Abstract
This study evaluated the influence of different vascular reconstruction kernels on the image quality of CT angiographies of the lower extremity runoff using a 1st-generation photon-counting-detector CT (PCD-CT) compared with dose-matched examinations on a 3rd-generation energy-integrating-detector CT (EID-CT). Inducing continuous extracorporeal perfusion in a human cadaveric model, we performed CT angiographies of eight upper leg arterial runoffs with radiation dose-equivalent 120 kVp acquisition protocols (CTDIvol 5 mGy). Reconstructions were executed with different vascular kernels, matching the individual modulation transfer functions between scanners. Signal-to-noise-ratios (SNR) and contrast-to-noise-ratios (CNR) were computed to assess objective image quality. Six radiologists evaluated image quality subjectively using a forced-choice pairwise comparison tool. Interrater agreement was determined by calculating Kendall’s concordance coefficient (W). The intraluminal attenuation of PCD-CT images was significantly higher than of EID-CT (414.7 ± 27.3 HU vs. 329.3 ± 24.5 HU; p < 0.001). Using comparable kernels, image noise with PCD-CT was significantly lower than with EID-CT (p ≤ 0.044). Correspondingly, SNR and CNR were approximately twofold higher for PCD-CT (p < 0.001). Increasing the spatial frequency for PCD-CT reconstructions by one level resulted in similar metrics compared to EID-CT (CNRfat; EID-CT Bv49: 21.7 ± 3.7 versus PCD-CT Bv60: 21.4 ± 3.5). Overall image quality of PCD-CTA achieved ratings superior to EID-CTA irrespective of the used reconstruction kernels (best: PCD-CT Bv60; worst: EID-CT Bv40; p < 0.001). Interrater agreement was good (W = 0.78). Concluding, PCD-CT offers superior intraluminal attenuation, SNR, and CNR compared to EID-CT in angiographies of the upper leg arterial runoff. Combined with improved subjective image quality, PCD-CT facilitates the use of sharper convolution kernels and ultimately bears the potential of improved vascular structure assessability.
Similar content being viewed by others
Introduction
Contrast-enhanced computed tomography angiography (CTA) constitutes the first-line diagnostic modality in the evaluation of the peripheral arterial circulation1,2. By providing a rapid depiction of the vascular status, CTA primarily aids in evaluating further treatment options and thus potential indications for surgical or interventional therapy to avoid unnecessary or probably unsuccessful procedures3. However, CTA bears certain limitations, especially for the depiction of the smaller vasculature, e.g., the arteries in the lower leg and the perforating femoral vessels. Furthermore, vessel lumen assessment is particularly limited in patients with heavy arterial wall calcifications. The introduction of dual-energy CT and associated postprocessing options have not been able to alleviate these drawbacks satisfactorily4,5,6. However, the latest clinical photon-counting detector CT (PCD-CT) technology seems to be able to address these issues. The semiconductor design allows for direct measurement of each photon’s energy, thus revoking the previously required intermediate step of converting the photons to light. This development also eliminates the need for isolating septa within the detector and allows for smaller overall pixel size, thus increasing spatial resolution7,8. Furthermore, background noise and beam hardening can be reduced significantly9. Independently, the selection of different reconstruction kernels is known to affect resolution and image noise10,11. While a variety of postprocessing options have been investigated for cardiac11,12,13,14,15,16, head/neck17,18, and aortic PCD-CT19,20,21,22,23, suchlike prospectively designed investigations regarding peripheral vessels remain scarce24.
To close this scientific gap, this study aimed to investigate the influence of different vascular convolution kernels on the image quality of PCD-CT in comparison to dose-matched energy-integrating detector CT (EID-CT) examinations in a continuous extracorporeal perfusion model.
Materials and methods
Cadaveric specimens and extracorporeal perfusion model
The anatomical institute of the local university provided four fresh-frozen body donors which were subsequently included in this experimental study. Donors had written informed consented to the posthumous use for study and research purposes. Experiments were approved by Institutional Review Board of the University of Würzburg (protocol number: 20220413 01), who waived the need for further informed consent, and were conducted according all applicable laws and regulations.
