Abstract
Spiral ligament fibrocytes generate potassium gradients, which hair cells require to convert mechanical sound waves into electrical palsy. Together with the stria vascularis, they regulate endolymph electrolyte homeostasis. Developing spiral ligament fibrocytes and generating endocochlear potential with an appropriate endolymph ion composition are essential for hearing. Understanding spiral ligament fibrocyte development is useful for studying age-related and genetic hearing loss, as well as for regenerative therapy and cochlear immunology. Despite interspecies differences, most studies of cochlear development have been conducted in rodent models due to the difficulty of using human fetal samples. This study investigated the cochlear development of spiral ligament fibrocytes in a small New World monkey species, the common marmoset (Callithrix jacchus). We examined the developmental expression of specific genes in spiral ligament fibrocytes, including those essential for the generation of endolymphatic potential. Our results showed that this animal model of spiral ligament fibrocyte development is similar to that of humans and is a suitable alternative for the analysis of human cochlear development. The time course established in this study will be useful for studying the primate-specific developmental biology of the inner ear, which may lead to novel treatment strategies for human hearing loss.
Similar content being viewed by others
Introduction
The inner ear is a peripheral sensory organ essential for hearing and balance. The cochlea, part of the inner ear, is mammals' peripheral organ responsible for hearing. In the cochlea, hair cells convert the mechanical sensation of sound into neuroelectric pulses. These pulses eventually reach the auditory cortex of the brain, where neurons can perceive the neuroelectric stimuli converted from sound waves. The generation of this intrinsic electrical impulse in the hair cells is driven by a concentration gradient of potassium ions between the hair cells and the endolymph facing the hair cells.
The spiral ligament fibrocytes of the cochlea are the major tissue that generates the concentration gradient of potassium ions essential for hearing. Spiral ligament fibrocytes also regulate the composition of endolymph in collaboration with the stria vascularis1,2. Therefore, the finely-tuned development of spiral ligament fibrocytes in the cochlea is essential for normal hearing3. These spiral ligament fibrocytes are composed of five cell types according to their location in the spiral ligament: type-IV fibrocytes. Depending on the type, these spiral ligament fibrocytes express specific transporters, channels, or gap junctions. They collaborate to maintain the electrolyte ion homeostasis in the cochlea. In addition, these spiral ganglion fibrocytes play important physiological roles in the cochlear immune response, inflammation, aging, regulation of cochlear blood flow, and glutamate homeostasis4,5.
Previous studies have mainly focused on cochlear development in rodent models. However, human cochlear development is poorly understood because it occurs in late gestation6,7,8, and ethical issues preclude the study of late-term fetal samples. Furthermore, species differences in cochlear development between rodents and humans have been reported, and findings on cochlear development in rodent models cannot necessarily be directly extrapolated to humans6. To overcome this sampling limitation and minimize species differences, the small primate model of the common marmoset (Callithrix jacchus) has been used to understand cochlear development9,10,11,12 and genetic or age-related hearing loss13. Similar approaches have been used to study the central nervous system14. We have previously reported on the basic anatomical stages of cochlear development in the common marmoset compared to humans and mice, including the expression patterns in hair cells, supporting cells, spiral ganglion neurons, stria vascularis, and other conventional markers of cochlear cells9,10,11,12. In addition, we identified several differences between rodents and marmosets in the development of hair cells, spiral ganglion neurons, and stria vascularis. Moreover, we demonstrated similarities to the human developmental process9,10,12.
Research on the development of spiral ligament fibrocytes in non-human primates is still in its infancy. Such studies would reveal interspecies differences in the development of spiral ligament fibrocytes. In addition, knowledge of primate development will be useful in regenerative studies of human spiral ligament fibrocytes.
In this study, we present a detailed description of the cochlear development in C. jacchus. Furthermore, as in humans, cochlear development in primates is slower than in rodents6,9. As precisely demonstrated in hair cells, spiral ganglion neurons, and stria vascularis, it is believed that this animal model is better suited to detect short or transient developmental changes in gene expression patterns.
Materials and methods
Specimens
Cadaverous temporal bone samples from common marmosets at E96 (n = 3), E109 (n = 3), E115 (n = 3), E120 (n = 3), and P0 (n = 3) were used in this study, as same as our previous reports9,12. Adult animals were anesthetized with isoflurane inhalation (1.5–4%) and Caesarion section was performed. Embryos were anesthetized on ice deeply. The animal experiments were approved by the Animal Experiment Committee of Keio University (Approval number: 11006, 08020) and performed according to the guidelines of the National Institutes of Health and the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Tissue preparation
Temporal bones were dissected immediately after euthanasia and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight. Specimens were embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) for cross-sectioning. P0 specimens were decalcified in Decalcifying Solution B (Wako, Osaka, Japan) for 1 week and then embedded in Tissue-Tek O.C.T. compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) for cross-sectioning, as same as our previous reports9,12. Seven-micrometer sections were used for immunohistochemical analysis.
Antibodies
The following primary antibodies were used:
Anti-CALD1 (mouse IgG1, MS-1251-P0, NeoMarkers, Fremont, CA, USA, 1:500), anti-COCH (rabbit IgG, HPA050122, Atlas Antibodies, Bromma, Sweden, 1:200), anti-CCN2 (goat IgG, sc14939, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:200), anti-ATP1B1 (mouse IgG2a, ab2873, Abcam, Cambridge, UK, 1:1000), anti-ATP1A1 (rabbit IgG, ab76020, Abcam, Cambridge, UK, 1:1000), anti-SLC12A2 (goat IgG, sc-21545, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:1000), anti-KCNJ16 (rabbit IgG, APC123, Alomone labs, Jerusalem, Israel, 1:1000), anti-CA2 (rabbit IgG, sc-25596, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:500), anti-GLUT1 (rabbit IgG, ab115730, Abcam, Cambridge, UK, 1:500), anti-COL2A1 (mouse IgG2b, sc-52658, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:100).
