Abstract
Dyslipidemia is a frequent side effect associated with nilotinib treatment. Patients with chronic myeloid leukemia (CML) under treatment with nilotinib who develop dyslipidemia have been shown to have a higher risk of presenting atherosclerotic cardiovascular disease (ACVD). Therapeutic discontinuation in selected individuals could be a strategy in order to prevent the development of ACVD. Observational study of patients with CML under nilotinib treatment. The lipid values were gathered before starting with nilotinib and after 3 months. Such values were also measured before discontinuation in patients who suspended nilotinib treatment, as well as 3 and 12 months later. 32 patients were included, 19 of them treated in monotherapy with nilotinib. The concentrations of total cholesterol and low-density lipoproteins (LDL) increased significantly after 3 months of treatment (27.29 mg/dL ± 22.88, p < 0.01). Of the total number of patients treated, 12 discontinued the treatment. LDL concentration was significantly reduced after 3 months of the nilotinib discontinuation (− 27.58 mg/dL ± 38.30, p = 0.030), remaining substantially lower after 12 months, compared to the time previous to discontinuation (− 24.58 mg/dL ± 37.31, p = 0.043). Nilotinib suspension reduces significantly LDL concentrations. These data support the strategy of therapeutic discontinuation in order to prevent future cardiovascular complications, especially in patients with prior cardiovascular risk factors.
Similar content being viewed by others
Introduction
The prognosis of chronic myeloid leukemia (CML) changed drastically after the introduction of tyrosine kinase inhibitors (TKI) in 2001, with the development of imatinib1. Subsequently, second (2GTKI) and third generation (3GTKI) TKI have been developed to be used in the front line and/or situations of intolerance or resistance to other TKI. 2GTKI have shown a faster and deeper molecular response than imatinib2, thus the option of discontinuing the treatment has been included in the specification sheet, provided that a series of clinical and analytical requirements are met.
A few years after the appearance of 2GTKI cardiovascular and metabolic side effects started to be notified3. More specifically, nilotinib has been associated with a significant increase of the lipid values in up to a 50% of the patients treated4, and also with peripheral arterial disease and ischemic cardiopathy, both in randomized clinical trials2 as well as real-life studies5. It has been observed that most cardiovascular events occur in patients with previous cardiovascular risk factors (CVRF) or atherosclerotic cardiovascular disease (ACVD). Thus, the current cardiovascular profile has an influence over the election of the TKI6. The treatment dose of nilotinib, as well as the former treatments with TKI also contribute to the pathogenesis of the ACVD7,8.
Dyslipidemia is one of the key factors in the development of atherosclerosis and cardiovascular disease. The atherogenesis is a progressive and complex process that takes place due to the interaction among the circulating cells and molecules, the vascular wall and the modifications in the characteristics of the bloodstream, resulting in the formation of lipid deposits in the arterial intima and the activation of the inflammatory response. Lipoproteins (LP) carry out a major role in this process, according to their number, size and proportion9.
LDL and other LP associated with apo B (IDL, VLDL) whose diameter is lower than 70 nm can pass freely through the intact arterial wall, interacting with the proteoglycans of the wall, thus being susceptible to remaining trapped in the extracellular matrix. Once the endothelial barrier is crossed, there can be a retention of LDL particles, especially those with a smaller size, due to their binding capacity with the proteoglycans of the extracellular matrix in the intima. This constitutes the primary event of the atherogenic process, triggering a series of modifications in the LP and local responses, including alterations of the endothelium with an increase of LDL particles permeability, monocyte recruitment (which will promote the formation of foam cells) and inflammatory changes that determine in turn a greater retention and progression of the atheromatous plaque10.
Given the effect that nilotinib has over the production of hypercholesterolemia, as well as its paramount role in the development of cardiovascular disease, different strategies are being implemented in order to achieve the correct evaluation and treatment of these patients11. Therapeutic discontinuation of nilotinib in those individuals with an adequate hematological response could represent another possibility for this population.
