Abstract
Previous studies have documented natural infections of SARS-CoV-2 in various domestic and wild animals. More recently, studies have been published noting the susceptibility of members of the Cervidae family, and infections in both wild and captive cervid populations. In this study, we investigated the presence of SARS-CoV-2 in mammalian wildlife within the state of Vermont. 739 nasal or throat samples were collected from wildlife throughout the state during the 2021 and 2022 harvest season. Data was collected from red and gray foxes (Vulpes vulples and Urocyon cineroargentus, respectively), fishers (Martes pennati), river otters (Lutra canadensis), coyotes (Canis lantrans), bobcats (Lynx rufus rufus), black bears (Ursus americanus), and white-tailed deer (Odocoileus virginianus). Samples were tested for the presence of SARS-CoV-2 via quantitative RT-qPCR using the CDC N1/N2 primer set and/or the WHO-E gene primer set. Surprisingly, we initially detected a number of N1 and/or N2 positive samples with high cycle threshold values, though after conducting environmental swabbing of the laboratory and verifying with a second independent primer set (WHO-E) and PCR without reverse transcriptase, we showed that these were false positives due to plasmid contamination from a construct expressing the N gene in the general laboratory environment. Our final results indicate that no sampled wildlife were positive for SARS-CoV-2 RNA, and highlight the importance of physically separate locations for the processing of samples for surveillance and experiments that require the use of plasmid DNA containing the target RNA sequence. These negative findings are surprising, given that most published North America studies have found SARS-CoV-2 within their deer populations. The absence of SARS-CoV-2 RNA in populations sampled here may provide insights in to the various environmental and anthropogenic factors that reduce spillover and spread in North American’s wildlife populations.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome associated coronavirus-2 (SARS-CoV-2), the virus that causes COVID-19, is most recognized for its ability to easily transmit from person-to-person. Recently, natural infections in a range of domestic and wild animals have also been documented1,2,3,4. With every new animal infected, the zoonotic potential of SARS-CoV-2 increases. Animal species that facilitate within-species transmission of SARS-CoV-2 are possible new reservoirs of the virus, and this transmission could lead to evolutionary changes in the virus that would pose a risk to humans upon reintroduction. In fact, this exact scenario occurred during 2020, with SARS-CoV-2 infection documented in farmed minks5,6. Notably, the Netherlands reported five different outbreak events in 2020, resulting in over 50% of mink farms having animals that tested positive for SARS-CoV-2. At over half of the farms with positive animals, employees also tested positive for SARS-CoV-2. Sequencing data from both the mink and humans suggests that both spillover, the transmission of disease from animals to humans, and spillback, the transmission of disease from humans to animals, occurred several times between these two populations6. Infected animals were detected in mink farms in multiple other countries which led to the selective culling of animals at affected farms, as well as the culling of all (> 17 million) mink in Denmark, to reduce the risk of spillover7,8.
Multiple recent studies in North America have shown that members of the Cervidae family are susceptible to SARS-CoV-2. We hypothesized that SARS-CoV-2 might be circulating in Vermont deer and wildlife, given the numerous reports of infections within wild deer populations4,9,10,11,12,13,14,15, and laboratory infections showing vertical16 and horizontal transmission17. North American deer are of particular concern as they are common, interact with humans, and are also domestically farmed. All three of these factors create opportunities for spillover and spillback events. The 2021 estimate for Vermont’s white-tailed deer population was approximately 133,00018, which is about a 1:5 deer-to-person ratio within the state19. While SARS-CoV-2 has been detected in wildlife in several US states and Canadian provinces, there is currently no published data on the virus in wildlife in the state of Vermont.
In this study, we examined the prevalence of SARS-CoV-2 viral RNA in a variety of animals native to Vermont via reverse transcription quantitative polymerase-chain reaction (RT-qPCR), using two different primer sets specific to SARS-CoV-2. We sampled fur-bearing animals including red and grey foxes (Vulpes vulples and Urocyon cineroargentus, respectively), fishers (Martes pennati), otters (Lutra canadensis), coyotes (Canis lantrans), bobcats (Lynx rufus rufus), and big-game animals including white-tailed deer (Odocoileus virginianus) and black bears (Ursus americanus) over the 2021 and 2022 hunting and trapping seasons.
