Abstract
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are rare neuroendocrine tumors. PGLs can further be divided into sympathetic (sPGLs) and head-and-neck (HN-PGLs). There are virtually no treatment options, and no cure, for metastatic PCCs and PGLs (PPGLs). Here, we composed a tissue microarray (TMA) consisting of 149 PPGLs, reflecting clinical features, presenting as a useful resource. Mutations in the pseudohypoxic marker HIF-2α correlate to an aggressive tumor phenotype. We show that HIF-2α localized to the cytoplasm in PPGLs. This subcompartmentalized protein expression differed between tumor subtypes, and strongly correlated to proliferation. Half of all sPGLs were metastatic at time of diagnosis. Cytoplasmic HIF-2α was strongly expressed in metastatic sPGLs and predicted poor outcome in this subgroup. We propose that higher cytoplasmic HIF-2α expression could serve as a useful clinical marker to differentiate paragangliomas from pheochromocytomas, and may help predict outcome in sPGL patients.
Similar content being viewed by others
Introduction
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are rare neuroendocrine tumors originating from neural crest-derived schwann cell precursors in the adrenal medulla, sympathetic ganglionic tissue in the abdomen or thorax, or parasympathetic ganglionic tissue mainly in the head- and neck region1,2.
Radical surgery is curative in most patients. Still, while most tumors display a non-metastatic phenotype, around 10% of pheochromocytomas and paragangliomas (PPGLs) develop metastases3. There are no reliable markers to conclusively demonstrate malignant potential of primary tumors. The 2017 WHO classification4 adopted the position that all PPGLs have malignant potential and instead made a distinction between non-metastatic and metastatic tumors. Since limited treatment options exist for patients with metastatic PPGL, better prognostic and predictive markers are needed for tailored treatment and follow-up.
PPGLs have the highest rate of heritability of all human solid tumors with up to 40% carrying germline mutations. The majority of familial PPGL tumors are caused by pathogenic variants in one of the four Succinate dehydrogenase subunit genes (SDHx). Sporadic PPGL harbor somatic driver mutations in some of the same genes as inherited predisposition syndromes (e.g., RET, VHL, NF1, SDHB), as well as in additional genes (e.g., HRAS, EPAS1)5,6,7,8,9.
Overall, there are over 20 susceptibility genes identified in PPGL10, and tumors can be divided into three broad groups; the pseudohypoxic cluster 1 with mutations in VHL, SDHx, EGLN1/PHD2, FH and EPAS1 genes11, the kinase signaling cluster 2 with mutations in RET, NF1, HRAS, MAX, TMEM127, and the wnt pathway cluster 3 with MAML3 fusions and mutations in CSDE17. Mutations in the first group lead to increased stabilization of hypoxia inducible factor (HIF)-2α and a metabolic switch into a persistent pseudohypoxic state (i.e., hypoxic phenotype in oxygenated conditions), while mutations in the kinase signaling group of genes activate the PI3K/AKT/mTOR and RAS/RAF pathways12,13.
Oxygen sensing is one of the most important evolutionary traits of multicellular organisms (reviewed in Hammarlund et al.14). During physiological settings when oxygen is present, HIFs are targeted for degradation. There are however mechanisms by which HIFs escape this, e.g., loss-of-function mutations in the genes encoding VHL or PHD, generating pseudohypoxic phenotypes. While both HIF-1α and HIF-2α respond to oxygen fluctuations, it is primarily HIF-2α that has been implicated in tumor progression. For example, the majority of clear cell renal cell carcinomas are driven by mutations in VHL, leading to complete HIF-2-driven tumor formation15.
Ten years ago, the first mutation in EPAS1 (encoding HIF-2α) in cancer was detected16. Two novel mutations were found in PGL patients, associated with increased protein half-life and HIF-2α activity16. Since then, a number of EPAS1 mutations have been detected in PPGL17,18,19,20,21,22,23. Fliedner et al. further defined EPAS1 mutated tumors as a subcluster of the pseuodohypoxic cluster 1 PGLs, separated from other hereditary pseudohypoxic PGLs driven by VHL or SDHx genes24. Recently, mutations in EPAS1 have been detected also in esophageal squamous cell carcinoma and colorectal cancer25,26.
HIF-2α acts as a nuclear transcription factor. However, emerging evidence show that HIF-2α can localize to the cytoplasm in neuroblastoma27,28 and glioblastoma29. In similarity to PPGL, neuroblastoma arises along the sympathetic ganglia and in adrenal glands, and originates from stem cells of the neural crest. We recently showed that HIF-2α is expressed in the cytoplasm of migrating neural crest cells30, demonstrating the importance of understanding HIF-2α expression and biology in PPGL.
We provide here a comprehensive PPGL TMA with an in-depth characterization of the material. All sympathetic paragangliomas (sPGL) specimens were positive for cytoplasmic HIF-2α and more than half of these patients presented with metastasis at time of diagnosis. Staining of Ki-67 as a proxy for proliferation is an important clinical parameter. We found that cytoplasmic HIF-2α strongly correlated with Ki-67, and high expression of cytoplasmic HIF-2α predicted poor outcome in sPGLs. Our results utilizing this TMA for biological analyses suggest that the cytoplasmic fraction of the HIF-2α protein separates metastatic from non-metastatic sPGLs, confers aggressive features, and predicts outcome. We propose further studies to establish and implement cytoplasmic HIF-2α expression as a clinical marker for poor outcome in sPGL.