The objective of this study is to verify whether the dyslipidemia produced by the nilotinib treatment is reversible after the suspension of the drug.
Materials and methods
Observational study of patients with CML under treatment with nilotinib during the last 12 months, at least. Patients belonged to the medical area of the Virgen de las Nieves Hospital in Granada, Spain. The information of the study was selected before the beginning of the TKI treatment, beginning discontinuation on the first of December of 2022.
The concentrations of total cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerides (TG) were measured before the start of the nilotinib treatment and 3 months later. In those patients eligible for discontinuation, a lipid profile was obtained before the suspension of the treatment, as well as after 3 and 12 months.
All samples were analyzed in the central laboratory of the Virgen de las Nieves University Hospital in Granada. Total cholesterol was determined in serum samples using enzymatic methods (Cholesterol Oxidase, Esterase, Peroxidase, Traceable to CDC Method). Reagent is certified to maintain traceability to the US Reference System for Cholesterol, based on the Abell-Kendall Reference Method in a network of Cholesterol Reference Method Laboratory Network, CRMLN) certified by the CDC. HDL cholesterol was determined by reaction with a specific accelerator detergent (direct method, with removal of particles and reaction with cholesterol esterase and colorimetric reading, traceable to the CDC method). The determination of LDL cholesterol was carried out by direct measurement, by colorimetric measurement using a liquid selective detergent. Triglycerides were determined using an enzymatic method (glycerol phosphate oxidase method, colorimetric) traceable to the IDMS method. The analyzer used was Alinity series C. by Abbott Diagnostics.
For the quantitative variables, their means ± standard deviation were obtained (except for age, which, due to moving away from a distribution close to a normal one, it was decided to estimate its median and interquartile range). For categorical variables, the proportions of each category were obtained. Given the normality of the distribution of cholesterol concentration and other parameters of the lipid profile, parametric tests were used. To assess the statistical significance of the differences in the concentrations of the lipid profile, Student’s t test was performed for related samples. The p threshold of statistical significance < 0.05 was taken. The software used was IBM® SPSS® Statistics v20.
This project was approved by the Provincial Ethics Committee for Research with Medicines of Granada (CEIm/CEI), Spain (code 5c07f325e14f4a852ee4d0047025daf9baaa41c7). Informed consent was obtained from all patients before being included in the study. All methods were performed in accordance with the relevant guidelines and regulations.
The datasets used and analyzed during the current study are available from the corresponding author for reasonable requests.
Results
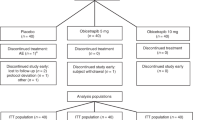
Data were collected from 32 patients who had been treated with nilotinib at some point. At the moment of the CML diagnosis the mean age was 48 years old (IQR 35.25–59.75). 56.3% of this population were men. 19 of them were treated in monotherapy with nilotinib, whereas 13 had also been treated with other TKI (9 imatinib, 3 dasatinib and 1 with both). The initial dose of nilotinib was 300 mg in 6.3% of the patients, 600 mg in 68.8% and 800 mg. Sokal score was low in 25 patients (78.10%), intermediate in 4 patients (12.50%), and high in 3 patients (9.40%). EUTOS score was low in 29 patients (90.60%) and high in 3 patients (9.40) (Table 1).
At the moment of the data collection, the mean number of months since diagnosis was 118 (IQR 74.75–178.75). Out of the total, 20 patients (62.50%) continued with the treatment with a mean of 76.50 months (IQR 48.25–120.75).