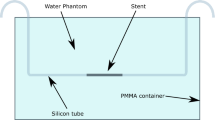
After surgical dissection of the common femoral and popliteal artery, as well as insertion of introduction sheaths, a continuous extracorporeal perfusion was established by means of a peristaltic pump. To this effect, a mixture of Ringer’s solution, glucose solution and iodine-containing contrast medium (Peritrast® 400 mg/ml, Dr. Franz Köhler Chemie GmbH, Bensheim, Germany) with an effective iodine concentration of 12.9 mg/ml was used. A schematic illustration of the experimental setup is provided in Fig. 1. Detailed background information on the establishment of the extracorporeal perfusion model and the necessary materials can be found elsewhere25.
Technical specifications and scan protocols
A clinical 1st-generation PCD-CT (Naeotom Alpha; Siemens Healthcare GmbH, Forchheim, Germany) and a 3rd-generation dual-source EID-CT (Somatom Force, Siemens Healthcare GmbH) were employed to perform CTA of the upper leg runoff. Scans were generated with a fixed tube voltage of 120 kVp and effective tube currents of 63 mAs (PCD-CT) and 74 mAs (EID-CT), respectively, to achieve matching radiation doses (CTDIVol = 5 mGy). Standard collimations of 144 × 0.4 mm (PCD-CT) and 96 × 0.6 mm (EID-CT) were used with an identical pitch factor of 1.0 and a rotation time of 0.5 s.
Image reconstruction parameters
Images were reconstructed individually for each leg with a field of view of 150 mm, a 512 × 512 pixel matrix and a slice thickness/increment of 1.0 mm, each. PCD-CT and EID-CT datasets were each reformatted with three vendor-specific vascular kernels characterized by different spatial frequencies (ρ50: spatial frequency at the 50% value of the modulation transfer function): For PCD-CT scans, Bv40 (ρ50 = 3.95 lp/cm), Bv48 (ρ50 = 5.40 lp/cm), and Bv60 (ρ50 = 8.79 lp/cm) were employed. Matched to the respective PCD-CT modulation transfer functions, Bv40 (ρ50 = 3.95 lp/cm), Bv49 (ρ50 = 5.62 lp/cm), and Bv59 (ρ50 = 8.32 lp/cm) were selected for EID-CT scans to achieve the best possible comparability. Furthermore, a sharp vascular kernel (Bv76, ρ50 = 16.47 lp/cm) only available for PCD-CT data was run. Of note, other relevant spatial frequency values (at 10% and 2% of the modulation transfer function) are included in Supplemental Table S1. Based on vendor information, comparable iterative reconstruction levels were chosen for PCD- and EID-CT datasets. EID-CT raw data was reformatted using a 3rd generation iterative reconstruction algorithm (level 3, ADMIRE, Siemens Healthcare GmbH) and likewise a 4th generation algorithm (level 3, QIR, Siemens) with PCD-CT. It must be noted that the latter algorithm prevents the counting of low-energy photons below a threshold of 20 keV, thereby suppressing electronic background noise.
Objective image analysis
Density in Hounsfield Units (HU) within vessel lumina and the surrounding muscle tissue was measured by means of standardized regions of interest (ROI). Each upper leg arterial runoff was evaluated at four consistent levels (proximal, middle, distal superficial femoral artery, and popliteal artery). In order to estimate image noise, the standard deviation of the attenuation (HU) in standardized ROIs within the arteries as well in subcutaneous fat tissue and surrounding air were used. All measurements were performed by one radiologist with 5 years of experience in the field with a clinical picture archiving and communication system (Merlin, Phönix-PACS, Freiburg, Germany) as is presented in Fig. 2. Signal-to-noise ratios (SNR) and contrast-to-noise-ratios (CNR) were computed as SNR = HUartery/SDfat or rather CNR = (HUartery – HUmuscle)/SDfat.
Subjective image analysis
Subjective image quality of the reconstructed image stacks generated was compared using a browser-based forced-choice pairwise comparison tool. Representative image slices of both legs at the proximal and distal levels were presented in random order (7 kernels × 2 legs × 2 levels × 4 donors = 112 images), resulting in 336 side-by-side comparisons (21 comparisons for 16 slice positions). Assessment was performed independently by six radiologists with 2 to 8 years of experience in CTA imaging without imposing a time limit or providing technical information. Raters judged overall image quality and luminal assessability using a certified diagnostic monitor (RadiForce RX660; EIZO, Hakusan, Japan). It may be noted that the pairwise comparison tool was chosen over a conventional rating-based analysis in order to simplify the assessment of images that show mostly minor differences. Furthermore, the setup allows for a significant reduction of individual reader effort and time requirement.
