MVD, the greater the advance of their DLMO; and vice versa, the higher the difference in light exposure in the PDW between ANT and MVD, the greater the delay of their DLMO (Fig. 4b). Therefore, this study emphasized the importance of comparing individual long-term light history with individual current light exposure as a means of assessing circadian phase shifts because: (a) the changes in light exposure in the PAW and the PDW between ANT and MVD were not homogeneous among participants (Fig. 4b); and (b) the light exposure change between ANT and MVD exhibited by the majority of participants was specific to one of the sensitive windows (PAW or PDW), thus influencing the valence of the individual circadian phase shift. This ecological study provides evidence on the association between long-term light history and circadian phase shifts.
Limitations
We recognize several limitations of this study, most of them derived from the fact that this is an ecological study. First, the reduced number of participants from a homogeneous population of university students prevents the generalization of the interpretation of our results and implies a restricted statistical power for the analyses used. Second, the Uruguayan Summer School on Introduction to Antarctic Research as a training course, in each edition carefully selects 12 undergraduate science students to travel to Antarctica. Thus, the volunteer participants in this study were not randomly selected and were highly motivated to be part of this study. Third, although all the procedures were personally supervised by the authors of this study, especially during ANT, light information provided by wrist actimeters could be underestimated because they may have eventually been covered by warm clothing (jackets and gloves). Also, the use of wrist actimeters to monitor light exposure instead of a device that does so in the vertical plane and close to the eyes may also affect the accuracy of light measurements. In addition, although human circadian system is more sensitive to short-wavelength optical radiation, the devices recorded photopic illuminance. Fourth, because the impact of ANT was contrasted with normal life (MVD), we did not control for other stimuli that could affect the circadian phase as confounding factors (food intake, stimulants, substance use, stress, etc.). However, we do not consider the lack of a completely controlled situation capturing participant’s daily lives in MVD as a limitation in this study. Instead, it enabled the emergence of individual differences, which are the focus of this study.
Concluding remarks
Our understanding of the complex interactions between photic and non-photic stimuli for the daily adjustment of the human circadian phase has made important progress in the last decade. First conceived as a rather stable individual trait, strong evidence has shown that, for example, the circadian phase can be very plastic60, light sensitivity is highly diverse among individuals11, and the power of light entrainment can be strong and fast even in ecological conditions17,21. People living in the same city (or even in the same house) can be subjected to very different light exposure patterns related to personal school/work schedules, chronotypes, and lifestyles. In this study, we show the importance of this individual long-term light history when it is challenged by a sudden and synchronized increase in light exposure affecting both light-sensitive circadian phase windows. It is not the absolute amount of light that all the participants received in a similar way in Antarctica what matters to explain the shifts of the circadian phase induced by this short trip to the Antarctic summer. Rather, each participant’s distinctive circadian phase shift relied on the unique change in light exposure he/she was subjected to between his/her baseline life in Montevideo and the Antarctic summer. We show that both light-sensitive windows were similarly capable of influencing the net circadian phase shift; i.e., the higher the light exposure difference in the PAW or in the PDW the higher the circadian phase advance or delay, respectively. This remarkable finding is supported by the diverse individual light history among participants: those participants whose difference in light exposure was similar in both the PAW and the PDW did not show a significant circadian phase shift, while those participants whose difference in light exposure was prevalent in the PAW advanced their circadian phase and those participants whose difference in light exposure was prevalent in the PDW delayed it. The association between long-term light history and circadian phase shifts reported in this study reinforces the need to pay attention to individual photic history in future studies interested in the modulation of the human circadian phase, especially in normal-life conditions.