The following secondary antibodies were used: donkey anti-mouse IgG, Alexa Fluor Plus 488 (A32766, Invitrogen, Waltham, MA, USA, 1:500); donkey anti-rabbit IgG, Alexa Fluor Plus 555 (A32794, Invitrogen, Waltham, MA, USA, 1:500); and donkey anti-goat Alexa Fluor Plus 647 (A32849, Invitrogen, Waltham, MA, USA, 1:500).
Immunohistochemistry
After a brief wash with PBS, the sections were heated (80 °C) in 10 µM citrate buffer (pH 6) for 15 min. After another brief wash, the sections were pre-blocked in PBS containing 10% normal serum for 1 h at room temperature, incubated with the relevant primary antibodies overnight at 4 °C, and then incubated with Alexa Fluor-conjugated secondary antibodies for 60 min at room temperature. The nuclei were counterstained with Hoechst 33258.
Ethical approval and consent to participate
The animal experiments were approved by the Animal Experiment Committee of Keio University (approval number: 11006, 08020) and were performed in accordance with ARRIVE guidelines and the guidelines of the National Institutes of Health and the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Results
Developmental expression patterns of CALD1, COCH and CCN2
The common marmoset has a gestation period of approximately 150 days15. Previously, we reported that E115 of the marmoset is equivalent to P9 of the mouse and 20 weeks of gestation in humans for cochlear development, and P0 of the marmoset is equivalent to P14 of the mouse and 24 weeks of gestation weeks in humans9. First, this study examined the expression patterns of CALD1, COCH, and CCN2 (Fig. 1), which have been reported to be highly expressed in spiral ligament fibrocytes of the adult marmoset cochlea16.
Expression of CALD1, COCH, and CCN2. (A) Expression of CALD1, COCH, and CCN2 in the E96 cochlea. Among them, only CALD1 expression was observed in the developing periotic mesenchymal tissue. (B) Expression of CALD1, COCH, and CCN2 in the E115 cochlea. While CALD1 expression was widely observed in the spiral ligament fibrocytes, only weak COCH expression was detected at this stage (arrow in B). No CCN2 expression was observed in spiral ligament fibrocytes at this stage. (C,D) Expression of CALD1, COCH, and CCN2 in the E120 cochlea. At this stage, CALD1 expression is widely observed in spiral ligament fibrocytes, and a relatively broader expression of COCH was detected compared with E115. However, CCN2 expression could not be detected at E120. (E,F) Expression of CALD1, COCH, and CCN2 in the P0 cochlea. At P0, both COCH and CCN2 expressions were observed ubiquitously in spiral ligament fibrocytes. CALD1 expression in type II, IV, and V fibrocytes was reduced and restricted to type I and III at this stage. Nuclei were counterstained with Hoechst (blue). Scale bar 50 µm in (A,D), 100 µm in (B,C,E,F). StV stria vascularis, I type I fibrocytes, II type II fibrocytes, III type III fibrocytes, IV type IV fibrocytes. (A–F) Basal turns.
CALD1 encodes Caldesmon, a calmodulin-binding protein. Caldesmon tonically inhibits the ATPase activity of myosin in smooth muscle. Caldesmon is expressed in type I and III fibrocytes in the spiral ligament fibrocytes of the mature cochlea in both rodents and primates16,17. CALD1 expression was previously detected in the developing cochlea of the common marmoset as early as E96 (Fig. 1A)9. Therefore, we used this protein as a conventional marker for spiral ligament fibrocytes. Its expression was observed in the developing periotic mesenchymal tissue, but not in the sensory epithelium. Until E120, CALD1 expression was widely observed in spiral ligament fibrocytes (Fig. 1B–D). However, at P0, CALD1 expression was restricted to type I and III fibrocytes (Fig. 1E).
COCH encodes Cochlin, an extracellular matrix (ECM) protein that is highly abundant in the cochlea and vestibule of the inner ear and is the major non-collagen component of the ECM of the inner ear18. COCH has also been identified as the causative gene for DFNA9, a hereditary hearing loss. Cochlin is also used clinically as a diagnostic marker for perilymphatic fistula19. In the developing cochlea of the common marmoset, a low level of COCH expression was detected at E115, whereas no expression was observed in the E96 cochlea (Fig. 1A,B). At E120, COCH expression was observed in presumptive type II and IV fibrocytes (Fig. 1C,D). At P0, COCH expression was widely observed in spiral ligament fibrocytes, as previously reported in the adult primate cochlea16 (Fig. 1E,F).
CCN2 encodes connective tissue growth factor (CTGF), a matricellular protein belonging to the CCN family of ECM-associated heparin-binding proteins20,21. CCN2 expression has been previously reported in spiral ligament fibrocytes of rodents22,23,24, and the common marmoset16. In the developing cochlea of the common marmoset, no CCN2 expression was detected until E120 (Fig. 1C,D). At P0, ubiquitous expression of CCN2 was observed in spiral ligament fibrocytes (Fig. 1E,F).
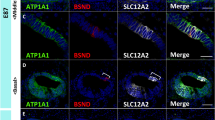
Developmental expression patterns of ATP1A1, ATP1B1, and SLC12A2
Homeostatic regulation of sodium and potassium in the endolymph, in conjunction with the stria vascularis, is one of the most important functions of spiral ligament fibrocytes. The expression of sodium and potassium transporters has been reported in the developing rodent cochlea25,26,27, and their expression during development is essential for normal hearing acquisition28,29. Therefore, this study examined the expression of ATP1A1, ATP1B1, and SLC12A2, which have been reported to be expressed in the developing stria vascularis12 and spiral ligament fibrocytes of the adult marmoset cochlea16 (Fig. 2).