Material and methods
Clinical material
A total of 149 patients diagnosed with PPGL at Sahlgrenska University Hospital, Gothenburg, Sweden, between 1984 and 2015 were included in the TMA. The study was approved by the local ethical board of Gothenburg and all methods performed were in accordance with the guidelines and regulations of the local ethical board of Gothenburg (ethical permit no. 833-18). The informed consent has been obtained from each patient or subject after full explanation of the purpose and nature of all procedures used. Patient information concerning age, sex, recurrent or metastatic disease as well as hormone profile of the tumors and causes of death when applicable was retrieved from in-patient charts and a pre-existing clinical database. Clinical pathology reports were searched for any further tumor specific data. Characterization of the material is summarized in Table 1.
Tissue microarray and immunohistochemical staining
PPGL patient material was retrieved from the pathology archives at Sahlgrenska University Hospital, Gothenburg. Tumor cores were formalin-fixed and paraffin-embedded into TMA blocks. From representative tumor areas selected by a board-certified pathologist (O.N.), tissue core biopsies were punched out and mounted in recipient blocks. A total of 175 donor tumor cores with 149 unique primary tumors were included in the TMA. Sufficient core quality was assessed by eosin staining. Sections (4 μm) were stained using AutostainerPlus (Dako). The TMA was stained for chromogranin A (CgA, MAB319, Merck Millipore 1:4000), synaptophysin (SYP, M0776, Agilent, 1:200 + Linker), tyrosine hydroxylase (TH, ab75875, Abcam, 1:300), vimentin (ab45939, Abcam, 1:200), HIF-1α (610959, BD Biosciences, 1:50 + Linker), HIF-2α (A700-003, Bethyl Labs, 1:200 + Linker; AB207607, Abcam, 1.50 + Linker), DEC1/BHLHE40 (nb100-1800, Novus Biologicals, 1:100), SDHB (459230, Invitrogen, 1:250), and Ki-67 (IR62661, Agilent, Ready-to-use + Linker). The slides were digitalized with Axio Scan Z.1 at 20 × magnification.
TMA staining of HIF-2α
Formalin-fixed paraffin-embedded PPGL patient TMA tissue cores were sectioned at 4 μm and prepared for further analysis. TMA slides were deparaffinised and rehydrated by incubation three times in Xylene, twice in 100% ethanol, twice in 96% ethanol, and twice in 70% ethanol for three minutes in each solution respectively, and rinsed with ddH2O. Slides were then incubated in pH 9 (TRIS–EDTA based) antigen retrieval buffer at 97 °C for 20 min in an Agilent PT-Link instrument. After the slides had cooled down for 30 min, slides were stained for HIF-2α using Agilent EnVision FLEX, High pH (Link) kit as well as Dako Cytomotion Autostainer. During the procedure, slides were incubated with EnVision FLEX Peroxidase-Blocking Reagent for 5 min to block endogenous peroxidases. Slides were incubated with HIF-2α antibody (A700-003, Bethyl Labs) diluted 1:200 in EnVision FLEX Antibody Diluent for 30 min at room temperature. Slides were then incubated with EnVision FLEX + Rabbit (LINKER) for 15 min for signal amplification, and treated with EnVision FLEX/HRP visualization reagent for 20 min. This was followed by incubation with EnVision FLEX DAB + Chromogen diluted in EnVision FLEX Substrate Buffer. In the last step, counterstaining was performed by incubating the slides in EnVision FLEX Hematoxylin for three minutes. Slides were finally mounted using organic solvent-based mounting medium. Between all steps, slides were washed with EnVision FLEX Wash Buffer. Antibody specificity was confirmed by staining hypoxic (1% oxygen) and normoxic (21% oxygen) SK-N-BE(2)c neuroblastoma, and VHL negative renal cell carcinoma 786-O and RCC-4 cells (Supplementary Fig. S1). To confirm cytoplasmic HIF-2α expression in PPGL, we also conducted immunofluorescent staining with PPGL cell line, hPheo1, using two different antibodies against HIF-2α (Supplementary Fig. S2).