Methods
Participants
Eleven healthy students from Facultad de Ciencias, Universidad de la República, Uruguay participated in this study out of the twelve members of the Second Uruguayan Summer School on Introduction to Antarctic Research held in January 2016, in the Uruguayan Antarctic Scientific Base Artigas. Although the twelve students agreed to participate in the research, the actimetry record of one participant were not validated, so these data were excluded from the analysis. All participants were clinically assessed to confirm they were healthy, and none showed sleep disturbances or depression signs (Beck’s Depression Inventory score < 1072). All procedures were approved by the ethics committee at the Instituto de Investigaciones Biológicas Clemente Estable, Ministerio de Educación y Cultura, Uruguay (CEP/HCPA 14-0057; approval date: December 16, 2013) and complied with the principles outlined by the Declaration of Helsinki73. All participants signed an informed consent form stating that they had been told about the aims and procedures of the study, their right to end participation without any explanatory statement at any time, their data being coded as to maintain anonymity, and their data being communicated for scientific purposes only.
We compared two different conditions: baseline situation, which corresponded to the start of university semester in the austral fall equinox 2016 (March 7–17, 2016, Montevideo, Uruguay, 34°540 S; 56°110 W, LD 12:12) and the Antarctic trip, which corresponded to the 2016 Uruguayan Summer Antarctic School (January 17–27, 2016, King George Island, 62°110 S; 58°520 W, LD 20:4). In the Montevideo baseline situation (MVD), students developed their regular lives, attending university courses with no restrictions nor special instructions. During the Antarctic trip (ANT), all the participants were living together, followed the same daily agenda (mealtimes at 08:00, 13:00 and 21:00, type of food intake, activities, etc.), and had an intense work schedule that was carried out regardless of the day of the week (workdays or weekend), including outdoor field trips on each morning and lab work and seminars in the afternoons. In MVD sampled weekends were excluded and only workdays were analyzed.
Chronotype
Chronotypes were assessed using the Spanish versions of both the Munich Chronotype Questionnaire (MCTQ)74,75 and the Morningness—Eveningness Questionnaire (MEQ)76,77. These questionnaires were answered during the first day of the MVD. MCTQ reports were used to calculate the mid-sleep point on free days corrected for sleep debt on work days (MSFsc)33 as an indicator of individual chronotype, in which higher values indicate later chronotypes. MEQ scores were calculated directly from the answers about sleep/activity preferences, and in this case, higher scores indicate earlier circadian preferences77.
Sleep logs (SL)
Participants were instructed to fill sleep logs with questions about the previous night, every morning during sample periods on both conditions. From the self-reported time of sleep onset and sleep end we calculated sleep duration (time interval from sleep onset to sleep end) and mid-sleep point (midpoint between sleep onset and sleep end). We also estimated sleep quality from a 100 mm subjective visual analogue scale (VAS) extending from “very bad night” to “very good night”78.
Melatonin
Hourly saliva samples (18:00–00:00 h) were collected in dim light (< 30 lx) on the last night of each sample period. Following Lewy13,14, saliva samples were obtained in the same settings for all participants in both conditions; i.e. participants remained in resting position in dark rooms, except for brief trips to the also dark bathroom from 17:00 to 00:00. Dim light was assessed by switching lights off (dark rooms) with led candles distributed in the corners of the room. We measured illuminance with a light meter to assure that light exposure remained below 30 lx in all places at all heights. Samples were then frozen and stored at − 20 °C, and later assayed for melatonin using the salivary melatonin competitive ELISA kit from Salimetrics following the manufacturer’s instructions. Tests were carried out by the same technician at the same time for 3 consecutive days, and control and treatment samples were combined in each ELISA plate. The concentration–response curves obtained were indistinguishable from those reported by the manufacturer. The same applied to the IC10 values (melatonin concentrations inhibiting 10% of the absorbance signal observed in the absence of melatonin), taken as an indication of assay sensitivity. This validates the use of the analytical sensitivity value reported by the manufacturer (1.37 pg/mL melatonin).