Data analysis and statistics
Generated data were processed in a Jupyter Notebook environment using Python version 3.8.15 and additional freely available libraries (pandas 1.3.5, numpy 1.21.6, choix 0.3.5, kendall w 1.0.0). Dedicated software (DATAtab e.U., Graz, Austria) was utilized for statistical analysis. Continuous variables are given as mean ± standard deviation. Significance is indicated by an alpha level of p < 0.05. Objective image parameters were compared using paired t-test (pair-by-pair) or one-way analyses of variance (ANOVA) and Bonferroni-corrected pairwise post-hoc tests (group). For comparison of the subjective image quality ratings, Friedman tests were employed. By fitting a Bradley-Terry-Model in the choix library26, preference ranks were calculated for the entire dataset and for each rater individually, resulting in a final hierarchic ranking of the compared subgroups from 1 (best) to 7 (worst). Bump chart diagrams and Boxplots were computed to visualize the results of objective and subjective image quality analyses. Inter-reader agreement was computed by calculation of Kendall’s concordance coefficient (W).
Results
Objective image analysis
The intraluminal attenuation showed no significant differences within the PCD-CT (p ≥ 0.100) or EID-CT group (p ≥ 0.591) for different kernels, while PCD-CT exceeded EID-CT attenuation (414.7 ± 27.3 HU vs. 329.3 ± 24.5 HU; p < 0.001). Image noise (intraluminal) increased significantly with sharper kernels for both scanners, regardless of measurement location (PCD-CT Bv40/48/60: 7.7 ± 3.1/11.0 ± 3.7/22.0 ± 5.7 HU vs. EID-CT Bv40/49/59: 10.0 ± 4.9/16.5 ± 5.7/30.5 ± 8.3 HU; p < 0.001). However, PCD-CT provided significantly lower arterial noise levels compared to EID-CT for matched kernels (p ≤ 0.044) as is exemplified in Fig. 3. The increase in noise to the next sharper kernel was also significantly lower in PCD-CT than in EID-CT. Representative CT images of one body donor reconstructed with all investigated convolution kernels are given in Fig. 4.
Image quality comparison between photon-counting and energy-integrating-detector CT. Upper row Photon-counting detector CT (PCD-CT). Lower row Energy-integrating detector CT (EID-CT). Lower right corner Direct comparison of a sharp kernel PCD-CT image (Bv60; left half) and a medium-sharp kernel EID-CT image (Bv49; right half) with equivalent SNR/CNR values in the same individual.
SNRfat (PCD-CT—Bv40/48/60: 22.7 ± 3.0/39.3 ± 5.5/52.3 ± 4.6 versus EID-CT—Bv40/49/59: 12.4 ± 1.9/22.8 ± 3.5/37.4 ± 3.3) and CNRfat (21.5 ± 3.0/38.2 ± 5.4/51.3 ± 4.6 versus 10.5 ± 1.7/21.7 ± 3.7/36.3 ± 3.3) were significantly higher in PCD-CT than in the EID-CT at comparable kernels (p < 0.001). Of note, the next sharper reconstruction kernel with PCD-CT reached the approximate values of the lower EID-CT kernel (e.g. SNRfat; EID-CT – Bv49: 22.8 ± 3.5 versus PCD-CT – Bv60: 21.4 ± 3.5) (Figs. 5 and 6). Detailed measurements of attenuation and noise, as well as computations of SNR and CNR are summarized in Table 1.
Subjective image analysis
Overall image quality of CTA acquired with PCD-CT was favored over EID-CT in rank-wise comparison, irrespective of convolution kernels. With the sole exception of one rater, the PCD-CT scan with the ultra-sharp, vessel-optimized vascular kernel (Bv76) was rated higher than all available EID-CT datasets (Fig. 7). Image quality of PCD-CT scans reformatted with Bv60 received the highest ratings, while EID-CT scans with Bv40 were rated worst. The reader agreement was good (W = 0.78) and the differences found were significant (p < 0.001).
Discussion
Establishing continuous extracorporeal perfusion in a human cadaveric model, this investigation compared kernel-related image quality of dose-matched 120 kVp CTA of the upper leg runoff between a 1st-generation PCD-CT and 3rd-generation EID-CT system. Our results confirm the superiority of the new detector technology regardless of the used convolution kernel in objective analysis, as well as subjective image ratings using a browser-based pairwise forced-choice comparison setup.