Expression of ATP1B1, ATP1A1, and SLC12A2. (A) Expression of ATP1B1, ATP1A1, and SLC12A2 in the E109 cochlea. No expression of ATP1B1, ATP1A1, and SLC12A2 expressions was detected in spiral ligament fibrocytes. (B) Expression of ATP1B1, ATP1A1, and SLC12A2 in E115 cochlea. While SLC12A2 expression could not be observed in the spiral ligament fibrocytes, no ATP1B1 and ATP1B1 expression could be detected at this stage. (C) Expression of ATP1B1, ATP1A1, and SLC12A2 in E120 cochlea. At this stage, ATP1B1 expression is observed in type II and IV spiral ligament fibrocytes (asterisk in C). In contrast, no ATP1A1 expression was detected at this stage. (D) Expression of ATP1B1, ATP1A1, and SLC12A2 in the E120 cochlea. At P0, ATP1B1, ATP1A1, and SLC12A2 expression was observed in type II and IV fibrocytes. The nuclei were counterstained with Hoechst (blue). Scale bar 100 µm, StV stria vascularis, II type II fibrocytes, IV type IV fibrocytes. (A–D) Basl turns.
ATP1A1 and ATP1B1 encode the Na+/K+-transporting ATPase subunits alpha-1 and beta-130,31 that are responsible for establishing and maintaining the electrochemical gradients of Na+ and K+ ions across the plasma membrane. The cooperative activities of proteins in the basal membrane of marginal cells in the stria vascularis are essential for K+ cycling and the formation of the endocochlear potential in the lateral wall32. In developing marmoset spiral ligament fibrocytes, neither ATP1A1 nor ATP1B1 expression could be detected by E115, whereas both were detected in the stria vascularis (Fig. 2A,B). ATP1B1 expression was detected in spiral ligament type II and IV fibrocytes of the cochlea at E120, but no ATP1A1 expression was detected at this stage (Fig. 2C). At P0, both ATP1A1 and ATP1B1 expression was detected in spiral ligament type II and IV fibrocytes (Fig. 2D).
SLC12A2 (Solute Carrier Family 12 Member 2) encodes the Na+/K+/2Cl− cotransporter (NKCC1), which is essential for normal hearing29,33. Developmental expression patterns of the rodent cochlea have been reported25. In the spiral ligament fibrocytes of the developing cochlea of the common marmoset, no expression was observed until E109 (Fig. 2A). At E115, SLC12A2 expression was observed in type II fibrocytes, and a weak expression was detected in type IV fibrocytes (Fig. 2B). At E120 and P0 cochlea, SLC12A2 expression was observed in type II and IV fibrocytes, similar to that in the adult marmoset cochlea (Fig. 2C,D).
Developmental expression patterns of KCNJ16
Next, we investigated another potassium recycling-related gene in cochlear spiral ligament fibrocytes; KCNJ16 (Fig. 3). KCNJ16 encodes the Kir 5.1 protein, an integral membrane protein, and an inward-rectifier-type potassium channel. Kir5.1 expression has been reported in type II, IV, and V spiral ligament fibrocytes in rodents34. While defects in KCNJ16 gene cause sensorineural hearing loss in human patients35; however, in mice, the KCNJ16 gene is not essential for auditory function36. In the developing cochlea of the common marmoset, no KCNJ16 expression was observed in the spiral ligament fibrocytes at E120, while KCNJ16 expression in the root cells was detected at E120 (Fig. 3A,B). In the P0 cochlea, KCNJ16 expression was observed in type II and IV spiral ligament fibrocytes (Fig. 3C).
Expression of KCNJ16. (A) Expression of KCNJ16 in the E115 cochlea. No KCNJ16 expression could be detected in the spiral ligament fibrocytes. (B) Expression of KCNJ16 in the E120 cochlea. No expression of KCNJ16 could still be detected in the spiral ligament fibrocytes, while KCNJ16 expression could be detected in outer sulcus cells at this stage. (C) Expression of KCNJ16 in the P0 cochlea. KCNJ16 expression could be detected in type II and IV spiral ligament fibrocytes at this stage. The nuclei were counterstained with Hoechst (blue). Scale bar 100 µm, II type II fibrocytes, IV type IV fibrocytes. (A–C) Basal turns.
Developmental expression patterns of CA2 and SLC2A1
In cochlear spiral ligament fibrocytes, several characteristic expressions of the enzymes, such as CA2, SLC2A1, AQP1, and creatinine kinase are expressed, which are related to the regulation of endolymph homeostasis and transporters or channels to maintain normal hearing, are expressed4. In this study, we analyzed the developmental expression of CA2 and SLC2A1 (Figs. 4, 5).
Expression of CA2. (A,B) Expression of CA2 in the E115 cochlea. No CA2 expression could be detected in the spiral ligament fibrocytes, while its expression in the stria vascularis could be observed. (C,D) Expression of CA2 in the E120 cochlea. Broad expression of CA2 in the spiral ligament fibrocytes could be detected at this stage. (E,F) Expression of CA2 in the P0 cochlea. At this stage, CA2 expression could be detected in type I, II, IV, and V spiral ligament fibrocytes. The nuclei were counterstained with Hoechst (blue). Scale bar 100 µm, StV Stria vascularis, I type I fibrocytes, II type II fibrocytes, III type III fibrocytes, IV type IV fibrocytes, V type V fibrocytes. (A–F) Basal turns.