Circadian timing was determined by calculating the dim light melatonin onset (DLMO)14,79,80, as a marker of the individual circadian phase and, in particular, as an indicator of the beginning of the biological night. Following Silva et al.34 and Coirolo et al.68, individual basal melatonin levels were calculated as the average of the melatonin levels before the nocturnal increase. Individual DLMO was calculated as the interpolated point in time at which the quadratic fit surpassed 2 SD above the individual basal melatonin level in MVD samples. For each participant, the melatonin level corresponding to MVD DLMO was considered the individual melatonin threshold. ANT DLMO was calculated as the time at which the melatonin level reached the individual threshold. To measure the individual impact of ANT on the circadian phase we calculated the circadian phase shift as the difference between DLMO values for each participant: ΔDLMO = DLMOANT − DLMOMVD.
Actigraphy
Participants wore ambulatory wrist activity and light monitors on the non-dominant arm (GENEactiv Originals, Activinsights) continuously during both sample periods. Each participant wore the same device during both phases of the study. The device incorporated an expandable wristband allowing use over outdoor clothing. All devices were configured on the same computer and set to record at 10 Hz. Data was exported and converted to 1-min epochs using GENEActiv Windows Software from Activinsights. We analyzed actigraphy data with the integrated software El Temps (© Antoni Díez-Noguera, Barcelona, CA, Spain). From the activity and light exposure, we obtained individual actograms for all participants in both conditions. We also performed cosinor and non-parametric analyses on both physical activity and average light exposure data (in lux). These analyses yielded linear variables, including the average activity of the most active 10 h period (M10), the average light exposure of the brightest 10 h period (MB10), the average activity of the least active 5 h period (L5) and the average light exposure of the least bright 5 h period (LB5)81,82. We then estimated the activity relative amplitude (RA) as M10-L5/M10 + L582,83. We also estimated phase variables, including acrophase (the moment of the day in which the cosinor function reaches its maximum for both physical activity and light exposure); M10c (the midpoint of the most active 10 h period); MB10c (the midpoint of the brightest 10 h period); L5c (the midpoint of the least active 5 h period); and LB5c (the midpoint of the least bright 5 h period)20. For physical activity, the raw 10-Hz triaxial data were first summarized into the gravity-subtracted sum of vector magnitudes for each 1-min epoch intervals. The resulting units for this outcome variable are g·min84,85. We then calculated the average gravity-subtracted signal vector magnitude for each minute, thus converting physical activity values from a time-dependent unit (g·min) to the time-independent milligravitational units (mg) in order to compare with previously reported cut-points (moderate-vigorous physical activity above 100 mg, MVPA)86,87. To measure the impact of sleep timing as determinant of the change of individual circadian phase between ANT and MVD, we calculated the difference between L5c values for each participant: ΔL5c = L5cANT − L5cMVD.
We calculated for each participant the hourly average of light exposure across each sample period in both conditions in two 3 h-phase-relevant windows centered by individual DLMO5,57,68: in the phase-delay window (PDW, 3 h before the DLMO) and in the phase-advance window (PAW, 3 h before the DLMO antipode). To measure the impact of long-term light history on individual circadian phase shift between ANT and MVD, we calculated the difference between the average light exposure in both the PDW (Δ light exposure PDW = light exposure PDWANT − light exposure PDWMVD) and the PAW (Δ light exposure PAW = light exposure PAWANT − light exposure PAWMVD) for each participant.
Statistical analysis
Data are expressed as mean values ± standard deviation throughout the text unless otherwise stated and were compared by parametric test (paired-samples t-test) for comparison between ANT and MVD. When data did not comply with normality and/or homoscedasticity, statistical comparisons were analyzed by non-parametric test (Wilcoxon signed-rank test) for paired-samples comparison between ANT and MVD. Effect size was assessed by Cohen’s d estimate. Data of the sleep logs and actigraphy were aggregated per participant per condition except for the linear model that included repeated measures of self-reported mid-sleep point (estimated by sleep logs) and light exposure in the two 3 h-phase-relevant windows centered by individual DLMO. Linear model fitting was performed using the most recent shell of Rstudio88 (version: 2023.03.0386) and the “nlme” R-package for linear and non-linear Mixed-Effects Modeling. Associations between variables were assessed by Pearson correlations. Statistical procedures were carried out the software PAST89 and R Core Team. Values of p ≤ 0.05 were considered statistically significant.