Results
Our SARS-CoV-2 surveillance effort covered the state of Vermont through the hunting and trapping seasons of 2021 (Oct 2021–March 2022) and the hunting season of 2022 (Oct–Nov 2022). We prioritized white-tailed deer during the 2022 season, given their abundance, potential interaction with humans, and our ability to collect high-quality samples for processing. In addition to white-tailed deer, the 2021 season also included a variety of fur-bearing animals that are commonly trapped in VT, including foxes, fishers, otters, coyotes, and bobcats. In 2021, we sampled 17 white-tailed deer as well as 250 fur-bearers (Table 1). However, most of our white-tailed deer sampling occurred during the 2022 season, where we were able to sample 470 white-tailed deer as well as 2 black bears (Table 1). Sampled animals were harvested across the state of Vermont, generating samples from a broad geographic range (Fig. 1, Fig. S1). At the conclusion of the 2021 season, we extracted RNA from all collected samples and performed RT-qPCR using the Centers for Disease Control and Prevention (CDC) SARS-CoV-2 N1 and N2 primer set for the structural nucleocapsid protein20 to test for the presence of viral RNA. We found no detectable SARS-CoV-2 RNA within any sample from the 2021 season (n = 272). Positive control wells on each plate amplified as expected, as did the internal control included in each sample extraction to confirm RNA integrity and rule out PCR inhibition. (Dataset S1).
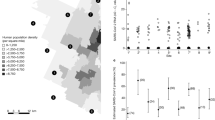
Geographic distribution of specimen harvest. Geographic distribution of wildlife sampled for SARS-CoV-2 in Vermont during the 2021 and 2022 hunting seasons is shown. Specimens are shown based on the reported town where the harvest occurred and colored according to the number of samples collected from each location. Graphs were generated using QGIS version 3.28.2 (Firenze) https://qgis.org/.
Surprisingly, when we analyzed samples from the 2022 season (n = 472), we observed a positivity rate of 28.2% of samples positive for both the N1 and N2 primers (133/472). The average cycle threshold (CT) for these samples was 36.6 for N1 and 38.0 for N2 (SD = 1.3 and 1.4, respectively). There were multiple additional samples positive by either the N1 or N2 primer sets, but not by both (N1 only = 28 samples, N2 only = 56 samples) (Dataset S1). The suddenly high number of positive samples, paired with the high average CT values and the lack of any samples with a CT < 30 for N1 or CT < 33 for N2 raised concerns that these initial numbers from the 2022 season may have been the result of contamination. In the period between processing the 2021 and 2022 samples, The University of Vermont’s laboratory began a separate project that involved in vitro expression of the SARS-CoV-2 nucleocapsid protein, and thus a DNA construct containing the sequences recognized by the N1 and N2 primer sets was newly present in the general laboratory environment.
Therefore, we set out to determine if the positive results seen with the N1/N2 primers were authentic or the result of plasmid DNA contamination in the laboratory environment from the University of Vermont during the sample aliquoting, before any sample analysis. First, we performed environmental swabbing of commonly used items and surfaces within the laboratory, including within the biosafety cabinet used to aliquot the wildlife specimens before RT-qPCR testing, the pipettes used for aliquoting, the laboratory bench, and pipettes. We detected SARS-CoV-2 N nucleic acids on all surface swabs with both the N1 and N2 primers with CTs as low as 23.6 (Dataset S2). None of the negative controls for the RT-qPCR reaction amplified. To determine if we were detecting RNA or DNA contamination, we next performed a quantitative polymerase chain reaction (qPCR) in which the typical incubation for reverse transcription was omitted, instead beginning directly with a 95 °C step to deactivate RT and activate hot-start Taq. The positive controls (remnant SARS-CoV-2 positive clinical specimen) included in these experiments exhibited an average N1 CT 5.4 ± 0.6 cycles higher in qPCR experiment than in RT-qPCR, as expected for samples where the input material was RNA rather than DNA (Fig. 2). Two of the three positive controls were undetectable with the N2 primer set in qPCR experiments; for the third, the N2 CT was 1.8 cycles higher in qPCR experiment than in RT-qPCR. In contrast, all laboratory sites sampled (except the biosafety cabinet floor, which had the highest CT originally) showed consistent CTs between RT-qPCR and qPCR reactions (average N1 CT 0.3 ± 0.9 cycles higher in qPCR experiment than in RT-qPCR), suggesting that the surface contamination consisted of DNA rather than RNA (Fig. 2).