TMA scoring
Immunohistochemical (IHC) staining was viewed and examined blindly by two independent persons using the PathXL software. A second independent assessment was conducted by an independent person using QuPath-0.3.0 software31. The software’s TMA dearrayer was applied to each slide to detect individual tumor cores and manually corrected when necessary. For CgA, SYP, TH, VIM and HIF-2α-C a scoring intensity of negative (0), weak (1 +), median (2 +) and strong (3 +) tumor cell specific expression was manually determined (representative images in Supplementary Fig. S3). Additionally, H-Score between 0 and 300 was calculated for HIF-2α-C staining for each tumor core by using QuPath-0.3.0 software. For this, cell detection command was used to identify each cell based on nuclear hematoxylin staining in every tumor core, then, intensity thresholds were set to distinguish cells with negative, weak, intermediate, and strong cytoplasmic HIF-2α based on DAB staining. The cores having H-Score between 0 and 15 scored as negative (0), 16–99 scored as weak (1 +), 100–199 scored as intermediate (2 +) and 200–300 scored as strong (3 +). Four EPAS1 mutated tumors (1 PCC, 3 sPGL, all non-metastatic) from the CNIO cohort32 were stained for HIF-2α using the same protocol as above. Scoring was performed by an independent person using the QuPath-0.3.0 software as described above. For HIF-1α, DEC1/BHLHE40 and nuclear HIF-2α staining, tumor cell specific expression scoring was determined semi-automatically by using QuPath-0.3.0 software. Each TMA core and cells were detected as above and then tumor cells with nuclear DAB staining were manually counted. The tumor cores with positive cancer cell percentages between 0 and 1% scored as negative (0), 1–5% scored as weak (1 +), 5–10% scored as intermediate (2 +) and > 10% scored as strong (3 +). For the Ki-67 staining, the number of positive and negative cells in the hotspot regions detected by a pathologist was manually counted and percentage of positive cells were calculated for each tumor core. The tumor cores with positive cancer cell percentages between 0% scored as negative (0), 0–1% scored as weak (1 +), 1–3% scored as intermediate (2 +) and > 3% scored as strong (3 +) (Kimura et al. 2018). Cells of necrosis, healthy tissue, scanning and staining artifacts and folded areas were manually removed from the calculation. Coherence between manual and semi-automated scoring by QuPath-0.3.0 software was analyzed for a fraction of markers by Kappa score. We could confirm a good agreement between the two ways of scoring (Supplementary Table S1).
Genetics
Germline mutational data of SDHA, SDHB, SDHC, SDHD, RET, VHL, and NF1 were collected from the two clinical genetics laboratories and oncogenetic clinics at the University hospitals in Gothenburg and Lund (Supplementary Table S2). Genetic screening of the proband/affected family member was performed using Sanger DNA sequencing or massive parallel sequencing panels, and for patients diagnosed from 2014 and onwards the analyses were combined with multiplex ligation-dependent probe amplification (MLPA, P081-D1 & P082-C2 (NF1), P226-D1 (SDHx), and P016-C2 (VHL), MRC-Holland, Amsterdam, The Netherlands) for the detection of large deletions or duplications. Whole exome sequencing (WES) data from 23 PPGL cases was extracted from previous published papers10,33. WES was run as either paired tumor tissue and normal tissue/blood (10 cases) or as single tumor tissue (13 cases), and identified additional germline and somatic variants in PPGL susceptibility genes (i.e., NF1, VHL, RET, EPAS1 and HRAS). Tumor samples with no apparent causative point mutation were further analyzed for exon/gene deletions or duplications by MLPA as described above. Only variants predicted to be pathogenic or likely pathogenic by the American College of Medical Genetics and Genomics34 were included in the final mutation list (Table 1)34. All tumor cores were stained for SDHB to determine SDHx loss-of-function for patients with unknown mutational status. Samples with negative SDHB staining were included in the list of patients with known mutations, designated as SDHx mutated (Table 1 and Supplementary Table S2).
mRNA expression analyses
For 23 PPGL samples analyzed in the current study, mRNA expression data from fresh tumor tissue run by 44 K Agilent Cy3/Cy5 2-color microarrays (Agilent) were available. Tumor samples were divided into expression groups based on previous unsupervised hierarchical clustering using a 153 discriminative gene set33. Normalized and log transformed expression data from different PPGL data sets (TCGA, (E-MTAB-733)35, (GSE67066)36) were downloaded from ‘R2: Genomics Analysis and Visualization Platform’ (http://r2.amc.nl).
Statistics
GraphPad Prism 9 software was used for correlation, statistical and survival analyses. Mann–Whitney test was used to determine statistical significance. Fisher’s exact test was used to determine the statistical difference in nuclear and cytoplasmic HIF-2α expression between patients diagnosed with sPGL, HN-PGL and PCC. p-value was employed as; p < 0.05 (*), p < 0.01(**), p < 0.001(***) and p < 0.0001(****) if not otherwise specified. Spearman’s correlation test was used to calculate the correlation coefficient and statistical significance (two-tailed P value for each correlation coefficient). Kaplan–Meier survival analysis and the Log-rank (Mantel–Cox) test were used to prepare and compare survival curves, respectively.
Results
Characterization of patient material
The TMA was constructed from donor blocks of 175 fixed primary, recurrent and metastatic tumor tissue from 149 patients diagnosed and operated for PPGL at Sahlgrenska University Hospital, Gothenburg, Sweden, between 1983 and 2015. After removal of recurrent, metastatic, and duplicate cores, 149 primary tumor cores obtained from individual patients were used in the analyses. The patient material (Table 1) is coherent with previously published population-based data37. The majority of patients were diagnosed with PCCs (n = 123), while the rest were diagnosed with PGLs (n = 26; 13 sPGLs and 13 head- and neck (HN)-PGLs). At time of diagnosis, 93% (n = 138) of the tumors were considered non-metastatic while 7% (n = 11) were diagnosed as metastatic. A substantially higher proportion of sPGLs were considered metastatic as compared to PCCs (46% vs. 4% respectively). None of the thirteen HN-PGLs were metastatic at time of diagnosis, in line with an overall low malignancy rate among these tumors38. In total, 47 patients (46%) were found to have somatic, or germline mutations known to be associated with PPGL (Table 1), in coherence with previous data39,40.
sPGLs are more proliferative than PCCs
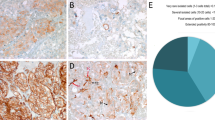
We stained our TMA for diagnostic PPGL markers Chromogranin A (CgA), tyrosine hydroxylase (TH), vimentin (VIM), and synaptophysin (SYP) (Supplementary Fig. S3). All proteins were expressed as expected (Supplementary Fig. S3 and Supplementary Table S3). None of these markers differentiated non-metastatic vs. metastatic PPGLs (Supplementary Table S4). Generally, PPGLs are slow growing tumors with low proliferation rates, and Ki-67 positive tumor cells typically reside in hot spot regions. We stained and analyzed such hot spot regions from each TMA donor. The fraction of Ki-67 positive cells was significantly higher in sPGL as compared to PCCs (Fig. 1A, B; Table 2).