Data availability
All data reported in this paper will be shared by the corresponding author upon request. Any additional information required to reanalyze the data reported in this paper is available from the corresponding author upon request.
References
Berson, D. M., Dunn, F. A. & Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295, 1070–1073 (2002).
Hattar, S. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
De Coursey, P. J. Daily light sensitivity rhythm in a rodent. Science 131, 33–35 (1960).
Honma, K., Honma, S. & Wada, T. Phase-dependent shift of free-running human circadian rhythms in response to a single bright pulse. Experientia 43, 1205–1207 (1987).
Khalsa, S. B. S., Jewett, M. E., Cajochen, C. & Czeisler, C. A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 549, 945–952 (2003).
Minors, D. S., Waterhouse, J. M. & Wirz-Justice, A. A human phase-response curve to light. Neurosci. Lett. 133, 36–40 (1991).
St Hilaire, M. A. et al. Human phase response curve to a 1 h pulse of bright white light: PRC to 1 h light pulses in humans. J. Physiol. 590, 3035–3045 (2012).
Blume, C., Garbazza, C. & Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 23, 147–156 (2019).
Lunn, R. M. et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 607–608, 1073–1084 (2017).
Stevens, R. G. & Zhu, Y. Electric light, particularly at night, disrupts human circadian rhythmicity: Is that a problem?. Philos. Trans. R. Soc. B Biol. Sci. 370, 20140120 (2015).
Phillips, A. J. K. et al. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.1901824116 (2019).
Duffy, J. F. & Wright, K. P. Entrainment of the human circadian system by light. J. Biol. Rhythms 20, 326–338 (2005).
Lewy, A. J. & Sack, R. L. The dim light melatonin onset as a marker for Orcadian phase position. Chronobiol. Int. 6, 93–102 (1989).
Lewy, A. J., Cutler, N. L. & Sack, R. L. The endogenous melatonin profile as a marker for circadian phase position. J. Biol. Rhythms 14, 227–236 (1999).
Wams, E. J. et al. Linking light exposure and subsequent sleep: A field polysomnography study in humans. Sleep 40, 165 (2017).
Moreno, C. R. C. et al. Sleep patterns in Amazon rubber tappers with and without electric light at home. Sci. Rep. 5, 14074 (2015).
Stothard, E. R. et al. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr. Biol. 27, 508–513 (2017).
Arendt, J. Biological rhythms during residence in polar regions. Chronobiol. Int. 29, 379–394 (2012).
Arendt, J. & Middleton, B. Human seasonal and circadian studies in Antarctica (Halley, 75°S). Gen. Comp. Endocrinol. 258, 250–258 (2018).
Lowden, A. et al. Delayed sleep in winter related to natural daylight exposure among arctic day workers. Clocks Sleep 1, 105–116 (2018).
Wright, K. P. et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 23, 1554–1558 (2013).
Chellappa, S. L. Individual differences in light sensitivity affect sleep and circadian rhythms. Sleep 44, zsaa214 (2021).
Spitschan, M. & Santhi, N. Individual differences and diversity in human physiological responses to light. eBioMedicine 75, 103640 (2022).
Vetter, C. et al. A review of human physiological responses to light: Implications for the development of integrative lighting solutions. LEUKOS 18, 387–414 (2022).
Akacem, L. D., Wright, K. P. & LeBourgeois, M. K. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol. Rep. 6, e13617 (2018).
Crowley, S. J., Cain, S. W., Burns, A. C., Acebo, C. & Carskadon, M. A. Increased sensitivity of the circadian system to light in early/mid-puberty. J. Clin. Endocrinol. Metab. 100, 4067–4073 (2015).