Previous feasibility studies have established the value of cadaveric models, which permit realistic CTA imaging and intra-individual comparative studies27. Suchlike setups allow for accurate direct comparisons of different scanner types, as characteristic sources of bias in matched-pair patient studies are absent. Otherwise, relevant confounders include different body weight, varying contrast agent amount and concentration, and variations in applied effective radiation dose28. Recent studies investigating CTA of the aorta19 or peripheral arterial runoffs24 have been based on retrospective data and therefore suffer from discrepancies in acquisition protocols between PCD-CT and EID-CT. To overcome this limitation, this experimental cadaveric study employed a constant tube potential of 120 kVp without automatic tube modulation and a fixed tube current resulting in identical CTDIVol values.
Regardless of utilized convolution kernels, intraluminal attenuation was higher in PCD-CT than in EID-CT datasets. This result is consistent with previous studies19,24 and is most likely due to the equal weighting of all photons regardless of their energy by the photon-counting detector and the resulting relative overweighting of low-energy photons compared to EID-CT28. Consistent with the above-mentioned previous studies, image noise was lower for comparable kernels in PCD-CT scans and increased with sharper reconstruction kernels. However, the increase in noise remained significantly lower in PCD-CT than in EID-CT. A plausible explanation to this finding lies in the combination of increased beam yield and threshold-based suppression of low-energy electronic noise inherent to the improved detector technology9. We also demonstrated that both SNR and CNR for comparable kernels in PCD-CT were significantly higher than with corresponding EID-CT datasets. Thereby, the values of PCD-CT reformatted with the next sharper kernel were comparable to the lower EID-CT kernel. This allows for the use of sharper kernels without a reduction in image quality, facilitating improved vessel assessment, especially in the presence of calcifications or inserted stents. This improves the evaluability of the arterial runoff while maintaining the assessability of the surrounding soft tissue.
Consistent with the results of the objective image quality analysis, all PCD-CT scans were attributed with better subjective ratings than EID-CT scans. However, the fact that one reader ranked images reconstructed with the PCD-specific ultra-sharp kernel worst needs to be mentioned. While this result may be attributed to individual taste, these images show an increased degree of hardening, and thus supposedly allow for sharper delineation of vessel lumina and stenosis evaluation in the presence of calcifications. However, the assessment of the surrounding tissue is certainly limited. Of note, this convolution kernel was primarily used for comparative reasons and requires further dedicated evaluation to assess suitable clinical applications.
In this context, future research is required to investigate the impact of PCD-CTA’s superior image quality on patient treatment by potentially increasing diagnostic accuracy for vascular pathologies, such as stenoses or occlusions. Considering that the potential benefits of ultra-high-resolution PCD-CT examinations providing spectral image information without dose penalty have been demonstrated in cardiac imaging12,14,16, dedicated investigation concerning peripheral arterial run-offs is warranted.
Certain limitations specific to this study must be mentioned. First, due to the experimental nature of this work, only 8 extremities of 4 body donors were investigated. Second, to increase comparability, we refrained from applying automatic tube voltage modulation on either scanner, although this could have resulted in a better iodine contrast in EID-CT, as the automatic modulation would have selected lower tube voltages between 70 and 100 kVp. Since fixed tube voltages below 100 kVp were not applicable with PCD-CT at the time of writing, performing low-kV scans solely on the EID-CT scanner would have prevented reasonable comparisons between the two detector techniques in our opinion. Further studies will be necessary to investigate other scan modes with different tube voltage settings once they become applicable. Third, we did not perform PCD-CT examinations in ultra-high resolution scan mode, since this setting could not be replicated on the EID-CT scanner without a considerable dose increase. Finally, to investigate the isolated influence of different convolution kernels, post-processing techniques like virtual monoenergetic imaging or polyenergetic bin imaging were not assessed, since the necessary reconstruction algorithms are not directly comparable.
Conclusion
PCD-CT offers superior intraluminal attenuation, SNR, and CNR compared to EID-CT in angiographies of the peripheral arterial runoff of the lower extremity. This allows for employing sharper convolution kernels without compromising image quality, hence improving both subjective image quality and assessability of vascular structures as well as surrounding soft tissue.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request. Due to the nature of this research, participants of this study did not agree for their data to be shared in a public repository.