Out of the 32 patients, 13 had suspended TKI treatment during their oncological history, although at the moment of the data collection only 12 patients (37.50%) were in a situation of therapeutic discontinuation. This was because one of them had to be reintroduced into the treatment due to failure in the discontinuation (loss of major molecular response) after 2 months. The mean duration of the nilotinib treatment in the cohort of discontinued treatment was 66.50 months (IQR 56.25–92), with a mean suspension time of 45 months (IQR 10.25–64.50). The dose of nilotinib previous to the suspension was 150 mg in 6.3% of the patients (2 patients), 300 mg in 56.3% (12 patients), 400 mg in 3.1% (1 patient) and 600 mg in 34.4% (11 patients). This information is summarized in Table 2.
Table 3 outlines the data referring to the prevalence of CVRF and atherosclerotic cardiovascular disease. The information is divided in 2 groups: before initiating the TKI treatment and at the time of the study (differentiating between the cohort continuing with the nilotinib treatment at that moment and the one that had suspended it).
Evolution of the lipid profile in patients treated with nilotinib
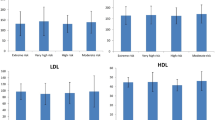
Data on the lipid profile before the TKI treatment were obtained in 29 of the 32 patients. The values of the different cholesterol fractions after 3 months of nilotinib treatment were gathered in 31 patients. The concentrations of total cholesterol, HDL, LDL and TG before starting the treatment with nilotinib and after 3 months appear in Table 4. Significant variations in the concentrations of total cholesterol, HDL and LDL were observed.
These changes were clinically significant. Before starting with the nilotinib treatment, 9 patients (31.03%) met the definition of hypercholesterolemia (CT > 200 mg/dL and/or LDL > 160 mg/dL), although only 2 of them were being treated with hypolipidemic agents. This number increased after 3 months of treatment up to 20 subjects (64.52%). There were no modifications in the hypolipidemic treatment before starting with the TKI, compared to the visit after 3 months of starting with nilotinib.
Regarding the 12 patients under TKI discontinuation, 7 of them (58.33%) presented dyslipidemia and all of them were being treated with statins and/or ezetimibe. There were no changes in the hypolipidemic treatment between the pre-discontinuation visit and the visits 3 and 12 months after the discontinuation. Table 5 shows the values of the different lipidic fractions before suspending the treatment with nilotinib, after 3 months and after 12 months. Significant differences were observed in the reduction of the concentration values of LDL, both after 3 months and 12 months after suspending treatment with TKI (Fig. 1).
Evolution of LDL cholesterol concentrations (mg/dL) in patients with discontinuation of nilotinib). Mean ± standard error is represented. There are statistically significant differences between the concentration of LDL cholesterol before discontinuation and 3 months post-discontinuation, as well as between the concentration of LDL cholesterol before discontinuation and 12 months post-discontinuation. *p < 0.05, compared with DISC-3M. DISC-3M discontinuation at 3 months, DISC-12M discontinuation at 12 months, PreDISC before discontinuing nilotinib.
Discussion
Treatment with TKI has improved the survival rate of patients with CML to the point of being equivalent to the general population, paired by age and gender1. This fact has put the spotlight on cardiovascular diseases in this population, since TKI have been associated with an increased incidence of cardiovascular disease, especially nilotinib6 y ponatinib13.
Nilotinib produces an early increase in the LDL levels, since the first month of the treatment, secondary to the higher concentration of proprotein convertase subtilisin/kexin type 9 (PCSK9), due to the mTORC1 inhibition, among other causes14. Besides, it has been observed that these patients present higher concentrations of LDL oxidatively modified (oxLDL) than the patients treated with imatinib and dasatinib, as demonstrated by the KIARO study in a recently published multicenter study15. The oxidative modification of the LDL particles increases the atherogenic potential and fosters the inflammatory response, objective in this population with the increase of inflammatory cytokines (TNF-alfa and IL-6), reduction of anti-inflammatory cytokines de (IL10) and increase in the expression of proatherogenic molecules of endothelial adhesion (E-selectin, VCAM-1, ICAM-1)4. Capture and accumulation of LDLox by the macrophages initiates a wide range of bioactivities that can boost the development of atherosclerotic injuries.