Higuchi, S., Nagafuchi, Y., Lee, S. & Harada, T. Influence of light at night on melatonin suppression in children. J. Clin. Endocrinol. Metab. 99, 3298–3303 (2014).
Eastman, C. I., Suh, C., Tomaka, V. A. & Crowley, S. J. Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci. Rep. 5, 8381 (2015).
Egan, K. J., Knutson, K. L., Pereira, A. C. & von Schantz, M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med. Rev. 33, 70–78 (2017).
Paech, G. M., Crowley, S. J., Fogg, L. F. & Eastman, C. I. Advancing the sleep/wake schedule impacts the sleep of African-Americans more than European-Americans. PLoS ONE 12, e0186887 (2017).
Chellappa, S. L., Steiner, R., Oelhafen, P. & Cajochen, C. Sex differences in light sensitivity impact on brightness perception, vigilant attention and sleep in humans. Sci. Rep. 7, 14215 (2017).
Cowan, R. L. et al. Sex differences in response to red and blue light in human primary visual cortex: A bold fMRI study. Psychiatry Res. Neuroimaging 100, 129–138 (2000).
Roenneberg, T. et al. Epidemiology of the human circadian clock. Sleep Med. Rev. 11, 429–438 (2007).
Silva, A. et al. Chronotype-dependent changes in sleep habits associated with dim light melatonin onset in the Antarctic summer. Clocks Sleep 1, 352–366 (2019).
Chang, A.-M., Scheer, F. A. J. L., Czeisler, C. A. & Aeschbach, D. Direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans depend on prior light history. Sleep 36, 1239–1246 (2013).
Hébert, M., Martin, S. K., Lee, C. & Eastman, C. I. The effects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res. 33, 198–203 (2002).
Smith, K. A., Schoen, M. W. & Czeisler, C. A. Adaptation of human pineal melatonin suppression by recent photic history. J. Clin. Endocrinol. Metab. 89, 3610–3614 (2004).
Kawasaki, A. et al. Impact of long-term daylight deprivation on retinal light sensitivity, circadian rhythms and sleep during the Antarctic winter. Sci. Rep. 8, 16185 (2018).
Owen, J. & Arendt, J. Melatonin suppression in human subjects by bright and dim light in Antarctica: Time and season-dependent effects. Neurosci. Lett. 137, 181–184 (1992).
Tassino, B., Horta, S., Santana, N., Levandovski, R. & Silva, A. Extreme late chronotypes and social jetlag challenged by antarctic conditions in a population of university students from Uruguay. Sleep Sci. 9, 20–28 (2016).
Collet, G. et al. Altitude and seasonality impact on sleep in Antarctica. Aerosp. Med. Hum. Perform. 86, 392–396 (2015).
Pattyn, N. et al. Sleep during an Antarctic summer expedition: New light on “polar insomnia”. J. Appl. Physiol. 122, 788–794 (2017).
Weissová, K., Škrabalová, J., Skálová, K., Bendová, Z. & Kopřivová, J. The effect of a common daily schedule on human circadian rhythms during the polar day in svalbard: A field study. J. Circadian Rhythms 17, 9 (2019).
Leppäluoto, J., Sikkilä, K., Meyer-Rochow, V. B. & Hassi, J. Low melatonin secretion associates with albedo in circumpolar environments: Seasonal melatonin. J. Pineal Res. 35, 158–162 (2003).
Nelson, K. L., Davis, J. E. & Corbett, C. F. Sleep quality: An evolutionary concept analysis. Nurs. Forum (Auckl.) 57, 144–151 (2022).
Figueiro, M. G. et al. Effects of a tailored lighting intervention on sleep quality, rest-activity, mood, and behavior in older adults with Alzheimer disease and related dementias: A randomized clinical trial. J. Clin. Sleep Med. 15, 1757–1767 (2019).
Juda, M., Liu-Ambrose, T., Feldman, F., Suvagau, C. & Mistlberger, R. E. Light in the senior home: Effects of dynamic and individual light exposure on sleep, cognition, and well-being. Clocks Sleep 2, 557–576 (2020).