Expression of SLC2A1. (A,B) Expression of SLC2A1 in the E120 cochlea. No SLC2A1 expression could be detected in the spiral ligament fibrocytes, except for blood vessels. (C,D) Expression of SLC2A1 in the P0 cochlea. At this stage, SLC2A1 expression could be detected in type I, II, IV, and V spiral ligament fibrocytes. The nuclei were counterstained with Hoechst (blue). Scale bar 100 µm, StV Stria Vascularis, I type I fibrocytes, II type II fibrocytes, III type III fibrocytes, IV type IV fibrocytes, V type V fibrocytes. (A–D) Basal turns.
CA2 encodes carbonic anhydrase II, one of the 16 human α-carbonic anhydrase forms. CA2 expression in the cochlea has been previously reported in rodents, humans, and common marmosets16,26,37,38,39,40,41. Carbonic anhydrase (CA) affects ion movement in spiral ligament fibrocytes by converting H2O and CO2 to H+ and HCO- ions, which exchange for Na+, K+, and Cl− and regulate the concentrations of these ions and pH in the cochlear fluid. Weak CA2 expression was observed in the spiral ligament fibrocytes of a 15-week-old human fetus, whereas strong CA2 expression was observed in the spiral limbus at this stage41.
In the developing cochlea of the common marmoset, CA2 expression was not observed in spiral ligament fibrocytes by E115, whereas it was detected in the stria vascularis (Fig. 4A,B). At E120, CA2 expression was detected in type I, II, IV, and V fibrocytes, similar to the expression pattern in the adult marmoset cochlea, as previously reported16 (Fig. 4C–F).
SLC2A1 encodes glucose transporter 1 (GLUT1), which is present in the endothelial cells of the capillaries of the stria vascularis7,42,43. In the rodent cochlea, SLC2A1 expression has also been observed in spiral ligament fibrocytes during late development42. In the developing cochlea of the common marmoset, SLC2A1 expression was not observed in the spiral ligament by E120, whereas it was observed in several capillaries as previously reported12 (Fig. 5A,B). In the P0 cochlea, SLC2A1 expression was detected in type I, II, IV, and V fibrocytes (Fig. 5C,D).
Developmental expression patterns of type II collagen
Finally, this study examined the developmental expression patterns of type II collagen, the most abundant collagen in the cochlea and spiral ligament (Fig. 6). COL2A1 encodes the alpha-1 chain of type II collagen, which is found in cartilage, the vitreous humor of the eye, and the inner ear. COL2A1 is one of the causative genes for Stickler syndrome, which is characterized by ocular, skeletal, orofacial, and auditory defects44. COL2A1 gene expression in spiral ligament fibrocytes in rodents45 and human fetuses46 has been reported previously.
Expression of COL2A1. (A) Expression of COL2A1 in the E115 cochlea. No COL2A1 expression could be detected in the spiral ligament fibrocytes. (B,C) Expression of COL2A1 in the E120 cochlea. At E120, COL2A1 expression in the thin layer lining the cochlear boney capsule (arrowheads in C) and slight expression can be detected in fibrocytes. (D,E) Expression of COL2A1 in the P0 cochlea. At P0 cochlear, abundant expression of the COL2A1 is detected broadly in the lateral wall fibrocytes. The nuclei were counterstained with Hoechst (blue). Scale bar 100 µm, StV Stria Vascularis. (A–E) Basal turns.
In the developing cochlea of the common marmoset, no COL2A1 expression was observed in spiral ligament fibrocytes at E115 (Fig. 6A). At E120, COL2A1 expression was observed in the thin layer lining the cochlear bone capsule and its low expression was observed in fibrocytes (Fig. 6B,C). At P0, abundant expression of COL2A1 was detected throughout the lateral wall fibrocytes (Fig. 6D,E).
Discussion
In this study, we investigated the development of spiral ligament fibrocytes in common marmosets. We demonstrated the developmental expression patterns of the characteristic genes reported in rodents and non-human primates. Although spiral ligament fibrocytes are essential for normal hearing ability, little is known about their development compared to other cochlear tissues, such as hair cells, supporting cells, and spiral ganglion neurons. In addition, most of the existing developmental studies are based on rodent models, and only a handful of studies have investigated the spiral ligament fibrocytes development in human fetuses7,41,46.
Recently, interspecies differences in cochlear development between primates (including humans and common marmosets) and rodents have been reported. Therefore, studying the development of spiral ligament fibrocytes in humans is important. However, in the human fetus, spiral ligament fibrocyte development and differentiation occur in the second trimester of gestation (e.g., COL2A1 expression in the human fetal cochlea is evident after 21–22 weeks of gestation46), making it more difficult to use due to ethical concerns in many countries recently. The common marmoset has been reported as an alternative research platform for human fetuses12 in the case of the development of the stria vascularis, an important developmental process that occurs in the late phase of gestation in humans7. In this study, we hypothesized that the common marmoset would be useful for studying the development of spiral ligament fibrocytes. We examined the gene expression patterns of developing spiral ligament fibrocytes.
Schematic diagrams of spiral ligament fibrocyte development in the common marmoset are shown in Fig. 7. Most of the expression of the characteristic genes and differentiation of spiral ligament fibrocytes, supported by the gene expression patterns, were detected in this primate at a relatively late stage of development, as predicted by previous observations obtained from in the human fetus7. Among the genes examined in this study, while CALD1 expression was observed at the earliest stages, most of the initial expression of these characteristic markers, including transporters of channels required for essential functions of spiral ligament fibrocytes, was observed between E115 and P0. This indicates that the functional maturation of spiral ligament fibrocytes is evident during late phase of cochlear development in this primate. At the same time, characteristic markers for each subtype of spiral ligament fibrocytes appeared after E115. This indicates that the differentiation of these subtypes is evident during the late gestation phase in this primate. While the general developmental time course of the lateral fibrocytes of common marmosets was unveiled in this study, the tonotopic gradient of their development could not be investigated in detail. A future study will be awaited.
