Abstract
We evaluated the association between gestational age at threatened preterm birth (TPTB) diagnosis and preterm birth (PTB) incidence using a nationwide birth cohort. Data of 94,236 women with singleton deliveries from the Japan Environment and Children’s Study (enrolled between 2011 and 2014) were analysed. Participants were divided based on parity and gestational age at TPTB diagnosis (22–24, 25–27, 28–30, 31–33, and 34–36 weeks). Multivariable logistic regression models were used to calculate the odds ratios (ORs) for PTB before 37 and 34 weeks in women from all groups, using participants without TPTB as the reference. The adjusted ORs for PTB before 37 weeks were the highest in the latest gestational age group in nulliparous and multiparous women without previous PTB, while those before 34 weeks were the highest in the earliest and latest gestational age group in multiparous women without previous PTB and in the earliest gestational age group in multiparous women with previous PTB. The association between gestational age at TPTB diagnosis and PTB incidence varies based on maternal parity and PTB before 37 or 34 weeks. Further studies with detailed clinical data and a unified TPTB diagnosis protocol are necessary to clarify this association.
Similar content being viewed by others
Introduction
Preterm births (PTBs) account for 75% of perinatal mortality cases and more than half of long-term morbidity cases1,2,3. The rate of PTB is increasing globally4. In Japan, the PTB rate had increased annually, from 4.1% in 1980 to 5.3% in 20005, but since then it has remained stable. There has been no unified protocol for preventing PTB, and the research for strategies to reduce PTB is ongoing. Identifying women at risk remains a challenge6. Preventing PTB by addressing causal factors is paramount for improving neonatal outcomes. A detailed evaluation of women at risk of PTB is required for tailored treatment2. Thus, clarification of the association of maternal factors with PTB is required to reduce the incidence of PTB and improve the outcomes of infants.
Threatened preterm births (TPTBs) are a major risk factor for PTB, although 50% of women hospitalised for TPTB give birth at term7. TPTB is defined according to the clinical criteria of regular uterine contractions accompanied by a change in cervical dilatation, effacement, or both, or initial presentation with regular uterine contractions and cervical dilatation of at least 2 cm7. Multiple testing in women with TPTB has been proposed to detect women at risk of PTB, including measurement of cervical length (CL) and foetal fibronectin testing6,8. However, the clinical usefulness of these tests is primarily the ability to identify women who are least likely to deliver6. Moreover, TPTB has a wide range of characteristics, and the type of TPTB that presents the highest risks for PTB remains unknown7.
One characteristic of TPTB is gestational age at diagnosis, which is easy to identify. However, the clinical significance of this marker with stratified gestational ages has not been clarified. Moreover, the characteristics of mothers stratified by gestational ages at TPTB diagnosis remain unclear. We aimed to determine whether gestational age at TPTB diagnosis could predict the risk of PTB. A previous study reported that the risk of another PTB may be inversely correlated with the gestational age at the previous PTB1. Another previous study reported that early gestational age at TPTB diagnosis may be a risk factor for PTB9. Thus, we hypothesised that an earlier age at TPTB diagnosis is correlated with a higher risk of PTB based on these previous studies1,9.
We analysed the association between participants stratified by gestational age at diagnosis of TPTBs and the incidence of PTB before 37 and 34 weeks of gestation, using data from a nationwide Japanese birth cohort study. We also considered the difference of maternal parity in this analysis because nulliparous women without previous pregnancy details would need more information regarding the risk of PTB compared to multiparous women.
Methods
Study design
We analysed data from the Japan Environment and Children’s Study (JECS), which was a nationwide, government-funded, prospective birth cohort study started in January 2011 (participant enrolment: between January 2011 and March 2014) to investigate the effects of environmental factors on children’s health10,11. Briefly, the JECS was funded directly by the Ministry of the Environment, Japan and involved collaboration between the Programme Office (National Institute for Environmental Studies), Medical Support Centre (National Centre for Child Health and Development), and 15 Regional Centres (Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu/Okinawa)10,11. For inclusion in the JECS, expectant mothers had to meet the following criteria: (1) residence within the study area at the time of recruitment, with an expectation to continue residing in Japan in the foreseeable future; (2) expected due date between 1 August 2011 and mid-2014; and (3) the ability to participate in the study without difficulty (i.e. ability to comprehend the Japanese language and complete a self-administered questionnaire).
