Abstract
Increasing carbon dioxide (CO2) concentration in the atmosphere is considered one of the most important challenges today. Therefore, capturing CO2 and producing alternative energy sources through Power-to-X (PtX) approaches have become relevant scientific topics in recent years. However, there is a significant research gap regarding water management in PtX processes, particularly in offshore operations. The present study evaluates relevant aspects and possible challenges with respect to water management as well as mass and energy balances in conceptual offshore methane and methanol production platforms. The results show that 1600 m3 of seawater must be desalinated to supply the electrolyzer and reach a daily 50-Megagram (Mg) hydrogen production. Around 1100 m3 of brine coming out of the desalination plant may be discharged to the sea as long as prior environmental impact assessments are conducted. Additionally, 273 Mg and 364 Mg CO2 need to be generated daily by direct air capture to produce 99 Mg day−1 methane and 265 Mg day−1 methanol, respectively. The daily produced methane and methanol wastewater is estimated to be 223 and 149 m3, respectively. Based on the scant literature on methanol wastewater, this is expected to contain toxic substances. Zero liquid discharge (ZLD) is proposed as wastewater method. The corresponding energy demand for the water management facilities is projected to be negligible compared to the other PtX processes. The presented management of water streams in PtX platforms would not only help recover some of the resources (water, hydrogen and methanol), but also substantially contribute to the production cycle itself while leading toward a more sustainable approach.
Similar content being viewed by others
Introduction
The current emission of anthropogenic carbon dioxide (CO2) together with its increasing concentration in the atmosphere and oceans is certainly one of the most challenging global issues today1. A recent report from the Intergovernmental Panel on Climate Change shows that, among all major groups of greenhouse gases, CO2 holds the largest share of global net emissions from anthropogenic sources (around 75% in 2019)2. The Paris Agreement of the United Nations Framework Convention on Climate Change came to the light to establish a road map aiming to reduce CO2 emissions from fossil fuel burning and limit global warming to well below 2 °C3. As a result, several approaches as well as governmental incentives and strategies have been established to promote and develop renewable energies and concepts such as Power-to-X4,5,6,7. Power-to-X (PtX) consists in the production of hydrogen (Power-to-Hydrogen)8 or other clean fuels and energy carriers, such as methane, methanol and other synthetic fuels9,10,11,12, with the use of electricity coming from wind or solar energy, among other renewable sources. Offshore platforms are considered as potential areas of implementation for PtX technologies as they provide optimal conditions in terms of availability of existing infrastructure and resources such as water, CO2 and renewable energy6,13. In order to move away from fossil fuels and reduce the carbon footprint, an improvement in the efficiency of energy production is fundamental. One major issue of renewable sources, such as wind energy, is their fluctuating operation. However, the generation of energy carriers in the form of PtX products offers a way to easily store and transport surplus or intermittent electricity14,15.
Offshore Power-to-X platforms generally comprise of seawater desalination followed by a water electrolysis unit to produce pure hydrogen8. Hydrogen can be used as fuel or further processed along with CO2 in a synthetic fuel unit, where a final product and wastewater are generated. The most relevant units and technologies for PtX production of methane and methanol are presented hereafter.
Currently, hydrogen is largely produced from fossil fuels; a source that not only is unsustainable but generates significant CO2 emissions16. Recent advances in alternative technologies, such as water electrolysis, have allowed the use of renewable energies for emissions-free hydrogen generation. Electrolysis involves the splitting of water molecules into hydrogen and oxygen by applying an electrical current.
Alkaline electrolyzers (AEL), proton exchange membrane (PEM) electrolyzers, and solid oxide electrolyzers (SOEL) are some examples of water electrolysis technologies. In principle, water electrolysis consists of two electrodes submerged in or separated by an electrolyte4. AEL and PEM are advanced and commercially available technologies, as opposed to SOEL, which is still in early demonstration phases16. Comprehensive studies from the International Renewable Energy Agency4,16 provided a comparison between AEL and PEM along with forecasts on their performance and techno-economic characteristics. The studies presented the advantages that make PEM a more suitable technology for offshore operations over its commercial counterpart; including its fast response times, reduced overall footprint and flexible operation4,16,17. PEM electrolyzers are not built to operate directly with seawater but require ultra-pure water with a feed quality below 0.5 ppm total dissolved solids (approximately 1 μS cm−1)17,18. In consequence, any unreacted water which is internally recirculated must be regularly removed and retreated to avoid concentration of substances within the electrolyzer. In offshore environments, such high quality can be achieved by means of desalination technologies.