PPGLs express low levels of Ki-67 and HIF-1α. (A) Representative images from tumor cores scored as negative (0), weak (1 +), intermediate (2 +) or strong (3 +) for Ki67. (B) Quantification of Ki67 positive cells (in %). (C) Representative images from tumor cores scored as negative (0), weak (1 +), intermediate (2 +) or strong (3 +) for HIF-1α. (D) Quantification of HIF-1α positive cells (in %). Tumor cores were scored manually by three independent researchers as well as semi-automatically using the QuPath-0.3.0 software. Statistical significance was determined by the Mann–Whitney test. sPGL, sympathetic paraganglioma; HN-PGL, head- and neck paraganglioma; PCC, pheochromocytoma. The scale bar represents 100 μm in core images and 50 μm in zoomed images.
Since a large fraction of PPGLs presents with mutations affecting oxygen sensing, we stained our TMA for HIF-1α and HIF-2α. While 38% of HN-PGLs were positive, only 9% of PCCs and 0% of sPGLs expressed HIF-1α (Fig. 1C,D; Table 2). There was no difference in HIF-1α staining between non-metastatic and metastatic tumors (Supplementary Table S4).
HIF-2α expression is mainly cytoplasmic in all paragangliomas
HIF-2α positive tumor cores showed a high degree of cytoplasmic staining (Fig. 2A; Table 2), in concordance with findings in glioblastoma29, neuroblastoma28, and neural crest development30. We therefore decided to divide our analyses into nuclear and cytoplasmic HIF-2α. Evaluation of staining intensity of HIF-2α revealed that the majority of tumors scored negative for nuclear HIF-2α, while highly intense cytoplasmic HIF-2α staining was observed in 69% of sPGLs, 92% of HN-PGLs and 33% of PCCs (Fig. 2B–E; Table 2). To address HIF-2 activity in these tumors, we stained for HIF-2 specific target gene DEC1/BHLBE40, and found that the majority of tumors from all subtypes expressed this protein (Supplementary Table S3).
PPGLs express high levels of cytoplasmic HIF-2α and cytoplasmic HIF-2α is highly expressed in metastatic sPGLs. (A) Representative images from tumor cores scored as negative (0), weak (1 +), intermediate (2 +) or strong (3 +) for HIF-2α. (B) Quantification of positive cells for nuclear HIF-2α (HIF-2α-N) (in %). (C) H-score for cytoplasmic HIF-2α (HIF-2α-C). (D) Quantification of fraction (in %) of positive tumor cores for nuclear and cytoplasmic HIF-2α. (E) Quantification of fraction (in %) of tumor cores staining with high intensity (scored 2–3) for nuclear and cytoplasmic HIF-2α. (F)–(G) Expression of nuclear (HIF-2α-N) (F) and cytoplasmic (HIF-2α-C) (G) HIF-2α as divided by low (0–1) and high (2–3) staining intensity in sPGL, HN-PGL and PCC tumor subgroups. Tumor cores were scored manually by three independent researchers as well as semi-automatically using the QuPath-0.3.0 software. Statistical significance was determined by the Mann–Whitney test in B-C and Fisher’s exact test in D-G. sPGL, sympathetic paraganglioma; HN-PGL, head- and neck paraganglioma; PCC, pheochromocytoma. The scale bar represents 100 μm in core images and 50 μm in zoomed images. Note that there are no metastatic HN-PGLs in this cohort.
Metastatic sPGLs express high levels of cytoplasmic HIF-2α
We analyzed cytoplasmic HIF-2α in non-metastatic and metastatic PPGL patients based on their mutational status and observed that expression was not affected (Supplementary Table S4). When analyzing the sPGL cohort for cytoplasmic HIF-2α in non-metastatic and metastatic cases in depth, we observed that virtually all tumors from patients with metastasis expressed HIF-2α with high intensity (Supplementary Table S5). This was true for patients with SDHx mutations as well as without any known mutations (Supplementary Table S4), suggesting that staining for cytoplasmic HIF-2α may be useful to detect aggressive tumors that would otherwise have been missed using SDH screening immunohistochemistry.
Expression of nuclear HIF-2α was unchanged in non-metastatic vs. metastatic tumors in all three PPGL entities (Fig. 2F). In coherence with cytoplasmic HIF-2α staining at low intensity in PCCs overall, there was no difference in expression in non-metastatic as compared to metastatic tumors (Fig. 2G; Supplementary Table S5). Notably, while 57% of non-metastatic sPGLs strongly expressed cytoplasmic HIF-2α, this number increased to 83% in metastatic tumors (Fig. 2G; Supplementary Table S5).