Abbreviations
- CNR:
-
Contrast-to-noise ratio
- CTA:
-
Computed tomography angiography
- CTDIVol :
-
Volume computed tomography dose index
- EID-CT:
-
Energy-integrating detector CT
- PCD-CT:
-
Photon-counting detector CT
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
References
Gerhard-Herman, M. D. et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 135, e686–e725. https://doi.org/10.1161/CIR.0000000000000470 (2017).
Norgren, L. et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 45(Suppl S), S5-67. https://doi.org/10.1016/j.jvs.2006.12.037 (2007).
Napoli, A. et al. Peripheral arterial occlusive disease: Diagnostic performance and effect on therapeutic management of 64-section CT angiography. Radiology 261, 976–986. https://doi.org/10.1148/radiol.11103564 (2011).
Gruschwitz, P. et al. Noise-optimized virtual monoenergetic reconstructions of dual-energy CT angiographies improve assessability of the lower leg arterial segments in peripheral arterial occlusive disease. Radiography 29, 19–27. https://doi.org/10.1016/j.radi.2022.09.002 (2022).
Kosmala, A. et al. Dual-energy CT angiography in peripheral arterial occlusive disease: Diagnostic accuracy of different image reconstruction approaches. Acad. Radiol. 29(Suppl 4), S59–S68. https://doi.org/10.1016/j.acra.2020.10.028 (2022).
Klink, T., Wilhelm, T., Roth, C. & Heverhagen, J. T. Dual-energy CTA in patients with symptomatic peripheral arterial occlusive disease: Study. Studie über die diagnostische Genauigkeit und limitierende Faktoren. Fortschr. Gebiet. Rontgenstr. Nukl. 189, 441–452. https://doi.org/10.1055/s-0043-101526 (2017).
Tortora, M. et al. Spectral photon-counting computed tomography: A review on technical principles and clinical applications. J. Imaging. https://doi.org/10.3390/jimaging8040112 (2022).
Willemink, M. J., Persson, M., Pourmorteza, A., Pelc, N. J. & Fleischmann, D. Photon-counting CT, technical principles and clinical prospects. Radiology 289, 293–312. https://doi.org/10.1148/radiol.2018172656 (2018).
Meloni, A. et al. Photon-counting computed tomography (PCCT). Technical background and cardio-vascular applications. Diagnostics https://doi.org/10.3390/diagnostics13040645 (2023).
Gassenmaier, T. et al. Next generation coronary CT angiography: In vitro evaluation of 27 coronary stents. Eur. Radiol. 24, 2953–2961. https://doi.org/10.1007/s00330-014-3323-6 (2014).
Petritsch, B. et al. Photon-counting computed tomography for coronary stent imaging: In vitro evaluation of 28 coronary stents. Investig. Radiol. 56, 653–660. https://doi.org/10.1097/RLI.0000000000000787 (2021).
Bratke, G. et al. Spectral photon-counting computed tomography for coronary stent imaging: Evaluation of the potential clinical impact for the delineation of in-stent restenosis. Investig. Radiol. 55, 61–67. https://doi.org/10.1097/RLI.0000000000000610 (2020).
Mannil, M. et al. Photon-counting CT: High-resolution imaging of coronary stents. Investig. Radiol. 53, 143–149. https://doi.org/10.1097/RLI.0000000000000420 (2018).
Mergen, V., Eberhard, M., Manka, R., Euler, A. & Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 9, 981012. https://doi.org/10.3389/fcvm.2022.981012 (2022).
Mergen, V. et al. Ultra-high-resolution coronary CT angiography with photon-counting detector CT feasibility and image characterization. Investig. Radiol. 57, 780–788. https://doi.org/10.1097/RLI.0000000000000897 (2022).
Rotzinger, D. C. et al. Performance of spectral photon-counting coronary CT angiography and comparison with energy-integrating-detector CT: Objective assessment with model observer. Diagnostics https://doi.org/10.3390/diagnostics11122376 (2021).
Michael, A. E. et al. Photon counting CT angiography of the head and neck: Image quality assessment of polyenergetic and virtual monoenergetic reconstructions. Diagnostics https://doi.org/10.3390/diagnostics12061306 (2022).