Reverse osmosis (RO) is currently the main commercially-available technology for the production of fresh water from brackish and seawater19,20. RO is a pressure-driven process in which water is pumped through a series of semi-permeable membranes, thereby generating a low salinity product (permeate) along with a residual concentrated brine (concentrate). Apart from the pressurized RO membrane modules, RO plants may include a pretreatment step consisting of filtration and addition of chemicals to protect the RO membranes, as well as a post-treatment to adjust and reach the target quality. The required water quality for PEM electrolysis cannot be achieved with RO alone, thus a polishing post-treatment, typically through ion exchange or electrodialysis, is needed. RO entails electricity to achieve high operating pressures. As a way to fulfill the energy demand for these systems and take advantage of renewable sources, membrane desalination plants have been coupled with renewable energies in the recent years21. For instance, Serna and Tadeo22,23 simulated the operation and integration of RO desalination with both fluctuating wave energy and PEM electrolysis. Other desalination technologies, such as electrodialysis, have also been shown to be suitable alternatives for fresh water supply with variable power sources (hybrid photovoltaic/wind)24.
Methane is an energy carrier of significant importance to the industry, energy, and transportation sectors worldwide. Two major approaches exist to produce synthetic methane: biological methanation and catalytic methanation. Catalytic methanation involves the hydrogenation of either carbon monoxide (CO) or CO2 to produce methane and water14,25. Catalytic reactors, in particular fixed-bed reactors, are considered the state of the art for large-scale applications26. In the Power-to-X concept, CO2 from point sources or air is the target feedstock along with hydrogen produced by electrolysis. From a stochiometric standpoint, CO2 methanation generates twice as much wastewater—which must be handled before disposal or reuse—for every mol of methane produced compared to CO methanation. In addition, both approaches are largely exothermic and therefore heat management is required.
Methanol is used as an energy carrier for hydrogen storage and transportation and has several applications in the chemical industry27,28,29. Currently, 90% of the total methanol is produced from natural gas through high-pressure (the BASF process) and low-temperature methods30. Additional available approaches include methanol production from coal, biomass31 and the catalytic hydrogenation of CO232. Similar to methane synthesis, the generation of methanol through green hydrogen and CO2 is considered as a suitable and promising approach for PtX. Likewise, the reaction of hydrogenation of CO2 to methanol is slightly exothermic and requires cooling as well as generates wastewater as by-product.
In offshore environments, the generation of methane and methanol wastewater together with a large brine production after desalination through RO have the potential to be an environmental bottleneck of these operations. Research has shown that slight changes in salinity and temperature caused by brine disposal practices may have an adverse impact on marine ecosystems33. A previous study on the feasibility of offshore desalination highlighted the environmental impact as an important aspect to consider in platform-based facilities34. Water intake, backwash/concentrate neutralization and strategic discharge are of high relevance. To the best of the authors’ knowledge, there are no studies in the current literature that provide information on the characteristics or the precise management of process wastewaters from catalytic methanation reactors. A single study by Schirrmeister et al.35 has reported that wastewater from a methanation demonstration plan was processed and reused for water electrolysis without further details. Likewise, literature about methanol wastewater is highly limited. Methanol wastewaters are considered as toxic and proven to negatively impact the human and ecological environment36,37. Table 1 summarizes the characteristics of wastewater coming from methanol synthesis. The wide range of values shows the variability of the reported qualities, depending on the production process. Apart from the substances listed in Table 1, formaldehyde and methanoic acid have also been observed in methanol wastewater36.
Therefore, a sustainable wastewater treatment is the need of the hour. Zero liquid discharge (ZLD) has previously been considered for treating brine17,38 as well as wastewater39. Conventional ZLD approaches consist of a series of thermal processes, often in combination with membrane technologies40. Although ZLD is becoming very popular in industrial wastewater treatment in several countries, the energy consumption and the implementation cost remain very high. However, globally increasing freshwater scarcity and the rising cost of wastewater disposal made ZLD an attractive water treatment and management strategy41. Hence, ZLD approach was followed in this research study.
Currently, there is a lack of literature that incorporates water management as a main topic of Power-to-X. Available publications on inland or offshore water electrolysis42,43 and/or generation of methane, methanol and other PtX products12,13,44 primarily limit their focus on the processes themselves, their integration or modelling. Hence, the management, disposal or treatment of water are barely addressed17 or not mentioned. Nevertheless, water is highly relevant in PtX operations as it is an important input and the main byproduct. Particularly in remote locations like offshore platforms, a proper and self-sufficient water management is essential. This work looks into the relevant aspects that surround the use and handling of water on offshore PtX platforms and how these compare to other processes in terms of energy consumption. Additionally, this study aims to provide an initial overview of the mass balance and different water streams in such operations by evaluating a conceptual PtX platform with a daily 50-Megagram (Mg) hydrogen production in which methane and methanol are considered as two separate target product scenarios.