Cytoplasmic HIF-2α correlates to proliferation proxy Ki-67
To determine the putative clinical impact of cytoplasmic HIF-2α, we investigated possible relationships with PPGL diagnostic markers. When investigating PPGLs as one entity, we found that cytoplasmic HIF-2α positively correlated with SYP, DEC1/BHLHE40 and Ki-67, while there was an inverse correlation with CgA expression (Fig. 3A,B). Correlation between HIF-2α and Ki-67 was strongest in sPGLs (Fig. 3C).
Cytoplasmic HIF-2α correlates to proliferation in sPGLs and EPAS1 mRNA expression is higher in sPGL as compared to PCC. (A) Compilation of correlations between all markers stained for in this cohort. (B) Compilation of correlations only between cytoplasmic HIF-2α and the other markers for visualization. (C) Correlations between cytoplasmic HIF-2α and Ki-67 as divided into the different subgroups of PPGL. (D) HIF1A and EPAS1 mRNA expression in a subset of tumors from our TMA cohort, analyzed by whole exome sequencing. (E) Expression of EPAS1 mRNA in the pseudohypoxic and kinase-signaling groups from (D). (F) Quantification of fraction of TMA cores scored as low or high for cytoplasmic HIF-2α in the pseudohypoxic and kinase-signaling groups. (G)–(I). Expression of EPAS1 mRNA as determined by RNA sequencing in sPGLs and PCCs in three different cohorts; TMA (n = 21, G), TCGA (n = 177, H) and Korpershoek (n = 49, I). (J) Expression of EPAS1 mRNA as determined by RNA sequencing in pseudohypoxic as compared to all other subgroups in the Favier cohort (n = 188). Correlation coefficient was calculated using Spearman’s correlation test in GraphPad Prism 9 and statistical significance (two-tailed P value for each correlation coefficient) was computed automatically by the software. Statistical significance was determined using Mann–Whitney test in E, G-J and Fisher’s exact test in F. PCC, pheochromocytoma; sPGL, sympathetic paraganglioma.
EPAS1 expression is higher in sPGLs
We analyzed expression of HIF-2α in five tumors with EPAS1 mutations from two different cohorts (one tumor from TMA; four tumors from the CNIO study cohort32). All of these tumors (1 PCC and 4 sPGLs) expressed cytoplasmic HIF-2α (Supplementary Fig. S4 and Supplementary Table S6).
We have previously performed whole exome sequencing and expression microarray on a subset of PPGLs included in our TMA33. Here we utilized this material to analyze HIF1A and EPAS1 expression in relation to mutation status and clinical features. Expression of HIF1A mRNA was slightly higher in the pseudohypoxic cluster, but half of the tumors in this group still displayed low levels (Fig. 3D). We could not detect any correlations between HIF1A mRNA and mutation status, metastasis, or diagnosis (Fig. 3D). Analysis of EPAS1 expression showed substantially higher expression levels in tumors belonging to the pseudohypoxic cluster (Fig. 3D–E). We did not find a correlation between mRNA and cytoplasmic HIF-2α expression as most tumors in the kinase signaling cluster express the protein, but low mRNA levels (Fig. 3A,F).
We further investigated mRNA expression in correlation to tumor subtype and found that EPAS1 levels were higher in sPGLs as compared to PCCs (Fig. 3G), in line with protein data (Fig. 2). We confirmed these findings in two independent cohorts (TCGA, n = 177 and Korpershoek, n = 49; Fig. 3H–I). While we could not assess EPAS1 expression between PPGL subtypes in the Favier data set (n = 188) due to lack of such data, we detected higher mRNA levels in pseudohypoxic tumors as compared to all other groups (Fig. 3J).
High expression cytoplasmic HIF-2α predicts worse survival in sPGL patients
To investigate the survival status in our cohort, we used clinical follow-up data where 25 patients had died at an endpoint of 10 years (Supplementary Table S7). Dividing the cohort according to diagnosis, 15% of PCC, and as many as 46% of sPGL patients were deceased. Out of these 25 patients in total, 9 had died from cancer (5 from metastatic PPGL and 4 from other cancers), while the remaining 16 patients died of other causes (heart-related, infections, multi-organ failure, stroke, or other or unknown reasons). The survival probability did not differ between PCC and PGL patients when taking all causes of death into consideration (Fig. 4A). However, when further dividing into PGL subtypes, sPGLs displayed a significantly worse prognosis than PCCs and HN-PGLs (Fig. 4B). Since it is difficult to determine whether the cause of death from other reasons than metastatic PPGLs were due to their primary tumor, we stratified survival probability only in patients that died from metastatic disease. There was no pronounced difference when comparing PCC and PGL (Fig. 4C). Although few patients in this cohort died from their disease, when further stratifying PGL patients, there is a separation where sPGL patients have worse prognosis (survival rate only 68% for sPGL, while 100% for HN-PGL and 98% for PCC) (Fig. 4D).