Lemola, S., Ledermann, T. & Friedman, E. M. Variability of sleep duration is related to subjective sleep quality and subjective well-being: An actigraphy study. PLoS ONE 8, e71292 (2013).
Pye, J. et al. Irregular sleep-wake patterns in older adults with current or remitted depression. J. Affect. Disord. 281, 431–437 (2021).
Redline, S., Redline, B. & James, P. Sleep epidemiology: An introduction. In Social Epidemiology of Sleep (Oxford University Press, 2019).
Stepanski, E. J. & Wyatt, J. K. Use of sleep hygiene in the treatment of insomnia. Sleep Med. Rev. 7, 215–225 (2003).
Weymouth, W. & Steel, G. D. Sleep patterns during an antarctic field expedition. Mil. Med. 178, 438–444 (2013).
Reiter, A. M., Sargent, C. & Roach, G. D. Finding DLMO: Estimating dim light melatonin onset from sleep markers derived from questionnaires, diaries and actigraphy. Chronobiol. Int. 37, 1412–1424 (2020).
Najjar, R. P. et al. Chronic artificial blue-enriched white light is an effective countermeasure to delayed circadian phase and neurobehavioral decrements. PLoS ONE 9, e102827 (2014).
Sletten, T. L., Revell, V. L., Middleton, B., Lederle, K. A. & Skene, D. J. Age-related changes in acute and phase-advancing responses to monochromatic light. J. Biol. Rhythms 24, 73–84 (2009).
Reiter, A. M., Sargent, C. & Roach, G. D. Concordance of chronotype categorisations based on dim light melatonin onset, the morningness-eveningness questionnaire, and the munich chronotype questionnaire. Clocks Sleep 3, 342–350 (2021).
Ruiz, F. S. et al. Early chronotype with advanced activity rhythms and dim light melatonin onset in a rural population. J. Pineal Res. 69, 12675 (2020).
Crowley, S. J., Acebo, C., Fallone, G. & Carskadon, M. A. Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep 29, 1632–1641 (2006).
Razavi, P. et al. Shift work, chronotype, and melatonin rhythm in nurses. Cancer Epidemiol. Biomark. Prev. 28, 1177–1186 (2019).
Zerbini, G., Winnebeck, E. C. & Merrow, M. Weekly, seasonal, and chronotype-dependent variation of dim-light melatonin onset. J. Pineal Res. 70, 12723 (2021).
Prayag, A. S., Najjar, R. P. & Gronfier, C. Melatonin suppression is exquisitely sensitive to light and primarily driven by melanopsin in humans. J. Pineal Res. 66, e12562 (2019).
Rea, M. S., Nagare, R. & Figueiro, M. G. Modeling circadian phototransduction: Retinal neurophysiology and neuroanatomy. Front. Neurosci. 14, 615305 (2021).
Hashimoto, S. et al. Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci. Lett. 221, 89–92 (1997).
Kozaki, T., Kubokawa, A., Taketomi, R. & Hatae, K. Light-induced melatonin suppression at night after exposure to different wavelength composition of morning light. Neurosci. Lett. 616, 1–4 (2016).
Kripke, D. F., Elliott, J. A., Youngstedt, S. D. & Rex, K. M. Circadian phase response curves to light in older and young women and men. J. Circadian Rhythms 5, 4 (2007).
Takasu, N. N. et al. Repeated exposures to daytime bright light increase nocturnal melatonin rise and maintain circadian phase in young subjects under fixed sleep schedule. Am. J. Physiol. 291, R1799–R1807 (2006).
Gooley, J. J. et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 96, E463–E472 (2011).
Coirolo, N., Casaravilla, C., Tassino, B. & Silva, A. Evaluation of environmental, social, and behavioral modulations of the circadian phase of dancers trained in shifts. iScience 25, 104676 (2022).