Methods
Offshore platform concept
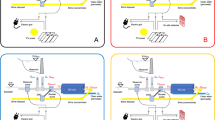
The selected location for the conceptual offshore Power-to-X platform is the German Bay region in the North Sea. Two production scenarios were selected for the study: one platform for methane and another one for methanol as final product. The results presented in this work were performed with the assumption that energy is supplied by offshore wind parks without interruption or varying loads, so the entire platform was in continuous operation. A production of 50 Mg of hydrogen a day from the electrolyzer was defined as the base size of the platform. Accordingly, the design and estimation of all processes in the two scenarios were derived from this parameter. Figure 1 shows a graphical depiction of a wind-powered PtX platform, in which seawater is pumped into a desalination step to later be used to produce hydrogen and methane or methanol from CO2 in catalytic reactors. In this concept, wastewater from the synthesis process is treated to avoid its disposal back into the sea. The only outputs of the platform are brine from the desalination, solid waste and the target product.
Seawater desalination
A desalination plant was designed to determine the input water quantity needed to supply the electrolyzer with ultra-pure water as well as to estimate the energy demand of the overall desalination process. The plant consists of pretreatment, desalination through reverse osmosis and post-treatment with ion exchange. The desalination process was simulated in Water Application Value Engine (WAVE, DuPont) to estimate the recovery rate and specific energy consumption. Feed seawater composition and conditions for the design were taken from available literature from the selected offshore location. The permeate required to cover the desired daily hydrogen production was estimated by the general chemical reaction in an electrolyzer:
with the assumption that 90% of the water is split, as described for commercial electrolyzers45, while the unreacted 10% is returned to the ion exchange system. Additional water demands for cooling or steam generation in the other processes was not considered for the design since data on the required quantities and qualities are limited in literature. Moreover, cooling systems in the different processes may also be operated with locally abundant seawater or cooling oil and not strictly with purified water.
Electrolysis
The rated power \({P}_{el,PEM}\) [W] for the electrolyzer was calculated by Faraday’s law46:
where \({\dot{n}}_{{H}_{2}}\) is the amount of hydrogen produced [mol s−1], \(z\) is the valence number of ions of hydrogen (2), \(F\) is the Faraday constant and \({\eta }_{F}\) the faraday efficiency (up to 99%46). The applied cell voltage \({U}_{el}\) has to exceed the thermoneutral voltage needed to split a water molecule (1.48 V)18. Commercial electrolyzers are typically operated between 1.6 to 2 V depending on the current density and operating temperature; a low current density is equivalent to a low voltage but results in lower production rates and a larger electrolyzer47. Since space is a limiting aspect in offshore platforms, a voltage of 2 V was used in this study. The assumed operating pressure and temperature were 30 bar and 80 °C.
Direct air capture (DAC)
DAC using solid-based adsorption and desorption was considered in this study because it requires lower temperature, approximately 80–100 °C which allows the integration of waste heat from electrolyzers and synthetic fuel synthesis. Commercial solid-base DAC can capture 0.14 Mg CO2 day−1 while consuming 2.5 MWhth thermal energy per Mg CO2 and 0.5 MWhel per Mg CO248.
The theoretical amount of CO2 [g] needed for either methane synthesis or methanol synthesis can be determine based on Eq. (3). The ideal stoichiometric ratios (\(SR\)) of H2/CO2 are 4 and 3 for methane and methanol, respectively. The governing parameter here is the daily amount of H2 produced by the electrolyzer, \({n}_{{H}_{2}}\) [mol].
where \({MW}_{{CO}_{2}}\) is the molecular weight of CO2. The resulting mass of carbon dioxide \({m}_{{CO}_{2}}\) was then used to estimate the necessary electrical and thermal energy demand.
Methane synthesis
Fixed-bed methane synthesis based on honeycomb catalysts was selected for this study due to its good load flexibility26. The methanation process was adapted from a demonstration plant operated by Mörs et al.49 to convert H2 and CO2 to methane (CH4) as described by the Sabatier reaction:
The two-phase methanation consisted in a reactor with multi-tube channels coated with a catalyst followed by a polishing reactor. The total energy demand of the methane synthesis was taken as 1.2 MWhel per Mg CH4. Assuming 100% conversion of hydrogen, the daily estimated methane production [g] was calculated using Eq. (5).
where \(SR\) equals 4, and \({MW}_{{CH}_{4}}\) is the molecular weight of methane.