Intense staining of cytoplasmic HIF-2α predicts poor outcome in sPGLs. (A) Survival probability (10-year follow-up) of PPGL patients when including all causes of death. (B) Survival probability (10-year follow-up) of patients when only including patients dead from PPGL disease comparing PCC and PGL subgroups. (C)–(D) Survival probability (10-year follow-up) of PPGL patients divided into PGL and PCC subgroups when including all causes of death (C) and only PPGL caused deaths (D). (E)–(F) Survival probability stratified by low (0–1) and high (2–3) staining intensity of cytoplasmic HIF-2α in the entire PPGL cohort (E) and PCC (F). (G)–(H) Survival probability (10-year follow-up) of patients when only including patients dead from PPGL disease in PGL (G) and sPGL (H) comparing low (0–1) and high (2–3) staining intensity of cytoplasmic HIF-2α. Statistical significance was calculated using the Log-rank (Mantel–Cox) test. PCC pheochromocytoma, sPGL sympathetic paraganglioma, HN-PGL head- and neck paraganglioma, PGL paraganglioma, PPGL pheochromocytoma and paraganglioma.
While there was no correlation between cytoplasmic HIF-2α and outcome in PPGL or PCC entities (Fig. 4E,F), high intensity of cytoplasmic HIF-2α predicts poor outcome in PGL patients (Fig. 4G), with a more pronounced effect in sPGL patients specifically (Fig. 4H).
Discussion
There is a lack of accessible material for biological analyses of PPGL. We present a TMA of cores from 149 individual patients with PCCs, HN-PGLs and sPGLs, reflecting patient distribution of all clinical parameters. We demonstrate that this material is valuable by extensive characterization of protein markers used in diagnostics in connection to primary tumor location, non-metastatic vs. metastatic disease, mutation status, biochemical phenotype, and importantly 10-year follow-up data. In addition, we add RNA expression data from a selection of these patients.
Half of sPGL patients in our cohort present with metastatic disease, and in coherence, patients from this tumor entity have a worse prognosis and lower survival rate as compared to PCC and HN-PGL. This strengthens the benefit of the WHO classification of these three subgroups for diagnostics, when assessing treatment regimens and patient follow-up schemes. It has recently been further demonstrated that metastatic HN-PGLs have a longer disease-specific survival than metastatic PPGLs, and that extra-adrenal PPGLs (i.e., PGL as compared to PCC) have a worse prognosis41, in line with our data.
PPGLs within the pseudohypoxic group present with a more aggressive behavior and worse prognosis. Mutations in the EPAS1 gene confer such a pseudohypoxic profile and represent a subgroup of its own within the pseudohypoxic group of tumors24. We have, in previous studies on neural crest cells during embryogenesis as well as in neuroblastoma, observed that the HIF-2α protein non-canonically localizes to the cytoplasm28,30. There was a clear distinction between the percentage of tumors positive for cytoplasmic vs. nuclear protein in PPGLs, and considering their proposed shared ancestor cells, the neural crest, with neuroblastoma (shown in e.g., composite tumors42), these findings are of importance to further understand PPGL initiation and subtype distinction.
The relatively high expression of HIF-1α and nuclear and cytoplasmic HIF-2α in HN-PGLs is explained by the fact that these tumors are located in the oxygen sensing carotid body. In contrast, sPGLs express no or very low levels of HIF-1α and nuclear HIF-2α, while all of them express substantial levels of cytoplasmic HIF-2α. This suggests that the presence of cytoplasmic HIF-2α in sPGLs is not due to a hypoxic environment in these tumors, but rather present with a bona fide pseudohypoxic phenotype mediated via non-canonical mechanisms for regulation of HIF-2α and downstream activity.
PPGLs are to a large extent slow growing, and the assessment of Ki-67 expression as a proxy for proliferation in clinical assessment of these tumors is of significant value for prognostics. Sympathetic PGLs display substantially higher expression of Ki-67 and in coherence with this more proliferative phenotype predicts poor outcome for patients that die from their primary tumor. Cytoplasmic HIF-2α correlates positively to Ki-67, and is highly and intensely expressed in the majority of metastatic sPGLs cases. While all sPGL patients that present with low tumor intensity of cytoplasmic HIF-2α survive, the fraction of patients with high intensity-expressing tumors presents with a survival probability of 68%. It is however important to note that the number of patients that die from their primary or metastatic tumor is low, and additional studies with other cohorts should be performed.
The mechanism-of-action for cytoplasmic HIF-2α is yet unknown, and ongoing functional studies aim to assess its direct role in PPGL subtype initiation and progression. Our data show that EPAS1 mRNA expression is substantially increased in the pseudohypoxic- as compared to the kinase PPGL subgroup. However, both groups express high cytoplasmic HIF-2α, suggesting that HIF-2α is regulated at a (post-)translational-rather than transcriptional level.
To assess the role of HIF-2α in PPGL progression, Bechmann and colleagues knocked down or overexpressed HIF-2α in cell line models, and showed that HIF-2α induced a pro-metastatic phenotype43. The HIF-2α-driven effects on the PPGL cells were due to protein function per se and not hypoxia-induced mechanisms43. These data on overall HIF-2α protein expression in PPGL as one tumor entity, in combination with our results on the dependence of cytoplasmic HIF-2α in the different tumor subtypes, suggest an important tumor-driving role of HIF-2α in these tumors. sPGLs and neuroblastomas arise along the sympathetic chain ganglia and share a common ancestor cell in the trunk neural crest. It is therefore of particular interest that the HIF-2α protein is expressed in oxygenated cells, is mainly cytoplasmic, and that this fraction of cells predicts poor outcome in both of these tumor types. In addition, the high expression of cytoplasmic HIF-2α in metastatic sPGLs is coherent with the correlation between intensely HIF-2α positive tumor cells and distal metastasis in neuroblastoma. Since sPGL patients present with significantly worse survival than PCC and HN-PGL patients, routinely assessing HIF-2α expression in sPGL could represent a new diagnostic biomarker and aid in treatment decisions and patient follow-up.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Furlan, A. et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 357, 6346. https://doi.org/10.1126/science.aal3753 (2017).