Environmental Swabs Show Contamination of SARS-CoV-2 Nucleocapsid DNA in Laboratory Environment. Samples from residual clinical SARS-CoV-2 positive specimens (pos #1–3), laboratory surfaces and equipment used to process/aliquot field samples (BSC = Biosafety Cabinet) and a selection of deer that initially tested positive for SARS-CoV-2 nucleic acid with the N1 primer set were analyzed by RT-PCR (to detect either RNA or DNA) and PCR (to detect RNA). The difference in cycle threshold (CT) between PCR and RT-PCR for each sample is shown. Positive control clinical samples shown in orange circles, environmental swabs shown in blue squares, deer samples shown in gray triangles.
Next, we compared qPCR and RT-qPCR amplification on select deer specimens that showed amplification with either the N1 or N2 primer sets (see Fig. 2 for a subset and Dataset S2 for complete data). In each case, we were still able to detect viral nucleic acids, and as seen in the surface swabs the CTs were consistent between RT-qPCR and qPCR reactions (average N1 CT 1.1 ± 1.4 cycles lower in qPCR experiment than in RT-qPCR). This result indicated that the original N1/N2 results were most likely detecting DNA contamination. The contamination likely occurred during the aliquoting step (after specimen collection), and illustrates the great difficulty posed by performing RT-qPCR based surveillance efforts in tandem with experiments that require the handling of plasmid DNA or PCR product without a physically separate facility, as previously reported21,22,23,24. Given the similar CT values for both the RT-qPCR and qPCR of laboratory surfaces and deer specimen samples, as well as the presence of N gene plasmid DNA (and associated contamination) in the laboratory where the samples were aliquoted, we concluded that the 2022 N1/N2 results were false positives.
To accurately detect the presence of SARS-CoV-2 viral RNA in the 2022 season samples, we repeated our RT-qPCR analysis using a new and independent set of primers, this time targeting the E gene25 rather than N. There was no E gene plasmid DNA present in the laboratory in which these samples were processed and aliquoted, and no E amplification products were present at any point in the study. All 474 samples from the 2022 season were undetectable by the E gene primer/probe set, indicating that there was no detectable SARS-CoV-2 viral RNA in any Vermont wildlife surveilled during the 2021 or 2022 seasons (Dataset S1).
Discussion
White-tailed deer can both successfully be infected with and transmit SARS-CoV-2. This has been demonstrated by both laboratory studies16,17 and several reports of naturally infected deer in multiple states and provinces within the United States and Canada4,9,10,11,12,13,14,15. Since prior surveillance studies have reported RT-qPCR positivity rates of upwards of 30% in nasal swabs4 and seropositivity rates of more than 40%12,26, it was initially surprising that no animals within the Vermont sample set were positive, especially during the 2022 season. However, recent work from Diel et al. describing the spread of SARS-CoV-2 within deer in New York state during the 2021 and 2022 seasons showed only sporadic positives during 2021 and a significant increase (up to 20%) in the 2022 season15. Furthermore, the majority of positive cases were detected in the western half of New York and near New York City, the farthest regions geographically from the Vermont border14. A second study furthers this argument, revealing the relatively low positivity rate of 1.2% within the Quebec province in Canada, directly north of Vermont27. Therefore, SARS-CoV-2 may be circulating in Vermont deer at a low level. To assess our ability to detect this, we performed a power analysis to calculate the probability of detecting one case of SARS-CoV-2 within our 472 samples from the 2022 deer season as a function of underlying SARS-CoV-2 prevalence. If we were to repeat our surveillance efforts, we would expect to find at least one positive sample 80% of the time if the underlying prevalence were at least 0.34%; similarly, we have 95% power to detect from a population that was 0.64% positive, and there is only a 1% chance of our sampling no positives if the population were 0.97% positive (Fig. S2).