Methanol synthesis
Methanol synthesis through catalytic hydrogenation of CO2 methods were considered in this study and estimated by following the proposed offshore model from Patterson et al.12, where Eq. (6) was considered as the main production formula50.
The stoichiometric ratio (\(SR\)) of H2/CO2 was calculated as 3. Therefore, ideally 1 mol of CO2 and 3 mol of hydrogen are needed to produce 1 mol of methanol (CH3OH) and 1 mol of water. With 100% conversion rate of the reactants, the daily estimated methanol production [g] was calculated by Eq. (7).
where \({MW}_{{CH}_{3}OH}\) is the molecular weight of methanol.
Wastewater treatment
Considering the conversion assumptions followed for methane and methanol production, the daily volume of wastewater [m3] from each synthesis processes was estimated as follows:
in which \({MW}_{{H}_{2}O}\) is the molecular weight of water, and \(SR\) was taken as 2 and 3 for methane and methanol wastewater, respectively.
ZLD was considered for the calculations as the worst-case scenario in terms of energy consumption. The calculation approach for energy demand of the ZLD process was focused on treatment of the methane and methanol wastewaters, followed by reuse of the recovered water. A normalized energy demand of 58.6 kWhel m-3 was taken into consideration for methane and methanol wastewater treatment as assumed in literature for wastewater treatment through conventional thermal ZLD technologies39. The energy consumption by ZLD of the respective wastewater volumes was calculated as follows:
Results
The process chains of the PtX platform are depicted in Fig. 2. Likewise, the figure summarizes the main daily inputs and outputs of each process unit for both production scenarios (i.e., methane and methanol as final products).
Desalination
As seen in Fig. 2, the desalination plant treats under average conditions around 1600 m3 of seawater per day at an overall recovery of around 32%, thus delivering 500 m3 day−1 of ultra-pure water (< 0.1 μS cm−1) to the electrolyzer. The plant was designed to operate in a two-pass mode in order to reduce the amount of chemicals required for the post-treatment and maintain offshore storage and disposal challenges to the minimum. The energy consumption of the reverse osmosis system was estimated at 5.9 kWhel per m3 of produced water, while the consumption for the ultrafiltration pretreatment and post-treatment through ion exchange mixed beds was 0.2 and 0.1 kWhel m−3, respectively, resulting in a total specific energy consumption of is 6.2 kWhel m−3.
Around 1100 m3 of brine are produced daily by the desalination plant and returned to the sea. This stream contains a salinity level higher than the local conditions of the North Sea along with additional treatment chemicals. Concentrate discharge into the open-ocean is a common disposal practice in coastal desalination plants and may be a solution in offshore operations19. The Oslo-Paris Convention for the protection of the marine environment of the North-East Atlantic (OSPAR) strictly monitors and regulates the disposal of waste streams in offshore oil and gas installations. Thus, OSPAR regulations may apply for chemicals contained in the brine depending on their toxicity and biodegradability, however, the Convention currently does not state any discharge limits for salinity levels51. Nevertheless, an effective disposal management would certainly require site-specific assessments and the proper discharge equipment (i.e., piping and diffuser systems) to reduce its environmental impact as much as possible52,53.
Electrolysis
The PEM electrolysis system was designed to operate at 111.9 MWel in order to produce 50 Mg of hydrogen per day. This estimates that around 54 kWhel are needed to produce 1 kg of hydrogen, which corresponds to current energy demand values reported for PEM plants of similar magnitudes17,42,54. Moreover, the efficiency of the plant lies within the typical range and was found to be 62% with respect to the lower heating value of hydrogen16,17,54.
DAC
A daily production of 50 Mg of hydrogen corresponds to 24.8 × 106 mol of hydrogen. The required amount of CO2 to be capture and provided for synthetic fuels synthesis along with the energy demand required to capture this amount are shown in Table 2.
The total estimated energy demand in this study is 3.0 MWh Mg−1 CO2, which is slightly higher than the reported DAC industrial plant energy requirements from 1.8 to 2.45 MWh Mg−1 CO255.
Methane synthesis
Based on the ideal hydrogenation of CO2 in Eq. (4), the conversion of 273 Mg CO2 day−1 with 50 Mg H2 day−1 produces a maximum of 99 Mg CH4 day−1 and 223 m3 day−1 CH4 wastewater (Fig. 2a) as given by Eqs. (5) and (8), respectively. The total energy demand was estimated at 120 MWhel day−1.