Kameneva, P. et al. Single-cell transcriptomics of human embryos identifies multiple sympathoblast lineages with potential implications for neuroblastoma origin. Nat. Genet. 53(5), 694–706. https://doi.org/10.1038/s41588-021-00818-x (2021).
Ayala-Ramirez M. et al. Clinical Risk Factors for Malignancy and Overall Survival in Patients with Pheochromocytomas and Sympathetic Paragangliomas: Primary Tumor Size and Primary Tumor Location as Prognostic Indicators. J. Clin. Endocrinol. Metab. 96(3), 717–725. https://doi.org/10.1210/jc.2010-1946 (2011).
Lam, A. K. Update on adrenal tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 28(3), 213–227. https://doi.org/10.1007/s12022-017-9484-5 (2017).
Dahia, P. L. M. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat. Rev. Cancer 14(2), 108–119. https://doi.org/10.1038/nrc3648 (2014).
Fishbein, L. et al. Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat. Commun. 6(1), 6140. https://doi.org/10.1038/ncomms7140 (2015).
Fishbein, L. et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 31(2), 181–193. https://doi.org/10.1016/j.ccell.2017.01.001 (2017).
Dwight, T. et al. Functional significance of germline EPAS1 variants. Endocr. Relat. Cancer 28(2), 97–109. https://doi.org/10.1530/ERC-20-0280 (2021).
Wachtel, H. & Fishbein, L. Genetics of pheochromocytoma and paraganglioma. Curr. Opin. Endocrinol. Diabetes Obes. 28(3), 283–290. https://doi.org/10.1097/MED.0000000000000634 (2021).
Wilzén, A. et al. Malignant pheochromocytomas/paragangliomas harbor mutations in transport and cell adhesion genes: Sequencing of pheochromocytoma/paraganglioma. Int. J. Cancer 138(9), 2201–2211. https://doi.org/10.1002/ijc.29957 (2016).
López-Jiménez, E. et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol. Endocrinol. 24(12), 2382–2391. https://doi.org/10.1210/me.2010-0256 (2010).
Dahia, P. L. M. et al. A HIF1α Regulatory Loop Links Hypoxia and Mitochondrial Signals in Pheochromocytomas. PLoS Genet. 1(1), e8. https://doi.org/10.1371/journal.pgen.0010008 (2005).
Burnichon, N. et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 20(20), 3974–3985. https://doi.org/10.1093/hmg/ddr324 (2011).
Hammarlund, U. E., Flashman, E., Mohlin, S. & Licausi, F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science 370(6515), eaba3512. https://doi.org/10.1126/science.aba3512 (2020).
Choueiri, T. K. & Kaelin, W. G. Targeting the HIF2–VEGF axis in renal cell carcinoma. Nat. Med. 26(10), 1519–1530. https://doi.org/10.1038/s41591-020-1093-z (2020).
Zhuang, Z. et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N. Engl. J. Med. 367(10), 922–930. https://doi.org/10.1056/NEJMoa1205119 (2012).
Favier, J., Buffet, A. & Gimenez-Roqueplo, A. HIF2A mutations in Paraganglioma with polycythemia. N Engl J Med. 367(22), 2161–2162. https://doi.org/10.1056/NEJMc1211953 (2012).
Comino-Méndez, I. et al. Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum. Mol. Genet. 22(11), 2169–2176. https://doi.org/10.1093/hmg/ddt069 (2013).
Lorenzo, F. R. et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J. Mol. Med. 91(4), 507–512. https://doi.org/10.1007/s00109-012-0967-z (2013).
Pacak, K. et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J. Clin. Oncol. 31(13), 1690–1698. https://doi.org/10.1200/JCO.2012.47.1912 (2013).
Toledo, R. A. et al. In vivo and in vitro oncogenic effects of HIF2A mutations in pheochromocytomas and paragangliomas. Endocr. Relat. Cancer 20(3), 349–359. https://doi.org/10.1530/ERC-13-0101 (2013).
Welander, J. et al. Frequent EPAS1/HIF2α exons 9 and 12 mutations in non-familial pheochromocytoma. Endocr. Relat. Cancer 21(3), 495–504. https://doi.org/10.1530/ERC-13-0384 (2014).
Islam, F., Pillai, S., Gopalan, V. & Lam, A.K.-Y. Identification of novel mutations and expressions of EPAS1 in phaeochromocytomas and paragangliomas. Genes 11(11), 1254. https://doi.org/10.3390/genes11111254 (2020).
Fliedner, S. M. J. et al. Hypoxia-inducible factor 2α mutation-related paragangliomas classify as discrete pseudohypoxic subcluster. Neoplasia 18(9), 567–576. https://doi.org/10.1016/j.neo.2016.07.008 (2016).
Islam, F., Gopalan, V., Law, S., Lam, A. K. & Pillai, S. Molecular deregulation of EPAS1 in the pathogenesis of esophageal squamous cell carcinoma. Front. Oncol. 10, 1534. https://doi.org/10.3389/fonc.2020.01534 (2020).