While it has not been established how SARS-CoV-2 is introduced into wild deer populations, it seems likely that this occurs via human-to-deer transmission, deer-to-deer transmission, or a combination of the two26,28,29. Vermont may have multiple features that reduced the risk of human-to-deer transmission so far in the COVID-19 pandemic. First, the state of Vermont is sparsely populated in general, but especially in many of the places where deer are hunted, therefore reducing the potential for human-deer interaction. Additionally, the number of COVID-19 human infections within the state of Vermont was much lower than most other places within the USA (including neighboring states with higher levels of SARS-CoV-2 detected in deer) during the period in which we were conducting surveillance (Fig. 3, Dataset S3).
Case Counts and Prevalence of COVID-19 in Vermont and New York. Geographic distribution of COVID-19 cases (reported by the Vermont Department of Health and New York State Department of Health) during the during the surveillance period for the 2022 season (Oct 15th–Nov 15th 2022) at the county level. Raw case counts are shown on left and prevalence (case counts/county population) is shown on right (population counts are an estimate based on US Census 2022 data). Graphs were generated using QGIS version 3.28.2 (Firenze) https://qgis.org/.
Finally, Vermont lacks an established deer farm industry, with only three farms reported in 201730, all of which contain cervid species other than white-tailed deer since it is illegal to have captive white-tailed deer in Vermont. This agricultural set-up decreases the number and duration of direct contact between humans and cervids in the state. Transmission between farmed animals (such as mink) and farm employees that care for them has been well documented for viruses including SARS-CoV-2 and is a plausible route for the initial introduction of SARS-CoV-2 into deer populations as well31. Texas, Pennsylvania, Indiana, Ohio, and Michigan alone account for over 65% of deer farms within the United States30 and several of these states have also reported high rates of SARS-CoV-2 prevalence in captive and/or wild deer9,11,12,13,31.
A limitation of this study is the lack of samples other than nasal swabs, such as retropharyngeal lymph nodes or blood samples. Retropharyngeal lymph nodes (RPLNs) are commonly collected as part of surveillance efforts for chronic wasting disease; however, VT only conducts surveillance for this disease when warranted by clinical signs/symptoms currently, and not on hunter harvested deer. A 2022 study from Ontario, Canada reported a 2.3% (5/213) positivity in nasal swabs, compared to a 6% (17/298) positivity in retropharyngeal lymph nodes within the white-tailed deer they sampled, potentially demonstrating the increased sensitivity of RPLNs samples to detect SARS-CoV-2 in this species10. Since no blood samples or lymph nodes were collected in this study, we were unable to perform serology experiments to detect the presence of SARS-CoV-2 antibodies that would reveal SARS-CoV-2 disease history. The results reported here only represent a lack of active infections in the animals surveilled at a single discrete timepoint. While information into the natural history of SARS-CoV-2 infections in wildlife during the 2022 season would be highly informative, the lack of standard collection of blood samples at Vermont hunting check stations made collecting samples for serology logistically prohibitive for a number of reasons, including cost, training of individuals collecting samples, and the timeline of proposal to implementation of this project. Nonetheless, this sample represents the first data on SARS-CoV-2 RNA surveillance in Vermont wildlife and may provide a helpful baseline for future surveillance studies.