Schematic diagram of developmental gene expression patterns of spiral ligament fibrocytes. The expression of specific transporters became obvious after approximately E115. Expression of genes related to the ECM of spiral ligament fibrocytes is observed in relatively late stages of development, suggesting that functional differentiation precedes structural maturation of spiral ligament fibrocytes. HCs hair cells, StV stria vascularis, CXs Connexins, SLF spiral ligament fibrocytes. *1: Ref.9, *2: Ref.10, *3: Ref.12, *4: Ref.49.
The developmental maturation of spiral ligament fibrocytes is essential for the regulation of high potassium concentrations in the endolymph and for the generation of sufficient endolymphatic potential for hearing ability. Endocochlear potential has previously been observed in rodents just prior to the onset of hearing and coincides with the morphological maturation of gaps and tight junctions47,48. If the emergence of an endocochlear potential in the common marmoset fetus remains unknown, we hypothesize that endocochlear potential and hearing do not emerge in the marmoset fetus before E115, based on the observation that tight junction formation in basal cells of the stria vascularis becomes evident and gap junctions in lateral wall fibrocytes mature after E11512,49. However, based on the expression patterns of important transporters, this study showed that spiral ligament fibrocytes are not fully mature at E115 (Fig. 7). Our results suggest that the predicted time of the endocochlear potential generation is later than at E120. Future studies measuring endocochlear potential in the fetuses of this primate may benefit from our speculation about the timing of endocochlear potential formation based on our histologic findings.
This study suggests a relatively long gap between the maturation of hair cells and spiral ganglion neurons (around E96)9,10 and the predicted time of emergence of the endolymphatic potential (after E120) in this primate (Fig. 7). During this period, synapse formation between the hair cells and spiral ganglion neurons and pruning of spiral ganglion neurons have been reported to occur9,10. How neuronal connections are refined in this primate without the sufficient endolymphatic potential to influence hair cell activity remains an important scientific question to be elucidated in the future. It might be also useful in this primate that examining Ca2+ channels or relating genes, which are essential for initial synaptic formations before sufficient endolymphatic potential formation, as previously reported in rodents50.
Our results revealed both interspecies similarities and interspecies differences between rodents and primates. Most of the sequential expression patterns of the genes examined in this study were conserved between rodents and primates; however, several interspecies differences should be noted. Among the transporters examined in this study, it has been previously reported that Slc12a2 (P10 in gerbils25), Atp1a1 (P10 in gerbils26, P10 in rats27), and Ca2 (P8-12 in gerbils26, P5 in mice37) expression in spiral ligament fibrocytes precedes Atp1b1 (P15 in gerbils26, P14 in rats27) and Kcnj16 (P14 in rats34) expression in spiral ligament fibrocytes. In our observations of this primate, the sequential expression patterns between rodents and this primate were well preserved, with SLC12A2 and CA2 expressed relatively early and KCNJ16 expressed in the late phase. However, we observed interspecies differences in the expression patterns of ATP1A1 and ATP1B1; ATP1B1 expression preceded ATP1A1 expression in this primate, in contrast to rodents.
Genes related to the ECM and others were identified as having interspecies differences. Previous reports showed COL2A1 expression in developing spiral ligament fibrocytes at P13 in mice51, CCN2 expression at P0 in mice24, and SLC2A1 expression at P14 in gerbils42. We have previously reported that the P0 mouse cochlea is histologically equivalent to E101 in the common marmoset, P9–E115, and P14–D09. These results suggest that the expression patterns of COL2A1 and SLC2A1 are well conserved between rodents and this primate, whereas CCN2 expression occurs relatively late in this primate. Expression patterns in humans have been reported for COCH and COL2A1; COCH expression in spiral ligament fibrocytes has been reported at 22 weeks of gestation52, and COL2A1 at 21–22 weeks of gestation46. Furthermore, we have previously reported that the human fetal cochlea at 20 weeks of gestation is histologically equivalent to E115 in the common marmoset9. Thus, comparing previous findings with the current findings, we concluded that the timing of the expression patterns of COCH and COL2A1 in humans and common marmosets is well conserved.
In this study, we revealed the developmental time course of spiral ligament fibrocytes in this primate model as an alternative to the human fetal cochlea. This understanding of spiral ligament fibrocytes in the primate will be useful in several ways. First, cochlear spiral ligament fibrocytes are targets for regenerative medicine following noise trauma5,27,53. Previous studies have shown that type IV spiral ligament fibrocytes are the most sensitive to noise and degenerate before hair cell loss54,55. In addition, changes in the expression of ion transporters, such as ATP1A1, in spiral ligament fibrocytes after noise trauma have been reported56. Therefore, this study would be useful for future regenerative therapy targeting primate spiral ligament fibrocytes, including in human patients.
Second, relationships between age-related hearing loss and degeneration or morphologic changes in spiral ligament fibrocytes have been reported in rodents25,57,58 and humans59. Recently, Sun et al. reported that the common marmoset is a useful model for age-related hearing loss in non-human primates13. Our findings of spiral ligament fibrocytes in this study would be useful for future studies focusing on the aging of spiral ligament fibrocytes in this primate.