Methanol synthesis
The total conversion of 364 Mg CO2 day−1 with 50 Mg H2 day−1 produces 265 Mg CH3OH day−1 and 149 m3 day−1 CH3OH wastewater (Fig. 2b) by Eqs. (7) and (8), respectively. The electrical and thermal energy inputs in addition to the thermal energy output during methanol synthesis were adapted from the details shown in Fig. S6 of the supplementary information from Patterson et al.12. The calculated required electrical power consumed by the compressors was found 0.74 MWel while the thermal energy input was mainly required by the reboiler of the distillation column which requires 1.0 MWth per 1 Mg methanol production. These consumptions corresponded to approximately 196 MWhel and 265 MWhth. The thermal energy output equaled to the sum of thermal energies produced by compressors, steam drum, heat exchangers, hydrogen purge, and distillation column which resulted in 3.1 MWth. 1.0 MWth of the produced thermal energy was reused for the reboiler.
Wastewater treatment
The methane and methanol wastewater volume per day was calculated by following Eq. (8). Consequently, the daily required energy to treat methane and methanol wastewater was calculated by Eq. (9). The daily methane wastewater production was found 33.3% higher than the daily produced methanol wastewater. This led to consume 1.5 times higher energy to treat the methane wastewater as well (Fig. 3).
Discussion
As seen in Fig. 2, coupling methanol synthesis with a base daily production of 50 Mg of hydrogen would result in a larger amount of PtX product than having methane as final product. However, the total energy content of the expected production of methanol is just slightly higher than for methane despite the lower product quantity—namely, 1376 MWh for 99 Mg day−1 CH4 and 1479 MWh for 265 Mg day−1 CH3OH. The final products would need to be transported to the coast.
Moreover, a comparison of the cumulative daily energy consumption for both methane and methanol production platforms in Fig. 4 shows that the latter requires more energy to operate, as a consequence of its higher CO2 demand. In terms of electrical energy efficiency, there is no clear advantage between the two production scenarios. Around 47% of the input electrical energy in both process chains leaves in the form of the respective PtX product.
Electrolysis is the most energy demanding process of those evaluated, consuming nearly 87–91% of the total electrical energy. Whereas the desalination energy demands are practically negligible when compared to the rest of the processes with only 0.1%. This relatively low energy consumption has also been identified in Serna and Tadeo23 and Khan et al.17 showing the potential scalability of such a desalination treatment if additional water quantities are to be covered for heat management in other processes. Desalination is not expected to be significant in terms of energy demand regardless of the supply magnitudes of pure water required, but it may rather be limited by the space available as the plant itself and the required water and chemical storage capacity would be larger.
The treatment of the wastewater streams by ZLD required 0.3–0.5% of the total electrical energy (Fig. 4). From an energy perspective, the water management components in both production scenarios seem to be insignificant. Under variable electricity conditions, it may be expected that the operation will be adapted to the requirements of the other PtX processes.
ZLD would be an important measure to recover a substantial amount of hydrogen in the form of wastewater. As depicted in the normalized flow diagram of hydrogen in the process chain (Fig. 5a,b), 0.45 mol and 0.30 mol of H2 per mol of water produced by the desalination plant are lost as wastewater from the methane and methanol production, respectively. This constitutes 33–50% of the initial hydrogen input into the PEM. Treating methanol wastewater by ZLD would also remove the substantial amount of COD and aromatics along with formaldehydes and methanoic acids that are considered as highly harmful to the ecological system and human health37. This measure would be in accordance with the OSPAR Convention regulations51. In addition, ZLD could allow for the recovery of residual methanol. For instance, it may be estimated that up to 61.25 kg CH3OH day−1 may be recovered when considering a methanol concentration of 350 mg L−1 in the wastewater stream36,37.
RO brine treatment would also provide further recirculation of hydrogen, considering around 70% of the feed water is returned to the sea. ZLD would be a potential strategy for this, however, its implementation on brine treatment and management is still expected to see a progressive growth17,40. Previous studies on brine management by ZLD have reported energy consumptions of conventional brine concentrators of up to 39 kWh m−341, whereas conventional crystallizers may reach up to 70 kWh m−338. Furthermore, the energy required for a complete treatment of the daily 1100 m3 brine by either of these technologies would still correspond to less than 3% of the consumption from the PEM electrolyzer. The application of ZLD as brine treatment method would bring back substantial amounts of pure water into the hydrogen production cycle as well as recover other valuable resources. This could have a massive potential to help the overall economy as well17. Nevertheless, in offshore environments, space and load capacities of the platform would be the final limiting factors.