Symons, R. et al. Photon-counting computed tomography for vascular imaging of the head and neck: First in vivo human results. Investig. Radiol. 53, 135–142. https://doi.org/10.1097/RLI.0000000000000418 (2018).
Euler, A. et al. High-pitch photon-counting detector computed tomography angiography of the aorta: Intraindividual comparison to energy-integrating detector computed tomography at equal radiation dose. Investig. Radiol. 57, 115–121. https://doi.org/10.1097/RLI.0000000000000816 (2022).
Hagen, F. et al. Image quality and dose exposure of contrast-enhanced abdominal CT on a 1st generation clinical dual-source photon-counting detector CT in obese patients vs a 2nd generation dual-source dual energy integrating detector CT. Eur. J. Radiol. 151, 110325. https://doi.org/10.1016/j.ejrad.2022.110325 (2022).
Higashigaito, K. et al. Contrast-enhanced abdominal CT with clinical photon-counting detector CT: Assessment of image quality and comparison with energy-integrating detector CT. Acad. Radiol. 29, 689–697. https://doi.org/10.1016/j.acra.2021.06.018 (2022).
Decker, J. A. et al. Virtual non-contrast reconstructions of photon-counting detector CT angiography datasets as substitutes for true non-contrast acquisitions in patients after EVAR-performance of a novel calcium-preserving reconstruction algorithm. Diagnostics https://doi.org/10.3390/diagnostics12030558 (2022).
Symons, R. et al. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: An in vivo study. Med. Phys. 44, 5120–5127. https://doi.org/10.1002/mp.12301 (2017).
Rippel, K. et al. Evaluation of run-off computed tomography angiography on a first-generation photon-counting detector CT scanner: Comparison with low-kVp energy-integrating CT. Eur. J. Radio. 158, 110645. https://doi.org/10.1016/j.ejrad.2022.110645 (2023).
Gruschwitz, P. et al. Continuous extracorporeal femoral perfusion model for intravascular ultrasound, computed tomography and digital subtraction angiography. PLoS ONE 18, e0285810. https://doi.org/10.1371/journal.pone.0285810 (2023).
Bradley, R. A. & Terry, M. E. Rank analysis of incomplete block designs: I. The method of paired comparisons. Biometrika 39, 324–345. https://doi.org/10.2307/2334029 (1952).
Gruschwitz, P. et al. Photon-counting versus energy-integrating detector CT angiography of the lower extremity in a human cadaveric model with continuous extracorporeal perfusion. Invest. Radiol. https://doi.org/10.1097/RLI.0000000000000982 (2023).
Brazauskas, R. & Logan, B. R. Observational studies: Matching or regression?. Biol. Blood Marrow Transplant. 22, 557–563. https://doi.org/10.1016/j.bbmt.2015.12.005 (2016).
Funding
Open Access funding was enabled and organized by Projekt DEAL and supported by the Open Access Publication Fund of the University of Würzburg. Funding was provided by Interdisziplinäres Zentrum für Klinische Forschung, Universitätsklinikum Würzburg (Grant Nos. Z-02CSP/18, Z-3BC/02), Siemens Healthineers, and Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Contributions
P.G. designed and supervised the study, analyzed all data and prepared the manuscript. R.H. designed the pairwise comparison software. V.H., H.H., R.H., A.S.K., P.P. and A.M.A. performed observer analysis. H.H., V.H., P.J.K., A.M.A. and J.P.G. supported preparation of the manuscript and figures. P.G. performed statistical analysis and revised the manuscript. T.A.B., B.P. and J.P.G. contributed to preparation of the manuscript and provided quality control. S.E., F.K. and S.L. prepared the cadaveric specimen. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
PG (Grant Number Z-02CSP/18) and JPG (Grant Number Z-3BC/02) were funded by the Interdisciplinary Center of Clinical Research Wurzburg, Germany. JPG, BP and TAB serve as research consultants for Siemens Healthineers. The Department of Diagnostic and Interventional Radiology receives research funding by Siemens Healthineers. The authors of this manuscript declare no further relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gruschwitz, P., Hartung, V., Kleefeldt, F. et al. Standardized assessment of vascular reconstruction kernels in photon-counting CT angiographies of the leg using a continuous extracorporeal perfusion model. Sci Rep 13, 12109 (2023). https://doi.org/10.1038/s41598-023-39063-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39063-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.