An additional limitation of this study regards the acquisition of 2021 season samples. All fur-bearing specimens were collected throughout 2021, stored at −20 °C, and then thawed in a batch-wise fashion for sample collection. These specimens were generously made available to us by Vermont Agency of Natural Resources, Department of Fish & Wildlife but were independently collected for other surveillance purposes. As specimen collection was independent of our lab, specimen storage duration and temperature were factors beyond our scope to control. While SARS-CoV-2 RNA is relatively stable through repeated freeze–thaw cycles and storage at −20 °C)32, for the remainder of the study we chose to shift our focus to samples that we could collect from freshly deceased animals and quickly freeze at −80 °C to minimize unintended variables from the sample collection process. Thus, it should be noted that the samples collected in the 2021 and 2022 seasons represent largely two separate populations and cannot be directly compared.
While our findings indicate that there does not appear to be widespread SARS-CoV-2 in Vermont deer are reassuring at present, we do not expect this to continue indefinitely considering the increasing cases detected in the wildlife of neighboring regions15. Surveillance efforts to help detect the transmission and adaptation of SARS-CoV-2 in wildlife should be established throughout North America and should ideally prioritize species susceptible to infection. Ongoing surveillance studies will be required to understand not only the status of SARS-CoV-2 in Vermont wildlife populations, but also to understand the transmission and spread of the disease over time. Efforts to monitor the prevalence and mutational changes in SARS-CoV-2 viral genome are especially important within common and social species, such as white-tailed deer. The human health implication of deer as a SARS-CoV-2 reservoir is a sincere concern and warrants continued surveillance as a crucial measure in pandemic preparedness.
Methods
Wildlife specimen procurement
All samples were collected in collaboration with the Vermont Agency of Natural Resources, Department of Fish & Wildlife. All white-tailed deer and bear samples were collected during the Vermont hunting season. For the 2021 season, deer samples were collected on the opening weekend of rifle season (November 12th, 2021). For the 2022 season, samples were collected on youth weekend (Oct 22-23rd, 2022) and the opening weekend of rifle season (November 12th–13th, 2022). During these dates, samples were collected across the state of Vermont from deceased animals brought to big game reporting stations by hunters.
For fur-bearing animals (i.e., foxes, otters, coyotes, bobcats, and fishers), whole-animal carcass specimens were collected throughout the entirety of 2021 and stored at −20 °C until SARS-CoV-2 swab sample collection occurred in a batch-wise fashion during February–March 2022. Most whole-animal specimens were collected between October 2021 and March 2022 and therefore stored for only a few months (details for individual specimens available in Dataset S1).
2022 season samples were all collected from recently deceased animals brought by hunters to big game weigh stations. As animals are field dressed and brought directly to the game station, each sample was collected from an animal that had been deceased for a matter of hours at the time of collection and never frozen. A small number of deer were brought to the weigh stations the following day (8+ hours postmortem), but these are denoted in the data set.
For both the 2021 and 2022 season, nasal swabs were collected by inserting a dry, sterile swab (Copan #164KS01) approximately 1 inch into each nasal cavity of the specimen and making five passes around the interior of the nostril, ensuring even contact with the wall of the cavity. If the nasal cavity was inaccessible, throat swabs were taken by inserting the swab as far back into the throat as possible and making five passes around the entire circumference (denoted in Dataset S1). Samples were stored in 3 mL of phosphate-buffered saline (Gibco #10010023) on ice until returning to the laboratory where they were transferred to −80 °C until further use. At the conclusion of the deer sampling period in 2022, we thawed all collected samples and divided each sample into two aliquots (one for RT-qPCR and one for potential future viral isolation). As we did not detect any samples that were positive for SARS-CoV-2 viral RNA, we did not attempt virus isolation.
Environmental swabbing for laboratory plasmid contamination
Environmental contamination samples were collected by rubbing the surface of interest with a dry, sterile swab (Copan #164KS01) for approximately 10 s, rolling the swab during this time to ensure maximal surface contact. Samples were stored in 1 mL of phosphate-buffered saline (Gibco #10010023) and stored at −80 °C until nucleic acid extraction and amplification could occur.
Nucleic acid extraction and amplification
All 2022 season samples were thawed once and aliquoted at the University of Vermont between sample collection and extraction. All further processing and testing of swabs took place in a Clinical Laboratory Improvement Amendments (CLIA)- and College of American Pathologists (CAP)-certified facility at the University of Washington Virology Laboratory.