Third, lateral wall fibrocytes are essential for regulating the immune system as well as ion homeostasis. In recent years, the importance of the immune system in the cochlea has been reported, particularly in acoustic trauma or drug-induced hearing loss4. Maturation or activation of the cochlear immune system occurs predominantly in the fibrocytes of the spiral ligament5. For example, intercellular adhesion molecule-1 (ICAM1) expression in the spiral ligament fibrocytes, which would be related to macrophage recruitment in the cochlea in these pathological models, has been reported60,61,62. In addition, Therefore, information on spiral ligament fibrocytes obtained in this study would also be useful for future studies on the development or maturation of the cochlear immune system in this primate.
Conclusions
In conclusion, we have investigated the development of spiral ligament fibrocytes in the cochlea of a primate animal model. We have uncovered the underlying time course of spiral ligament fibrocyte development and the similarities and differences between these primates and rodents. The results of this study will be valuable for future developmental studies on the primate cochlea, as well as for studies on regenerative medicine or the aging studies of cochlear spiral ligament fibrocytes.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Von Bekesy, G. Resting potentials inside the cochlear partition of the guinea pig. Nature 169, 241–242. https://doi.org/10.1038/169241a0 (1952).
Nin, F. et al. The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Natl. Acad. Sci. USA 105, 1751–1756. https://doi.org/10.1073/pnas.0711463105 (2008).
Thulasiram, M. R., Ogier, J. M. & Dabdoub, A. Hearing function, degeneration, and disease: Spotlight on the stria vascularis. Front. Cell Dev. Biol. 10, 841708. https://doi.org/10.3389/fcell.2022.841708 (2022).
Furness, D. N. Forgotten fibrocytes: A neglected, supporting cell type of the cochlea with the potential to be an alternative therapeutic target in hearing loss. Front. Cell. Neurosci. 13, 532. https://doi.org/10.3389/fncel.2019.00532 (2019).
Peeleman, N., Verdoodt, D., Ponsaerts, P. & Van Rompaey, V. On the role of fibrocytes and the extracellular matrix in the physiology and pathophysiology of the spiral ligament. Front. Neurol. 11, 580639. https://doi.org/10.3389/fneur.2020.580639 (2020).
Locher, H. et al. Neurosensory development and cell fate determination in the human cochlea. Neural Dev. 8, 20. https://doi.org/10.1186/1749-8104-8-20 (2013).
Locher, H. et al. Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev. Neurobiol. 75, 1219–1240. https://doi.org/10.1002/dneu.22279 (2015).
Lavigne-Rebillard, M. & Bagger-Sjoback, D. Development of the human stria vascularis. Hear. Res. 64, 39–51. https://doi.org/10.1016/0378-5955(92)90166-k (1992).
Hosoya, M., Fujioka, M., Murayama, A. Y., Okano, H. & Ogawa, K. The common marmoset as suitable nonhuman alternative for the analysis of primate cochlear development. FEBS J. 288, 325–353. https://doi.org/10.1111/febs.15341 (2021).
Hosoya, M. et al. Neuronal development in the cochlea of a nonhuman primate model, the common marmoset. Dev. Neurobiol. 81, 905–938. https://doi.org/10.1002/dneu.22850 (2021).
Hosoya, M. et al. Early development of the cochlea of the common marmoset, a non-human primate model. Neural Dev. 17, 6. https://doi.org/10.1186/s13064-022-00162-8 (2022).
Hosoya, M. et al. Development of the stria vascularis in the common marmoset, a primate model. Sci. Rep. 12, 19811. https://doi.org/10.1038/s41598-022-24380-6 (2022).
Sun, Z. et al. Neural presbycusis at ultra-high frequency in aged common marmosets and rhesus monkeys. Aging (Albany NY) 13, 12587–12606. https://doi.org/10.18632/aging.202936 (2021).
Okano, H. Current status of and perspectives on the application of marmosets in neurobiology. Annu. Rev. Neurosci. 44, 27–48. https://doi.org/10.1146/annurev-neuro-030520-101844 (2021).
Hearn, J. P., Lunn, S. F., Burden, F. J. & Pilcher, M. M. Management of marmosets for biomedical research. Lab. Anim. 9, 125–134. https://doi.org/10.1258/002367775780994709 (1975).
Hosoya, M., Fujioka, M., Ogawa, K. & Okano, H. Distinct expression patterns of causative genes responsible for hereditary progressive hearing loss in non-human primate cochlea. Sci. Rep. 6, 22250. https://doi.org/10.1038/srep22250 (2016).
Suko, T., Ichimiya, I., Yoshida, K., Suzuki, M. & Mogi, G. Classification and culture of spiral ligament fibrocytes from mice. Hear. Res. 140, 137–144. https://doi.org/10.1016/s0378-5955(99)00191-4 (2000).
Ikezono, T. et al. Identification of the protein product of the Coch gene (hereditary deafness gene) as the major component of bovine inner ear protein. Biochim. Biophys. Acta 1535, 258–265. https://doi.org/10.1016/s0925-4439(00)00101-0 (2001).
Ikezono, T. et al. The diagnostic performance of a novel ELISA for human CTP (Cochlin-tomoprotein) to detect perilymph leakage. PLoS One 13, e0191498. https://doi.org/10.1371/journal.pone.0191498 (2018).
Hall-Glenn, F. & Lyons, K. M. Roles for CCN2 in normal physiological processes. Cell Mol. Life Sci. 68, 3209–3217. https://doi.org/10.1007/s00018-011-0782-7 (2011).
Abreu, J. G., Ketpura, N. I., Reversade, B. & De Robertis, E. M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 4, 599–604. https://doi.org/10.1038/ncb826 (2002).
Adams, J. C. Immunocytochemical traits of type IV fibrocytes and their possible relations to cochlear function and pathology. J. Assoc. Res. Otolaryngol. 10, 369–382. https://doi.org/10.1007/s10162-009-0165-z (2009).