Recently, Caocci et al.11 have demonstrated that the patients with CML under treatment with nilotinib with plasmatic levels of cholesterol > 200 mg/dL and LDL > 70 mg/dL after 3 months since the start of the treatment presented a higher incidence of ACVD. Furthermore, another mechanism proposed for the development of the cardiovascular disease is the augmented prothrombotic state, which would facilitate the platelet aggregation and the generation of thrombi16,17.
The first clinical essay that supported the therapeutical alternative of discontinuation was published in 2016. In the French study STIM18, approximately 50% of the patients that suspended treatment with TKI after achieving a steady deep molecular response (DMR) for at least 2 years, maintained it after discontinuation. Besides, the vast majority of the patients who suffered a loss of response recovered it after the reintroduction of the treatment.
Treatment with 2GTKI offers greater discontinuation success, since the probability of achieving a DMP is higher with imatinib. The clinical essay ENESTfreedom12 showed that the duration of the nilotinib treatment regarding discontinuation is inferior compared to the one needed for the imatinib treatment. This is why the possibility of interruption has been introduced in the specification sheet of nilotinib, currently being the only drug that includes this indication19. The clinical requirements are a minimum treatment of 3 years and deep molecular response sustained for at least a year before the suspension of the treatment.
One of the great advantages of therapeutical discontinuation is the cessation of the exposure to the side effects of nilotinib, especially to dyslipidemia. Nevertheless, to the best of our knowledge, up to now it had not been demonstrated that the concentration values of the LDL decreased after the suspension of the therapy.
In our study, carried out with patients under nilotinib treatment (both in monotherapy and in second or third line of treatment with imatinib and dasatinib) it was observed a statistically significant increase in the values of total cholesterol and LDL after 3 months of therapy with nilotinib. This increase in cholesterol particles was also clinically important, since the prevalence of dyslipidemia rose from 31.03% before starting with nilotinib to 65.63% after 3 months of therapy.
The subjects that discontinued treatment showed a statistically significant decrease in LDL concentrations, although not in the levels of total cholesterol (however, there exists a statistical tendency). No statistically significant differences were observed between the values of the lipidic fractions 3 and 12 months after the discontinuation. Statistically, a reduction in lipid levels has not been demonstrated in patients who discontinued nilotinib. The authors consider that this fact is due to the size of our sample, therefore future studies with a larger number of patients could demonstrate our theory.
Regarding the hypolipidemic treatment of the patients under nilotinib discontinuation, 58.33% required treatment with statins and/or ezetimibe. Only one of them was under hypolipidemic treatment before starting with TKI.
Only one out of the 13 patients in our cohort who were discontinued had to reintroduce nilotinib due to failure in the hematological response. Being considered an early failure and unable to measure the lipid concentrations after 3 and 12 months, it was not included in this group of patients when the lipid concentrations were analyzed. It is noteworthy, therefore, that among the 13 patients who discontinued treatment, just one of them had to be reintroduced, resulting in a success rate of 92.30%. We do not know yet if this percentage would be equivalent to that described in the large international series12,20,21 when increasing the sample size.
Our study presents certain limitations. The number of patients is low since the project was carried out in a single medical center; nevertheless, it has been able to demonstrate statistically significant changes, both in the development of dyslipidemia after starting treatment with nilotinib and the reduction of the LDL concentration after TKI suspension.
Conclusions
Nilotinib produces early dyslipidemia in a high percentage of patients treated with this drug. However, and for the first time to the best of our knowledge, it has been observed that the LDL concentrations are reduced in a statistically significant manner after therapy suspension. Thus, our data prove that the dyslipidemia produced by the nilotinib treatment is potentially reversible after the suspension of the treatment.
This fact is another reason to support the therapeutic discontinuation of TKI in patients treated for CML.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Bower, H. et al. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 34(24), 2851–2857 (2016).