There were two modes of recruitment: (1) at the time of the first prenatal examination at co-operating health care providers; and (2) at local government offices that issued a pregnancy journal called the Maternal and Child Health Handbook to all expecting mothers in Japan before they received municipal services for pregnancy, delivery, and childcare. Pregnant women were contacted through co-operating health care providers and/or local government offices issuing Maternal and Child Health Handbooks, and those who were willing to participate were registered. Self-administered questionnaires, which were completed by the women during the first and second/third trimester, were used to collect information on demographic factors, medical history, physical and mental health, lifestyle, occupation, environmental exposures at home and in the workplace, housing conditions, and socioeconomic status10,11.
Data collection
We used data released in October 2019 (dataset: jecs-ta-20190930). Participants with singleton pregnancies were included in the present study; however, women who had abortions, stillbirths, and missing information on exposures and outcomes were excluded from the analysis. Those with unreliable data regarding TPTB diagnosis and TPTB gestational age and missing data regarding maternal parity were also excluded. Parity was categorised into nulliparous and multiparous; multiparous women were further divided into two groups according to the presence of previous PTBs because women with previous PTBs had a 2.5-fold increased risk for their next pregnancy1. Moreover, we excluded cases with offspring chromosome abnormalities because those cases are related to PTBs.
Exposure variables
TPTB was diagnosed by each co-operating health care provider and derived from medical record transcripts. There were no unified clinical criteria for diagnosis of TPTB in the JECS. However, in Japan, TPTB was typically diagnosed based on the presence of regular uterine contractions, or changes in CL or dilatation, which was different from other countries7. Gestational age was typically verified based on accurate ultrasound examinations conducted during the first trimester, while gestational age at diagnosis of TPTB was derived from medical record transcripts. Participants with TPTB were divided into five categories based on gestational age at diagnosis (22–24, 25–27, 28–30, 31–33, and 34–36 weeks of gestation).
Main outcome measure
The main outcome measure was the incidence of PTB before 37 and 34 weeks of gestation. Gestational age at delivery was obtained from medical record transcripts. We also analysed PTB in participants without ischaemic placental disease (IPD) and chronic hypertension. IPD was based on the diagnosis of any of hypertensive disorders of pregnancy (HDP), small-for-gestational-age (SGA) infants, or placenta abruption (for which the data were derived from medical records). HDP was defined as persistently elevated blood pressure (≥ 140/90 mmHg) after 20 weeks of pregnancy in an otherwise normotensive woman12. SGA infants were defined as infants with birth weight < 1.5 standard deviations, corrected for parity, gestational age, and sex, based on the ‘New Japanese neonatal anthropometric charts for gestational age at birth’13. Although there is no consensus on the categorisation of PTBs14, cases of PTB with IPD would be the alternative for medically induced PTB.
Confounding factors
The following were included as confounding factors: maternal age, body mass index before pregnancy, maternal smoking status, maternal alcohol consumption status, maternal educational status, annual household income, marital status, assisted reproductive technology, and high maternal Kessler 6 scores at the first half of pregnancy. There was no multicollinearity, which was considered to be present under the following conditions: an association between independent variables with correlation coefficient r > 0.8 and/or variance inflation factor > 10.
Maternal ages were categorised into < 20, 20–29, 30–39, and ≥ 40 years. Body mass index before pregnancy was categorised into < 18.5, 18.5–19.9, 20.0–22.9, 23.0–24.9, and ≥ 25.0 kg/m2. The participants were requested to provide information regarding their smoking status by selecting one of the following: ‘currently smoking’, ‘never’, ‘previously did, but quit before realising current pregnancy’, and ‘previously did, but quit after realising current pregnancy’. The participants who chose ‘currently smoking’ were included in the ‘smoking’ category, whereas others were included in the ‘non-smoking’ category. The participants were also requested to provide information regarding their alcohol consumption status by choosing one of the following: never drank, quit drinking before pregnancy, quit drinking during the early stage of pregnancy, and kept drinking during pregnancy15. Maternal participants who chose ‘kept drinking during pregnancy’ composed the drinking category; all other participants composed the non-drinking category. Maternal educational status was categorised into the following four groups according to the number of years of education completed: junior high school (< 10 years); high school (10–12 years); technical junior college, technical/vocational college, associate degree, or bachelor’s degree (13–16 years); and graduate degree (master’s/doctor’s) (≥ 17 years). The annual household income was categorised into four levels: < 2,000,000, 2,000,000–5,999,999, 6,000,000–9,999,999, and ≥ 10,000,000 JPY. High maternal Kessler 6 scores were defined as scores ≥ 13 points16. For each factor, ‘no answer’ was analysed as a single item.