Adamsson, M., Laike, T. & Morita, T. Annual variation in daily light exposure and circadian change of melatonin and cortisol concentrations at a northern latitude with large seasonal differences in photoperiod length. J. Physiol. Anthropol. 36, 6 (2017).
Diekman, C. O. & Bose, A. Reentrainment of the circadian pacemaker during jet lag: East-west asymmetry and the effects of north-south travel. J. Theor. Biol. 437, 261–285 (2018).
Dumont, M. & Beaulieu, C. Light exposure in the natural environment: Relevance to mood and sleep disorders. Sleep Med. 8, 557–565 (2007).
Beck, A. T., Steer, R. A. & Carbin, M. G. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 8, 77–100 (1988).
World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 310, 2191 (2013).
Morales, J. F. D. & García, M. A. Relaciones entre matutinidad-vespertinidad y estilos de personalidad. An. Psicol. 19, 247–256 (2003).
Roenneberg, T., Wirz-Justice, A. & Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms 18, 80–90 (2003).
Adan, A. & Almirall, H. Adaptation and standardization of a Spanish version of the morningness-eveningness questionnaire: Individual differences. Personal. Individ. Differ. 11, 1123–1130 (1990).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 4, 97–110 (1976).
Corbett, R. W., Middleton, B. & Arendt, J. An hour of bright white light in the early morning improves performance and advances sleep and circadian phase during the Antarctic winter. Neurosci. Lett. 525, 146–151 (2012).
Wehr, T. A. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J. Clin. Endocrinol. Metab. 73, 1276–1280 (1991).
Wehr, T. A., Aeschbach, D. & Duncan, W. C. Evidence for a biological dawn and dusk in the human circadian timing system. J. Physiol. 535, 937–951 (2001).
McGowan, N. M. & Coogan, A. N. Sleep and circadian rhythm function and trait impulsivity: An actigraphy study. Psychiatry Res. 268, 251–256 (2018).
Van Someren, E. J. W. et al. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in alzheimer patients by application of nonparametric methods. Chronobiol. Int. 16, 505–518 (1999).
Lyall, L. M. et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91,105 participants from the UK Biobank. Lancet Psychiatry 5, 507–514 (2018).
Esliger, D. W. et al. Validation of the GENEA Accelerometer. Med. Sci. Sports Exerc. 43, 1085–1093 (2011).
Phillips, L. R. S., Parfitt, G. & Rowlands, A. V. Calibration of the GENEA accelerometer for assessment of physical activity intensity in children. J. Sci. Med. Sport 16, 124–128 (2013).
Hildebrand, M., Van Hees, V. T., Hansen, B. H. & Ekelund, U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exerc. 46, 1816–1824 (2014).
Okely, A. D. et al. Wrist acceleration cut points for moderate-to-vigorous physical activity in youth. Med. Sci. Sports Exerc. 50, 609–616 (2018).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021). https://www.R-project.org/.
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package for education and data analysis palaeontol. Electronica 4, 1–9 (2001).
Acknowledgements
We wish to thank the students of the Second Summer School on Introduction to Antarctic Research, who kindly volunteered as participants of this study. We are especially thankful to the Instituto Antártico Uruguayo for the logistic support. Financial support was provided by Comisión Sectorial de Investigación Científica (CSIC I+D_2016/623 and CSIC Grupos 2018 #92) and Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay. Julieta Castillo was supported by a scholarship from Agencia Nacional de Investigación e Innovación, Uruguay.
Author information
Authors and Affiliations
Contributions
Funding, project administration, experimental design, interpretation of data and writing original draft, B.T. and A.S..; data processing, statistical analysis and figure design, J.C.; supervision of actimetric data processing and review of the manuscript ACT and MPH. All authors contribute to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castillo, J., Tonon, A.C., Hidalgo, M.P. et al. Individual light history matters to deal with the Antarctic summer. Sci Rep 13, 12081 (2023). https://doi.org/10.1038/s41598-023-39315-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39315-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.