Islam, F., Gopalan, V., Lu, C. T., Pillai, S. & Lam, A. K. Identification of novel mutations and functional impacts of EPAS1 in colorectal cancer. Cancer Med. 10(16), 5557–5573. https://doi.org/10.1002/cam4.4116 (2021).
Holmquist-Mengelbier, L. et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell 10(5), 413–423. https://doi.org/10.1016/j.ccr.2006.08.026 (2006).
Persson, C. U. et al. ARNT-dependent HIF-2 transcriptional activity is not sufficient to regulate downstream target genes in neuroblastoma. Exp. Cell Res. 388(2), 111845. https://doi.org/10.1016/j.yexcr.2020.111845 (2020).
Li, Z. et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15(6), 501–513. https://doi.org/10.1016/j.ccr.2009.03.018 (2009).
Niklasson, C. U. et al. Hypoxia inducible factor-2α importance for migration, proliferation, and self-renewal of trunk neural crest cells. Dev. Dyn. 250(2), 191–236. https://doi.org/10.1002/dvdy.253 (2021).
Bankhead, P. et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 7(1), 16878. https://doi.org/10.1038/s41598-017-17204-5 (2017).
Monteagudo, M. et al. Analysis of telomere maintenance related genes reveals NOP10 as a new metastatic-risk marker in pheochromocytoma/paraganglioma. Cancers 13(19), 4758. https://doi.org/10.3390/cancers13194758 (2021).
Tomić, T. T. et al. MYO5B mutations in pheochromocytoma/paraganglioma promote cancer progression. PLOS Genet. 16(6), 1008803. https://doi.org/10.1371/journal.pgen.1008803 (2020).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17(5), 405–424. https://doi.org/10.1038/gim.2015.30 (2015).
Loriot, C. et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. J. Clin. Endocrinol. Metab. 97(6), 954–962. https://doi.org/10.1210/jc.2011-3437 (2012).
Evenepoel, L. et al. Expression of contactin 4 is associated with malignant behavior in pheochromocytomas and paragangliomas. J. Clin. Endocrinol. Metab. 103(1), 46–55. https://doi.org/10.1210/jc.2017-01314 (2018).
Muth, A. et al. Prevalence of germline mutations in patients with pheochromocytoma or abdominal paraganglioma and sporadic presentation: A population-based study in Western Sweden. World J. Surg. 36(6), 1389–1394. https://doi.org/10.1007/s00268-012-1430-6 (2012).
Rana, M. U. et al. Head and neck paragangliomas in Norway importance of genetics updated diagnostic workup and treatment. Acta Oto-Laryngologica 141(3) 303–308. https://doi.org/10.1080/00016489.2020.1845397 (2021).
Maher, E. R. The pressure rises: Update on the genetics of phaeochromocytoma. Hum. Mol. Genet. 11(20), 2347–2354. https://doi.org/10.1093/hmg/11.20.2347 (2002).
Pasini, B. & Stratakis, C. A. SDH mutations in tumorigenesis and inherited endocrine tumours: Lesson from the phaeochromocytoma-paraganglioma syndromes. J. Intern. Med. 266(1), 19–42. https://doi.org/10.1111/j.1365-2796.2009.02111.x (2009).
Pamporaki, C. et al. Determinants of disease-specific survival in patients with and without metastatic pheochromocytoma and paraganglioma. Eur. J. Cancer 169, 32–41. https://doi.org/10.1016/j.ejca.2022.03.032 (2022).
Tasaka, K. et al. Oncogenic FGFR1 mutation and amplification in common cellular origin in a composite tumor with neuroblastoma and pheochromocytoma. Cancer Sci. 113(4), 1535–1541. https://doi.org/10.1111/cas.15260 (2022).
Bechmann, N. et al. HIF2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr. Relat. Cancer 27(11), 625–640. https://doi.org/10.1530/ERC-20-0205 (2020).
Acknowledgements
The authors would like to thank Siv Beckman, Christina Möller, Gülay Altiparmak, and Yvonne Arvidsson for excellent technical assistance and Dr. Hans K. Ghayee for kindly providing hPheo1 cells.
Funding
Open access funding provided by Lund University. This work was supported by The Mrs Berta von Kamprad’s Foundation; the Swedish Cancer Society; the Swedish Childhood Cancer Fund; the Lions Cancer Fund West; and institutional research funds at the Department of Surgery, Sahlgrenska University Hospital.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.K., L.G., E.E., B.W., F.A., S.P., A.M., S.M.; Data curation: S.K., L.G., F.A.; Formal analysis: S.K., L.G.; Investigation: S.K., L.G., F.A., O.N., S.P.; Methodology: S.K., L.G., F.A., S.P., A.M., S.M.; Resources: M.R., O.N., B.W., F.A., A.M.; Validation: S.K., L.G., F.A., S.P.; Visualization: S.K., F.A.; Writing—original draft: S.M.; Writing—review & editing: S.K., L.G., E.E., P.M.S., M.R., O.N., B.W., F.A., S.P., A.M., S.M.; Supervision: A.M., S.M.; Project administration: A.M., S.M.; Funding acquisition: B.W., A.M., S.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karakaya, S., Gunnesson, L., Elias, E. et al. Cytoplasmic HIF-2α as tissue biomarker to identify metastatic sympathetic paraganglioma. Sci Rep 13, 11588 (2023). https://doi.org/10.1038/s41598-023-38606-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38606-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.