The proposed method reflected that the most important greenhouse gas (CO2) can be captured from natural or industrial source, human activity, or air by absorption, and chemically transformed into methane and methanol29. In addition, the production of methane and methanol from CO2 can be regarded as a completely carbon–neutral process, considering that the hydrogen necessary for this productive cycle can be originated from water dissociation by electrolysis56,57 and renewable electricity is employed.
The presented results clearly indicate the staggering energy consumption related to overall brine and wastewater treatment. Nevertheless, it is quite negligible when compared with the energy demand related to PtX products generation itself. In addition, the potential recovery of methanol and reincorporation of product water from the synthesis processes would contribute to the circular approach of the economy. Although considering the energy demand of ZLD in this approach may present the maximum energy demand for the wastewater treatment, its substantial impact may not be ignored when moving towards more sustainable treatment approaches.
Conclusion
A conceptual PtX platform for the generation of two separate product scenarios (methane and methanol) was evaluated to discuss the most important aspects that surround water management in offshore operations. Based on the results, a daily hydrogen production of 50 Mg from PEM electrolysis, together with 273 Mg and 364 Mg of captured CO2, can generate up to 99 Mg and 265 Mg of methane and methanol, respectively. It was estimated that the production chains would consume between 2945 and 3067 MWhel (plus 683 and 1175 MWhth) on a daily basis and result in comparable efficiencies with respect to the energy content of the final products. In addition, the assessment of the presented PtX platform led to the following relevant remarks with respect to water management:
-
The desalination step treats large amounts of water and around 70% comes out as brine. The brine contains the same local salt components as the raw water but at higher concentrations as well as treatment substances such as anti-scalants, cleaning agents and regeneration chemicals.
-
Brine discharge was proposed as disposal approach and may be implemented in real operations. Such measures should still include the provisions that local regulations are followed and proper environmental assessments and disposal strategies are performed to evaluate and reduce the impact on marine environments.
-
Between 30 to 45% of input hydrogen (in the form of water) into the PEM gets lost as wastewater during the methane and methanol generation processes. This is a reaction by-product from the hydrogenation of CO2 in both syntheses. However, data corresponding to wastewater volumes from real plants are not addressed in the available literature.
-
While no information is available on the composition of methane wastewaters, only a few studies provided data on wastewater from methanol production. Methanol wastewater is of environmental concern and therefore shall not be directly discharged.
-
Zero liquid discharge was proposed as the state-of-the-art solution to treat the waste streams in PtX-platforms as it provides the potential to eliminate all contaminants and ultimately reintegrate hydrogen as purified water into the processes.
-
Although zero liquid discharge as well as desalination are associated with high energy demands, their respective estimated energy consumptions was practically negligible compared to the rest of the processes considered for the PtX platform. Space and weight still remain as possible limiting factors of the water management facilities in offshore operations.
Decarbonization is certainly an important goal today and a shift to renewable sources and PtX products is one clear way toward it. However, it is essential that aspects such as resources management (water) and environmental protection by waste control in offshore operations are sensibly considered in order to claim and ensure that these processes are as sustainable and green as possible.
Data availability
All relevant data analyzed during the current study are included in the manuscript. Raw data are available from the corresponding author upon reasonable request.
References
United Nations Global Issues. https://www.un.org/en/global-issues (2022).
IPCC Sixth Assessment Report. Mitigation of Climate Change. Intergovernmental Panel on Climate Change (2022).
Stevens, C. J., Leach, A. M., Dale, S. & Galloway, J. N. Personal nitrogen footprint tool for the United Kingdom. Environ. Sci. Process. Impacts 16, 1563–1569 (2014).
International Renewable Energy Agency. Green hydrogen cost reduction: Scaling up electrolysers to meet the 1.5°C climate goal (2020).
Singlitico, A., Østergaard, J. & Chatzivasileiadis, S. Onshore, offshore or in-turbine electrolysis? Techno-economic overview of alternative integration designs for green hydrogen production into Offshore Wind Power Hubs. Renew. Sustain. Energy Transit. 1, 100005 (2021).
Wulf, C., Zapp, P. & Schreiber, A. Review of power-to-X demonstration projects in Europe. Front. Energy Res. 191 (2020).
H2Mare: offshore. How partners in the H2Mare flagship project intend to produce hydrogen on the high seas. https://www.wasserstoff-leitprojekte.de/projects/h2mare (2022).
Caparrós Mancera, J. J., Segura Manzano, F., Andújar, J. M., Vivas, F. J. & Calderón, A. J. An optimized balance of plant for a medium-size PEM electrolyzer: Design, control and physical implementation. Electronics 9, 871. https://doi.org/10.3390/electronics9050871 (2020).