Statistical analysis
Women were stratified by the presence of TPTB, and maternal characteristics and obstetric outcomes were compared. Chi-square tests were performed to analyse the statistical differences of the ratio of PTBs between the groups, after the stratification of the participants based on parity. The ratio of intrauterine infections was also compared. Intrauterine infection was clinically diagnosed by physicians at each institution. There were no unified criteria for intrauterine infection in the JECS. Moreover, univariable and multivariable logistic regression models were used to calculate the crude odds ratios (cORs), adjusted ORs (aORs), and 95% confidence intervals (CIs) for the incidence of PTB before 37 and 34 weeks of gestation in participants in each category for gestational age at TPTB diagnosis (participants with diagnosis of TPTB at 34–36 gestational weeks were excluded from the analysis for PTB before 34 weeks), using participants without TPTB as the reference group. ORs were adjusted for potential confounding factors. We performed the same analyses excluding participants with IPD. Statistical analysis was performed using SPSS version 26 (IBM Corp., Armonk, NY, USA).
Ethical approval
The JECS protocol was reviewed and approved by the Ministry of the Environment Institutional Review Board on Epidemiological Studies (approval no.: 100910001) and by the ethics committees of all participating institutions. The JECS was conducted in accordance with the Helsinki Declaration as well as with other national regulations and guidelines. Written informed consent was obtained from all participants.
Results
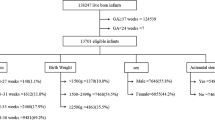
The total number of foetal records in the JECS was 104,062. Overall, 94,236 participants met the inclusion criteria (Fig. 1).
Table 1 summarises the maternal characteristics and obstetric complications according to TPTB diagnosis status. The characteristics of participants with and without TPTB diagnosis did not differ significantly, except for the ratios of PTB before 37 and 34 weeks of gestation.
Table 2 summarises the differences of outcomes based on the TPTB diagnosis status. The ratios of PTBs before 37 weeks in nulliparous and multiparous women without previous PTBs with TPTB diagnosis were maximised at 34–36 weeks of gestation. The PTB ratios before 37 weeks in multiparous participants with previous PTBs reached a plateau and did not show statistically significant results. There was no obvious trend in the increase of PTB ratios before 34 weeks according to the gestational age at TPTB diagnosis in nulliparous participants. The PTB ratios before 34 weeks in multiparous women without previous PTBs with TPTB diagnosis were maximised at 22–24 and 31–33 weeks of gestation. The ratios of PTBs before 34 weeks in multiparous women with previous PTBs with TPTB diagnosis were maximised at 22–24 weeks of gestation. The PTB ratios were much higher in multiparous women with previous PTBs compared with those in other groups. There were higher ratios of intrauterine infection in the early stage of TPTB in nulliparous women.
Table 3 summarises the cORs, aORs, and 95% CIs for PTB before 37 weeks of gestation for nulliparous and multiparous women in each TPTB gestational age category. The aORs for PTBs in nulliparous and multiparous women with TPTB diagnosis increased in every category of gestational age at diagnosis. The aORs for PTBs in nulliparous and multiparous women without previous PTBs with TPTB diagnosis were maximised at 34–36 weeks of gestation. There was no obvious trend in the increase of aORs according to the gestational age at TPTB diagnosis in multiparous participants with previous PTBs.
Table 4 summarises the cORs, aORs, and 95% CIs for PTB before 34 weeks of gestation for nulliparous and multiparous women in each TPTB gestational age category. The aORs for PTBs in nulliparous and multiparous women with TPTB diagnosis increased in every category of gestational age at diagnosis. The aORs for PTBs in nulliparous women reached a plateau. The aORs for PTBs in multiparous women without previous PTBs with TPTB diagnosis were maximised at 22–24 and 31–33 weeks of gestation. The aORs for PTBs in multiparous women with previous PTBs with TPTB diagnosis were maximised at 22–24 weeks of gestation.