Mahendrasingam, S., Bebb, C., Shepard, E. & Furness, D. N. Subcellular distribution and relative expression of fibrocyte markers in the CD/1 mouse cochlea assessed by semiquantitative immunogold electron microscopy. J. Histochem. Cytochem. 59, 984–1000. https://doi.org/10.1369/0022155411421801 (2011).
Friedrichsen, S. et al. CTGF expression during mouse embryonic development. Cell Tissue Res. 312, 175–188. https://doi.org/10.1007/s00441-003-0712-6 (2003).
Sakaguchi, N., Crouch, J. J., Lytle, C. & Schulte, B. A. Na-K-Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res. 118, 114–122. https://doi.org/10.1016/s0378-5955(98)00022-7 (1998).
McGuirt, J. P., Schmiedt, R. A. & Schulte, B. A. Na, K-ATPase and carbonic anhydrase expression in the developing gerbil cochlea. Audit. Neurosci. 2, 135–144 (1996).
Mutai, H., Nagashima, R., Fujii, M. & Matsunaga, T. Mitotic activity and specification of fibrocyte subtypes in the developing rat cochlear lateral wall. Neuroscience 163, 1255–1263. https://doi.org/10.1016/j.neuroscience.2009.07.059 (2009).
Watabe, T. et al. Time-controllable Nkcc1 knockdown replicates reversible hearing loss in postnatal mice. Sci. Rep. 7, 13605. https://doi.org/10.1038/s41598-017-13997-7 (2017).
Dixon, M. J. et al. Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum. Mol. Genet. 8, 1579–1584 (1999).
Schulte, B. A. & Steel, K. P. Expression of alpha and beta subunit isoforms of Na, K-ATPase in the mouse inner ear and changes with mutations at the Wv or Sld loci. Hear Res. 78, 65–76. https://doi.org/10.1016/0378-5955(94)90045-0 (1994).
Erichsen, S., Zuo, J., Curtis, L., Rarey, K. & Hultcrantz, M. Na, K-ATPase alpha- and beta-isoforms in the developing cochlea of the mouse. Hear. Res. 100, 143–149. https://doi.org/10.1016/0378-5955(96)00105-0 (1996).
Hibino, H. & Kurachi, Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 21, 336–345. https://doi.org/10.1152/physiol.00023.2006 (2006).
Delpire, E., Lu, J., England, R., Dull, C. & Thorne, T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat. Genet. 22, 192–195. https://doi.org/10.1038/9713 (1999).
Hibino, H. et al. Expression of an inwardly rectifying K+ channel, Kir5.1, in specific types of fibrocytes in the cochlear lateral wall suggests its functional importance in the establishment of endocochlear potential. Eur. J. Neurosci. 19, 76–84. https://doi.org/10.1111/j.1460-9568.2004.03092.x (2004).
Schlingmann, K. P. et al. Defects in KCNJ16 cause a novel tubulopathy with hypokalemia, salt wasting, disturbed acid-base homeostasis, and sensorineural deafness. J. Am. Soc. Nephrol. 32, 1498–1512. https://doi.org/10.1681/ASN.2020111587 (2021).
Lv, J. et al. Deletion of Kcnj16 in mice does not alter auditory function. Front. Cell Dev. Biol. 9, 630361. https://doi.org/10.3389/fcell.2021.630361 (2021).
Wu, L., Sagong, B., Choi, J. Y., Kim, U. K. & Bok, J. A systematic survey of carbonic anhydrase mRNA expression during mammalian inner ear development. Dev. Dyn. 242, 269–280. https://doi.org/10.1002/dvdy.23917 (2013).
Spicer, S. S. & Schulte, B. A. Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear. Res. 56, 53–64. https://doi.org/10.1016/0378-5955(91)90153-z (1991).
Ichimiya, I., Adams, J. C. & Kimura, R. S. Immunolocalization of Na+, K(+)-ATPase, Ca(++)-ATPase, calcium-binding proteins, and carbonic anhydrase in the guinea pig inner ear. Acta Otolaryngol. 114, 167–176. https://doi.org/10.3109/00016489409126037 (1994).
Weber, P. C., Cunningham, C. D. 3rd. & Schulte, B. A. Potassium recycling pathways in the human cochlea. Laryngoscope 111, 1156–1165. https://doi.org/10.1097/00005537-200107000-00006 (2001).
Yamashita, H., Sekitani, T. & Bagger-Sjoback, D. Expression of carbonic anhydrase isoenzyme-like immunoreactivity in the limbus spiralis of the human fetal cochlea. Hear. Res. 64, 118–122. https://doi.org/10.1016/0378-5955(92)90173-k (1992).
Ito, M., Spicer, S. S. & Schulte, B. A. Immunohistochemical localization of brain type glucose transporter in mammalian inner ears: Comparison of developmental and adult stages. Hear. Res. 71, 230–238. https://doi.org/10.1016/0378-5955(93)90039-4 (1993).
Ando, M., Edamatsu, M., Fukuizumi, S. & Takeuchi, S. Cellular localization of facilitated glucose transporter 1 (GLUT-1) in the cochlear stria vascularis: Its possible contribution to the transcellular glucose pathway. Cell Tissue Res. 331, 763–769. https://doi.org/10.1007/s00441-007-0495-2 (2008).
Acke, F. R., Dhooge, I. J., Malfait, F. & De Leenheer, E. M. Hearing impairment in Stickler syndrome: A systematic review. Orphanet. J. Rare Dis. 7, 84. https://doi.org/10.1186/1750-1172-7-84 (2012).
Yoo, T. J. & Tomoda, K. Type II collagen distribution in rodents. Laryngoscope 98, 1255–1260. https://doi.org/10.1288/00005537-198811000-00019 (1988).