Hoppe, W., Thonemann, N. & Bringezu, S. Life cycle assessment of carbon dioxide-based production of methane and methanol and derived polymers. J. Ind. Ecol. 22, 327–340 (2018).
Younas, M. et al. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2. Energy Fuels 30, 8815–8831 (2016).
Goeppert, A., Czaun, M., Jones, J.-P., Prakash, G. S. & Olah, G. A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 43, 7995–8048 (2014).
Patterson, B. D. et al. Renewable CO2 recycling and synthetic fuel production in a marine environment. Proc. Natl. Acad. Sci. 116, 12212–12219 (2019).
O’Kelly-Lynch, P. D., Gallagher, P. D., Borthwick, A. G., McKeogh, E. J. & Leahy, P. G. Offshore conversion of wind power to gaseous fuels: feasibility study in a depleted gas field. Proc. Inst. Mech. Eng. Part A J. Power Energy 234, 226–236. https://doi.org/10.1177/0957650919851001 (2020).
Götz, M. et al. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 85, 1371–1390 (2016).
Sterner, M. & Specht, M. Power-to-Gas and Power-to-X—The history and results of developing a new storage concept. Energies 14, 6594 (2021).
International Renewable Energy Agency. Hydrogen from renewable power: Technology outlook for the energy transition (2018).
Khan, M. A. et al. Seawater electrolysis for hydrogen production: A solution looking for a problem?. Energy Environ. Sci. 14, 4831–4839. https://doi.org/10.1039/d1ee00870f (2021).
Meier, K. Hydrogen production with sea water electrolysis using Norwegian offshore wind energy potentials. Int. J. Energy Environ. Eng. https://doi.org/10.1007/s40095-014-0104-6 (2014).
Greenlee, L. F., Lawler, D. F., Freeman, B. D., Marrot, B. & Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 43, 2317–2348. https://doi.org/10.1016/j.watres.2009.03.010 (2009).
Curto, D., Franzitta, V. & Guercio, A. A review of the water desalination technologies. Appl. Sci. 11, 670. https://doi.org/10.3390/app11020670 (2021).
Charcosset, C. A review of membrane processes and renewable energies for desalination. Desalination 245, 214–231. https://doi.org/10.1016/j.desal.2008.06.020 (2009).
Serna, Á. & Tadeo, F. Offshore desalination using wave energy. Adv. Mech. Eng. 5, 539857. https://doi.org/10.1155/2013/539857 (2013).
Serna, Á. & Tadeo, F. Offshore hydrogen production from wave energy. Int. J. Hydrogen Energy 39, 1549–1557. https://doi.org/10.1016/j.ijhydene.2013.04.113 (2014).
Campione, A. et al. Coupling electrodialysis desalination with photovoltaic and wind energy systems for energy storage: Dynamic simulations and control strategy. Energy Convers. Manag. 216, 112940. https://doi.org/10.1016/j.enconman.2020.112940 (2020).
Rönsch, S. & Ortwein, A. Methanation of synthetic gas—Fundamentals and process development. Chem. Ing. Tec. 83, 1200–1208. https://doi.org/10.1002/cite.201100013 (2011).
Held, M., Schollenberger, D., Sauerschell, S., Bajohr, S. & Kolb, T. Power-to-Gas: CO2 methanation concepts for SNG production at the Engler-Bunte-Institut. Chem. Ing. Tec. 92, 595–602 (2020).
Bozzano, G. & Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 56, 71–105 (2016).
Dalena, F. et al. Methanol production and applications: An overview. Methanol. 3–28 (2018).
Olah, G. A., Goeppert, A. & Prakash, G. S. Beyond Oil and Gas: The Methanol Economy (Wiley, 2018).
Bertau, M., Offermanns, H., Plass, L., Schmidt, F. & Wernicke, H.-J. Methanol: The Basic Chemical and Energy Feedstock of the Future (Springer, 2014).
Basile, A. & Dalena, F. Alcohols and Bioalcohols. Characteristics, Production, and Uses (Nova Publishers, 2015).
Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 31, 221–257 (2014).
Ahmad, N. & Baddour, R. E. A review of sources, effects, disposal methods, and regulations of brine into marine environments. Ocean Coast. Manag. 87, 1–7 (2014).
Archer, A., Graves, M., Noack, R. & Phillips, P. Assessing the feasibility of an offshore desalination facility. IDA J. Desalin. Water Reuse 1, 97–108. https://doi.org/10.1179/ida.2009.1.1.97 (2009).
Schirrmeister, S., Morstein, O. von & Föcker, H. Innovative large-scale energy storage technologies and Power-to-Gas concepts after optimisation. D2.3 Demonstration plant Falkenhagen commissioned/commissioning report (2017).