Table 5 summarises the cORs, aORs, and 95% CIs for PTB before 37 weeks of gestation in nulliparous and multiparous women with each TPTB gestational age category after the exclusion of IPD cases. The aORs for PTBs in nulliparous and multiparous women with TPTB diagnosis increased in every category of gestational age at diagnosis. The aORs for PTBs in nulliparous and multiparous women without previous PTBs with TPTB diagnosis were maximised at 34–36 weeks of gestation. The aORs for PTBs in multiparous participants with previous PTBs reached a plateau.
Table 6 summarises the cORs, aORs, and 95% CIs for PTB before 34 weeks of gestation for nulliparous and multiparous women in each TPTB gestational age category after the exclusion of IPD cases. The aORs for PTBs in nulliparous and multiparous women with TPTB diagnosis increased in every category of gestational age at diagnosis. There was no obvious trend in the increase of aORs according to the gestational age at diagnosis of TPTB in nulliparous participants. The aORs for PTBs in multiparous women without previous PTBs with TPTB diagnosis were maximised at 22–24 and 31–33 weeks of gestation. The aORs for PTBs in multiparous women with previous PTBs with TPTB diagnosis were maximised at 22–24 weeks of gestation.
Discussion
The present study revealed that the association between gestational age at TPTB diagnosis and the incidence of PTB varies based on maternal parity with a history of previous PTB and PTB before 37 or 34 weeks. Latest gestational age at diagnosis of TPTBs was associated with a higher incidence of PTBs before 37 weeks in nulliparous and multiparous women without previous PTBs. Meanwhile, earliest gestational age at TPTB diagnosis was associated with a higher incidence of PTBs before 34 weeks in multiparous women. The same tendency was confirmed in nulliparous and multiparous women after excluding the cases of patients with IPD. To the best of our knowledge, this is the first study to show the association between gestational age at TPTB diagnosis and the incidence of PTBs in a nationwide birth cohort with stratification of maternal parity and history of previous PTBs.
We speculate that one of the reasons for the difference in the results between PTB before 37 and 34 weeks of gestation could stem from differences in PTB aetiology between the early and late stages. The major aetiologies of PTB are infection in the early stage and hormonal changes in the late stage17. In this study, we identified higher ratios of intrauterine infection in the early stage of TPTB in nulliparous women; however, the number of participants with intrauterine infection was too small to draw a definitive conclusion that differences in PTB aetiology between the early and late stages would cause differences in the association between gestational age at diagnosis of TPTB and the incidence of PTBs. Regarding PTBs before 37 weeks, later TPTB diagnosis might be a predictor for PTB incidence. There would be a shorter CL and wider cervical dilatation at the time of TPTB diagnosis in the later TPTB group than in the early TPTB group because shorter CL and wider cervical dilatations are physiologically induced as gestational age progresses. Therefore, TPTB diagnosis at 34–36 weeks of gestation might be generally diagnosed based on uterine contractions and might lead to a higher rate of onset of labour immediately after TPTB diagnosis because cervical dilatation is strongly associated with the latency of pregnancy after TPTB diagnosis9,18. However, it should be noted that the majority of PTBs occur at a later gestational age; in particular, 73.2% of PTB cases occur at 34–36 weeks19. Regarding PTBs before 34 weeks, earliest TPTB diagnosis might be a predictor for PTB incidence in multiparous women. This is consistent with the findings of a previous study9 and our hypothesis. This information would be useful for multiparous women, because both clinicians and women may be predisposed to recommend or seek evaluation at a potentially lower threshold of symptoms when a previous PTB has occurred in multiparous women20. However, in clinical settings, the requirement for predicting of PTBs is greater in nulliparous than in multiparous women, because multiparous women already have important information concerning the prediction of PTBs (i.e. previous PTBs). The clear explanation of the plateau aORs for PTBs in nulliparous women and maximised aORs for PTBs in multiparous women without previous PTBs with TPTB diagnosis at 31–33 weeks of gestation is lacking. Therefore, the utility of gestational age at TPTB diagnosis for the prediction of PTBs before 37 and 34 weeks is questionable and needs further validation.