Khetarpal, U., Robertson, N. G., Yoo, T. J. & Morton, C. C. Expression and localization of COL2A1 mRNA and type II collagen in human fetal cochlea. Hear. Res. 79, 59–73. https://doi.org/10.1016/0378-5955(94)90127-9 (1994).
Steel, K. P. & Barkway, C. Another role for melanocytes: Their importance for normal stria vascularis development in the mammalian inner ear. Development 107, 453–463. https://doi.org/10.1242/dev.107.3.453 (1989).
Souter, M. & Forge, A. Intercellular junctional maturation in the stria vascularis: Possible association with onset and rise of endocochlear potential. Hear. Res. 119, 81–95. https://doi.org/10.1016/s0378-5955(98)00042-2 (1998).
Hosoya, M. et al. Dynamic spatiotemporal expression changes in connexins of the developing primate’s cochlea. Genes (Basel) https://doi.org/10.3390/genes12071082 (2021).
Johnson, S. L. et al. Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proc. Natl. Acad. Sci. 110, 8720–8725. https://doi.org/10.1073/pnas.1219578110 (2013).
Shpargel, K. B., Makishima, T. & Griffith, A. J. Col11a1 and Col11a2 mRNA expression in the developing mouse cochlea: Implications for the correlation of hearing loss phenotype with mutant type XI collagen genotype. Acta Otolaryngol. 124, 242–248. https://doi.org/10.1080/00016480410016162 (2004).
Robertson, N. G. et al. Inner ear localization of mRNA and protein products of COCH, mutated in the sensorineural deafness and vestibular disorder, DFNA9. Hum. Mol. Genet. 10, 2493–2500. https://doi.org/10.1093/hmg/10.22.2493 (2001).
Li, Y., Watanabe, K., Fujioka, M. & Ogawa, K. Characterization of slow-cycling cells in the mouse cochlear lateral wall. PLoS One 12, e0179293. https://doi.org/10.1371/journal.pone.0179293 (2017).
Hirose, K. & Liberman, M. C. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 4, 339–352. https://doi.org/10.1007/s10162-002-3036-4 (2003).
Wang, Y., Hirose, K. & Liberman, M. C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 3, 248–268. https://doi.org/10.1007/s101620020028 (2002).
Yamaguchi, T., Nagashima, R., Yoneyama, M., Shiba, T. & Ogita, K. Disruption of ion-trafficking system in the cochlear spiral ligament prior to permanent hearing loss induced by exposure to intense noise: Possible involvement of 4-hydroxy-2-nonenal as a mediator of oxidative stress. PLoS One 9, e102133. https://doi.org/10.1371/journal.pone.0102133 (2014).
Hequembourg, S. & Liberman, M. C. Spiral ligament pathology: A major aspect of age-related cochlear degeneration in C57BL/6 mice. J. Assoc. Res. Otolaryngol. 2, 118–129. https://doi.org/10.1007/s101620010075 (2001).
Mahendrasingam, S., Macdonald, J. A. & Furness, D. N. Relative time course of degeneration of different cochlear structures in the CD/1 mouse model of accelerated aging. J. Assoc. Res. Otolaryngol. 12, 437–453. https://doi.org/10.1007/s10162-011-0263-6 (2011).
Kusunoki, T. et al. Age-related histopathologic changes in the human cochlea: A temporal bone study. Otolaryngol. Head Neck Surg. 131, 897–903. https://doi.org/10.1016/j.otohns.2004.05.022 (2004).
Schmutzhard, J. et al. Apoptosis of the fibrocytes type 1 in the spiral ligament and blood labyrinth barrier disturbance cause hearing impairment in murine cerebral malaria. Malar. J. 11, 1–10 (2012).
Miyao, M., Firestein, G. S. & Keithley, E. M. Acoustic trauma augments the cochlear immune response to antigen. Laryngoscope 118, 1801–1808 (2008).
Tornabene, S. V., Sato, K., Pham, L., Billings, P. & Keithley, E. M. Immune cell recruitment following acoustic trauma. Hear. Res. 222, 115–124. https://doi.org/10.1016/j.heares.2006.09.004 (2006).
Acknowledgements
We thank Saki Ninomiya for providing technical support. We thank Ayako Y. Murayama, Sho Yoshimatsu, Junko Okahara for providing materials.
Funding
MH was supported by a Grant from the Japanese Government, MEXT KAKENHI (Grant-in-Aid for Scientific Research (B) 20H03836, Grant-in-Aid for Challenging Research (Exploratory) 21K19581), the Keio Medical Association, and Keio University Medical Science Fund, the Society for Promotion of International Oto-Rhino-Laryngology (SPIO), the Kanae Foundation for the Promotion of Medical Science, the Takeda Science Foundation, and Keio Gijuku Academic Development Funds. KI was supported by a Grant from the Japanese Government, MEXT KAKENHI (Grant-in-Aid for Scientific Research (C) 23K08973).
Author information
Authors and Affiliations
Contributions
M.H., K.I., T.K., T.N., N.O., H.O., and H.O., conceived and designed the experiments. M.H., K.I., and T.K. wrote the manuscript. M.H. performed most of the experiments. M.H., K.I., and T.K. analyzed the data. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
H. Okano is a founding scientist and a paid scientific advisory board member of San Bio Co., Ltd, and K Pharma Inc. MH was the founding scientist of Otolink, Inc. KI, TK, TN, NO and H. Ozawa have no conflicts of interest directly relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hosoya, M., Iwabu, K., Kitama, T. et al. Development of cochlear spiral ligament fibrocytes of the common marmoset, a nonhuman model animal. Sci Rep 13, 11789 (2023). https://doi.org/10.1038/s41598-023-39003-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39003-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.