Zhao, M., Jiang, J. H., Zhou, L. H., Jin, P. K. & He, D. The technical study on photo-catalytic oxidation treatment of low concentration methanol wastewater in Gas Field. Adv. Mater. Res. https://doi.org/10.4028/www.scientific.net/amr.671-674.2769 (2013).
Cao, G. et al. Production of a bioflocculant from methanol wastewater and its application in arsenite removal. Chemosphere 141, 274–281 (2015).
Panagopoulos, A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) desalination systems. Chem. Eng. Process. Process Intensification 176, 108944. https://doi.org/10.1016/j.cep.2022.108944 (2022).
Liang, L. & Han, D. Energy-saving study of a system for ammonium sulfate recovery from wastewater with mechanical vapor compression (MVC). Res. J. Appl. Sci. Eng. Technol. 3, 1227–1232 (2011).
Kieselbach, M. et al. Brines from industrial water recycling: New ways to resource recovery. J. Water Reuse Desalin. 10, 443–461 (2020).
Tong, T. & Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environ. Sci. Technol. 50, 6846–6855 (2016).
Dinh, V. N., Leahy, P., McKeogh, E., Murphy, J. & Cummins, V. Development of a viability assessment model for hydrogen production from dedicated offshore wind farms. Int. J. Hydrogen Energy 46, 24620–24631. https://doi.org/10.1016/j.ijhydene.2020.04.232 (2021).
Calado, G. & Castro, R. Hydrogen production from offshore wind parks: Current situation and future perspectives. Appl. Sci. 11, 5561. https://doi.org/10.3390/app11125561 (2021).
Fasihi, M., Bogdanov, D. & Breyer, C. Techno-economic assessment of Power-to-Liquids (PtL) fuels production and global trading based on hybrid PV-wind power plants. Energy Proc. 99, 243–268. https://doi.org/10.1016/j.egypro.2016.10.115 (2016).
Siemens Energy. Silyzer 300 (2020).
Gökçek, M. Hydrogen generation from small-scale wind-powered electrolysis system in different power matching modes. Int. J. Hydrogen Energy 35, 10050–10059. https://doi.org/10.1016/j.ijhydene.2010.07.149 (2010).
Larminie, J., Dicks, A. & McDonald, M. S. Fuel Cell Systems Explained (J. Wiley Chichester, 2003).
Viebahn, P., Scholz, A. & Zelt, O. The potential role of direct air capture in the German energy research program—Results of a multi-dimensional analysis. Energies 12, 3443 (2019).
Mörs, F., Schlautmann, R. E. & Gorre, J. Innovative large-scale energy storage tech-nologies and Power-to-Gas concepts after optimisation.
Sheldon, D. Methanol production-a technical history. Johnson Matthey Technol. Rev. 61, 172–182 (2017).
OSPAR Commission. OSPAR Convention for the protection of the marine environment of the North East Atlantic (1992).
Lattemann, S. & Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 220, 1–15. https://doi.org/10.1016/j.desal.2007.03.009 (2008).
Elsaid, K., Sayed, E. T., Abdelkareem, M. A., Baroutaji, A. & Olabi, A. G. Environmental impact of desalination processes: Mitigation and control strategies. Sci. Total Environ. 740, 140125. https://doi.org/10.1016/j.scitotenv.2020.140125 (2020).
Smolinka, T. et al. Study IndWEDe Industralisation of water electrolysis in Germany: Opportunities and challenges for sustainable hydrogen for transport, electricity and heat (2018).
Keith, D. W., Holmes, G., Angelo, D. S. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Take, T., Tsurutani, K. & Umeda, M. Hydrogen production by methanol–water solution electrolysis. J. Power Sources 164, 9–16 (2007).
Menia, S., Tebibel, H., Lassouane, F., Khellaf, A. & Nouicer, I. Hydrogen production by methanol aqueous electrolysis using photovoltaic energy: Algerian potential. Int. J. Hydrogen Energy 42, 8661–8669 (2017).
Acknowledgements
This research work is financially supported by the German Federal Ministry of Education and Research (03HY302G).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Y.M. and P.S. carried out the writing, investigation, interpretation and analysis. F.T. assisted in the investigation and drafting. F.S. and H.H. contributed to the conceptualization, supervision, critical review of the manuscript and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morales, Y., Samanta, P., Tantish, F. et al. Water management for Power-to-X offshore platforms: an underestimated item. Sci Rep 13, 12286 (2023). https://doi.org/10.1038/s41598-023-38933-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38933-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.