Total nucleic acids (TNA) were extracted using Roche MagNA Pure 96 instruments as previously described33, with 200µL of swab liquid extracted and eluted into 50µL. Each extraction plate included a positive control (pooled SARS-CoV-2-positive clinical remnants) and a negative control (cells derived from a HeLa cell line). All amplifications used AgPath ID One-Step RT-PCR enzyme and master mix (Life Technologies, ThermoFisher, Cat. #4387424 M) and 10µL of TNA per reaction and were carried out on ABI 7500 thermocyclers. In addition to the positive and negative controls from each extraction, each amplification plate contained a No-Template negative control (NTC; water). One of two primer/probe sets was used in all reactions: WHO-E25; or multiplexed CDC N1 and N234. The WHO-E primer probe set was utilized after the discovery of SARS-CoV-2 N plasmid contamination in the laboratory, to eliminate false-positive results. Only 2022 samples which were positive via the CDC N1/N2 primer/probe set were re-run using the WHO-E set. RT-qPCR amplifications consisted of 10’ at 48 °C (reverse transcription), 10’ at 95 °C (Reverse Transcriptase inactivation / polymerase hot-start), and 40 cycles of 15″ at 95 °C and 45″ at 60 °C. qPCR amplifications used the same cycling conditions but omitted the initial 10’ at 48 °C step. EXO RNA was added to all samples prior to extraction, and EXO amplification was included in every RT-qPCR reaction as an internal control to monitor for RNA degradation and PCR inhibition34.
Data availability
All code, supplemental manuscript metadata, and supporting information can be found in GitHub @emilybrucelab (https://github.com/emilybrucelab).
References
McAloose, D., Laverack, M., Wang, L., et al. From people to panthera: Natural SARS-CoV-2 infection in tigers and lions at the bronx zoo. mBio. 2020;11(5):e02220–20. https://doi.org/10.1128/mBio.02220-20
Schulz, C., Martina, B., Mirolo, M., et al. SARS-CoV-2–specific antibodies in domestic cats during first COVID-19 wave, Europe—Volume 27, Number 12 December 2021. Emerg. Infect. Dis. J. - CDC. https://doi.org/10.3201/eid2712.211252
Sit, T. H. C. et al. Infection of dogs with SARS-CoV-2. Nature 586(7831), 776–778. https://doi.org/10.1038/s41586-020-2334-5 (2020).
Hale, V. L. et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 602(7897), 481–486. https://doi.org/10.1038/s41586-021-04353-x (2022).
Oude Munnink, B. B. et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 371(6525), 172–177. https://doi.org/10.1126/science.abe5901 (2021).
Lu, L. et al. Adaptation, spread and transmission of SARS-CoV-2 in farmed minks and associated humans in the Netherlands. Nat Commun. 12(1), 6802. https://doi.org/10.1038/s41467-021-27096-9 (2021).
Mutant coronaviruses found in mink spark massive culls and doom a Danish group’s research. Accessed 10 March 2023. https://www.science.org/content/article/mutant-coronaviruses-found-mink-spark-massive-culls-and-doom-danish-group-s-research
Coronavirus: Denmark shaken by cull of millions of mink. BBC News. https://www.bbc.com/news/world-europe-54890229. Published November 11, 2020. Accessed 10 March 2023.
Kuchipudi, S. V., Surendran-Nair, M., Ruden, R. M., et al. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proc. Natl. Acad. Sci. 119(6), e2121644119. https://doi.org/10.1073/pnas.2121644119 (2022)
Pickering, B. et al. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nat Microbiol. 7(12), 2011–2024. https://doi.org/10.1038/s41564-022-01268-9 (2022).
Marques, A. D., Sherrill-Mix, S., Everett, J. K., et al. Multiple introductions of SARS-CoV-2 alpha and delta variants into white-tailed deer in Pennsylvania. mBio. 13(5), e02101–22. https://doi.org/10.1128/mbio.02101-22 (2022).