Hochhaus, A. et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 30(5), 1044–1054 (2016).
Aichberger, K. J. et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am. J. Hematol. 86(7), 533–539 (2011).
Bocchia, M. et al. Genetic predisposition and induced pro-inflammatory/pro-oxidative status may play a role in increased atherothrombotic events in nilotinib treated chronic myeloid leukemia patients. Oncotarget 7(44), 72311–72321 (2016).
Bondon-Guitton, E. et al. Cardiovascular risk profile of patients with peripheral arterial occlusive disease during nilotinib therapy. Target Oncol. 11(4), 549–552 (2016).
Chai-Adisaksopha, C., Lam, W. & Hillis, C. Major arterial events in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: A meta-analysis. Leuk. Lymphoma 57(6), 1300–1310 (2016).
Caocci, G. et al. Cardiovascular toxicity in patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors in the real-life practice: Identification of risk factors and the role of prophylaxis. Am. J. Hematol. 93(7), E159–E161 (2018).
Di Lisi, D. et al. The new HFA/ICOS risk assessment tool to identify patients with chronic myeloid leukaemia at high risk of cardiotoxicity. ESC Heart Fail. 9(3), 1914–1919 (2022).
Linton, M. F. et al. The Role of Lipids and Lipoproteins in Atherosclerosis. Endotext. https://www.ncbi.nlm.nih.gov/books/NBK343489/ (Accessed 21 December 2022) (MDText.com, Inc., 2019).
Borén, J. & Williams, K. J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 27(5), 473–483 (2016).
Caocci, G. et al. Low-density lipoprotein (LDL) levels and risk of arterial occlusive events in chronic myeloid leukemia patients treated with nilotinib. Ann. Hematol. 100(8), 2005–2014 (2021).
Radich, J. P. et al. Treatment-free remission following frontline nilotinib in patients with chronic phase chronic myeloid leukemia: 5-year update of the ENESTfreedom trial. Leukemia 35(5), 1344–1355 (2021).
Singh, A. P., Umbarkar, P., Tousif, S. & Lal, H. Cardiotoxicity of the BCR-ABL1 tyrosine kinase inhibitors: Emphasis on ponatinib. Int. J. Cardiol. 316, 214–221 (2020).
Abumiya, M. et al. Effects of proprotein convertase subtilisin/kexin type 9 and nilotinib plasma concentrations on nilotinib-induced hypercholesterolaemia in patients with chronic myeloid leukaemia. J. Clin. Pharm. Ther. 46(2), 382–387 (2021).
Sicuranza, A. et al. Pro-inflammatory and pro-oxidative changes during nilotinib treatment in CML patients: Results of a prospective multicenter front-line TKIs study (KIARO study). Front. Oncol. 12, 835563 (2022).
Alhawiti, N. et al. The tyrosine kinase inhibitor, nilotinib potentiates a prothrombotic state. Thromb. Res. 145, 54–64 (2016).
Pouwer, M. G. et al. The BCR-ABL1 inhibitors imatinib and ponatinib decrease plasma cholesterol and atherosclerosis, and nilotinib and ponatinib activate coagulation in a translational mouse model. Front. Cardiovasc. Med. 5, 55 (2018).
Etienne, G. et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J. Clin. Oncol. 35(3), 298–305 (2017).
Ritchie, E. K. Differentiating factors in treatment-free remission trials: Impact of study design on results and clinical applications. Leuk. Lymphoma 60(5), 1116–1125 (2019).
Rea, D. et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 129(7), 846–854 (2017).
Saussele, S. et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 19(6), 747–757 (2018).
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roa-Chamorro, R., Puerta-Puerta, J.M., Torres-Quintero, L. et al. Concentration of low-density lipoproteins (LDL) is significantly reduced after nilotinib discontinuation. Sci Rep 13, 11781 (2023). https://doi.org/10.1038/s41598-023-39057-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39057-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.