Chandler, J. C., Bevins, S. N., Ellis, J. W., et al. SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proc. Natl. Acad. Sci. 118(47), e2114828118. https://doi.org/10.1073/pnas.2114828118 (2021)
Palermo, P. M., Orbegozo, J., Watts, D. M. & Morrill, J. C. SARS-CoV-2 neutralizing antibodies in white-tailed deer from Texas. Vector-Borne Zoonotic Dis. 22(1), 62–64. https://doi.org/10.1089/vbz.2021.0094 (2022).
Vandegrift, K. J., Yon, M., Surendran Nair, M., et al. SARS-CoV-2 omicron (B.1.1.529) infection of wild white-tailed deer in New York City. Viruses 14(12), 2770. https://doi.org/10.3390/v14122770 (2022).
Caserta, L. C., Martins, M., Butt, S. L., et al. White-tailed deer (Odocoileus virginianus) may serve as a wildlife reservoir for nearly extinct SARS-CoV-2 variants of concern. Proc. Natl. Acad. Sci. 120(6), e2215067120. https://doi.org/10.1073/pnas.2215067120 (2023)
Cool, K. et al. Infection and transmission of ancestral SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. Emerg. Microbes Infect. 11(1), 95–112. https://doi.org/10.1080/22221751.2021.2012528 (2022).
Palmer, M. V. et al. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol. 95(11), e00083-e121. https://doi.org/10.1128/JVI.00083-21 (2021).
White-Tailed Deer FAQs | Vermont Fish & Wildlife Department. Accessed 6 March 2023. https://vtfishandwildlife.com/hunt/hunting-and-trapping-opportunities/white-tailed-deer/white-tailed-deer-faqs.
U.S. Census Bureau QuickFacts: Vermont. Accessed 6 March 6 2023. https://www.census.gov/quickfacts/VT.
Centers for Disease Control and Prevention Division of Viral Diseases. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. Atlanta: Centers for Disease Control and Prevention; 2020.
Evans, G. E. et al. Contamination of Qiagen DNA extraction kits with Legionella DNA. J Clin Microbiol. 41(7), 3452–3453. https://doi.org/10.1128/JCM.41.7.3452-3453.2003 (2003).
Koncan, R. et al. Learning from mistakes: Taq polymerase contaminated with β-lactamase sequences results in false emergence of Streptococcus pneumoniae containing TEM. J Antimicrob Chemother. 60(3), 702–703. https://doi.org/10.1093/jac/dkm239 (2007).
Montgomery, T. L. et al. Laboratory worker self-contamination with noninfectious SARS-CoV-2 DNA can result in false-positive reverse transcriptase PCR-based surveillance testing. J. Clin. Microbiol. 59(7), e00723-e821. https://doi.org/10.1128/JCM.00723-21 (2021).
Robinson-McCarthy, L. R. et al. Laboratory-generated DNA can cause anomalous pathogen diagnostic test results. Microbiol. Spectr. 9(2), e00313-e321. https://doi.org/10.1128/Spectrum.00313-21 (2021).
Corman, V., Bleicker, T., Brünink, S., et al. Diagnostic detection of 2019-nCoV by real-time RT-PCR. Published online 2020:13.
Bevins, S. N., Chipman, R. B., Beckerman, S. F., et al. SARS-CoV-2 occurrence in white-tailed deer throughout their range in the conterminous United States. Published online April 17, 2023. https://doi.org/10.1101/2023.04.14.533542
Kotwa, J. D., Massé, A., Gagnier, M., et al. First detection of SARS-CoV-2 infection in Canadian wildlife identified in free-ranging white-tailed deer (Odocoileus virginianus) from southern Québec, Canada. Published online January 21, 2022. https://doi.org/10.1101/2022.01.20.476458
McBride, D., Garushyants, S., Franks, J., et al. Accelerated evolution of SARS-CoV-2 in free-ranging white-tailed deer. Res Sq. Published online February 16, 2023:rs.3.rs-2574993. https://doi.org/10.21203/rs.3.rs-2574993/v1
Naderi, S., Chen, P. E., Murall, C. L., et al. Zooanthroponotic transmission of SARS-CoV-2 and host-specific viral mutations revealed by genome-wide phylogenetic analysis. Ogbunugafor CB, Weigel D, Rochman N, eds. eLife. 2023;12:e83685. https://doi.org/10.7554/eLife.83685
List of Reports and Publications | 2017 Census of Agriculture | USDA/NASS. Accessed 20 Feb 2023. https://www.nass.usda.gov/Publications/AgCensus/2017/index.php#full_report
Roundy, C. M. et al. High Seroprevalence of SARS-CoV-2 in white-tailed deer (Odocoileus virginianus) at one of three captive cervid facilities in Texas. Microbiol Spectr. 10(2), e00576-e622. https://doi.org/10.1128/spectrum.00576-22 (2022).
Dzung, A. et al. Prolonged unfrozen storage and repeated freeze-thawing of SARS-CoV-2 patient samples have minor effects on SARS-CoV-2 detectability by RT-PCR. J Mol Diagn. 23(6), 691–697. https://doi.org/10.1016/j.jmoldx.2021.03.003 (2021).
Bruce EA, Mills MG, Sampoleo R, et al. Predicting infectivity: comparing four PCR-based assays to detect culturable SARS-CoV-2 in clinical samples. 2021. https://doi.org/10.1101/2021.07.14.21260544.
Nalla, A. K., Casto, A. M., Huang, M. L. W., et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J. Clin. Microbiol. 58(6), e00557-20. https://doi.org/10.1128/JCM.00557-20
Acknowledgements
This study was supported by NIH grant P30GM118228-04 and UVM start-up funds (BAM, EAB) and an American Heart Association predoctoral fellowship (HWD; https://doi.org/10.58275/AHA.23PRE1020524.pc.gr.161030). We would like to acknowledge the Vermont Agency of Natural Resources, Fish & Wildlife Department for allowing us to participate in all their ongoing research efforts to collect these samples. We would also like to thank the Wildlife & Fisheries Club at the University of Vermont. Finally, a big thank you to all the Vermont hunters that were willing to participate in our efforts.
Author information
Authors and Affiliations
Contributions
Conceptualization (H.W.D., B.A.M., E.A.B.). Methodology (M.G.M., K.R.J., A.L.G.). Validation (H.W.D., E.A.B., M.G.M). Formal Analysis (H.W.D., M.G.M., E.R., D.J.S., B.A.M., E.A.B.). Investigation (H.W.D., M.G.M., M.M.S., J.G., Y.P., M.J., A.M.L.C., W.T.K., E.R., H.C.K., B.C.S., M.C.N., A.J.G., K.E.M., S.N., M.J.H., A.C.F., S.L.M., T.K.G., M.K.C., R.S.F., M.T., M.E.J., J.M., E.B.L., P.A.C., D.H.B., S.P.M., C.J.F., A.C.K., A.L.Y., J.H.F., J.W., E.C., G.U., R.S.R., E.M.V., M.C.T., K.E.G, O.C.D., K.R.P., V.C.R., M.W., T.W.B., C.C.G., S.D.S., A.C.S., C.E.R., A.M.D., S.L.M.). Resources (K.R.J., A.L.J., N.F., B.A.M., E.A.B.). Writing- Original Draft (H.W.D.). Writing- Review & Editing (all authors). Visualization (H.W.D., E.R., D.J.S.) Supervision (M.G.M., K.R.J., A.C.P.O., A.L.G., N.F., B.A.M., E.A.B.). Project Administration (H.W.D., M.G.M., B.A.M., E.A.B.). Funding Acquisition (B.A.M., E.A.B)
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Despres, H.W., Mills, M.G., Schmidt, M.M. et al. Surveillance of Vermont wildlife in 2021–2022 reveals no detected SARS-CoV-2 viral RNA. Sci Rep 13, 14683 (2023). https://doi.org/10.1038/s41598-023-39232-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39232-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.