Moreover, the difference between PTB before 37 and 34 weeks of gestation would be caused by the differences in methods used for diagnosing TPTB in Japan. There may be some differences in the criteria for patients with TPTB in Japan compared to those in other countries7; reports from other countries have shown that hospital admissions for threatened preterm labour was approximately 9% (129 women for TPTB diagnosis at 24–32 weeks and 105 women for TPTB diagnosis above 33 weeks among 2,534 participants)20. More participants were diagnosed with TPTB in the JECS compared to those in this previous study20. In Japan, many pregnant women would have been diagnosed as having TPTB based on just slight uterine contractions or short CL, and those patients with TPTB under these criteria are often treated with long-term maternal ritodrine hydrochloride administration21. The inconsistency of TPTB diagnosis in Japan might have led to the discrepancy between the present results and our hypothesis. Moreover, the higher prevalence of TPTB in the JECS would be partially attributed to the fact that we did not consider the difference between outpatients and hospitalisations. Further studies with unified protocols for diagnosing TPTB are warranted to clarify the true association between gestational age at TPTB diagnosis and PTB incidence.
The present study has some limitations. First, several clinical factors may affect the results. We did not account for detailed clinical scenarios, such as CL, cervical dilatation, uterine contractions, foetal fibronectin testing, genital bleeding during pregnancy, amniotic fluid levels, laboratory data including inflammatory cytokines, and treatments including tocolysis and cerclage. Further studies are required to clarify the association between gestational age at TPTB diagnosis and the incidence of PTB based on these clinical factors. Second, there is a possibility of selection bias, as several participants who had missing data were excluded. Although no significant differences were noted in the characteristics between those included and excluded from the analysis owing to missing data (data not shown), careful interpretation is needed based on considerations of these potential biases.
Conclusions
The association between gestational age at diagnosis of TPTB and the incidence of PTB varies based on maternal parity with a history of previous PTB and PTB before 37 or 34 weeks. This variety would be caused by differences in PTB aetiology between the early and late stages of pregnancy, differences in patient characteristics between the early and late stages of TPTB diagnosis, and differences in methods used for diagnosing TPTB in Japan compared to those in other countries. The utility of gestational age at TPTB diagnosis to predict PTB incidence in a clinical setting remains unclear. Further studies with detailed clinical data and a unified TPTB diagnosis protocol are necessary to clarify the association between gestational age at TPTB diagnosis and PTB incidence.
Data availability
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Epidemiological Research enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr. Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Abbreviations
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- CL:
-
Cervical length
- cOR:
-
Crude odds ratio
- HDP:
-
Hypertensive disorders of pregnancy
- IPD:
-
Ischaemic placental disease
- JECS:
-
Japan Environment and Children’s Study
- PTB:
-
Preterm birth
- SGA:
-
Small-for-gestational-age
- TPTB:
-
Threatened preterm birth
References
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Wen, S. W., Smith, G., Yang, Q. & Walker, M. Epidemiology of preterm birth and neonatal outcome. Semin. Fetal Neonatal Med. 9, 429–435 (2004).
López Bernal, A. Overview. Preterm labour: Mechanisms and management. BMC Pregnancy Childbirth 7, S2 (2007).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Sakata, S., Konishi, S., Ng, C. F. S. & Watanabe, C. Preterm birth rates in Japan from 1979 to 2014: Analysis of national vital statistics. J. Obstet. Gynaecol. Res. 44, 390–396 (2018).
Radan, A. P. et al. Cervico-vaginal placental α-macroglobulin-1 combined with cervical length for the prediction of preterm birth in women with threatened preterm labor. Acta Obstet. Gynecol. Scand. 99, 357–363 (2020).
Simhan, H. N. Practice Bulletin no. 171: Management of preterm labor. Obstet. Gynecol. 128, e155–e164 (2016).
Kyozuka, H. et al. Utility of cervical length and quantitative fetal fibronectin for predicting spontaneous preterm delivery among symptomatic nulliparous women. Int. J. Gynaecol. Obstet. 145, 331–336 (2019).
Chao, T. T., Bloom, S. L., Mitchell, J. S., McIntire, D. D. & Leveno, K. J. The diagnosis and natural history of false preterm labor. Obstet. Gynecol. 118, 1301–1308 (2011).
Kawamoto, T. et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25 (2014).
Michikawa, T. et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 28, 99–104 (2018).
Brown, M. A. et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72, 24–43 (2018).
Itabashi, K., Miura, F., Uehara, R. & Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 56, 702–708 (2014).
Goldenberg, R. L. et al. The preterm birth syndrome: Issues to consider in creating a classification system. Am. J. Obstet. Gynecol. 206, 113–118 (2012).
Yokoyama, Y. et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) protocol area. J. Epidemiol. 26, 420–432 (2016).
Furukawa, T. A. et al. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int. J. Methods Psychiatr. Res. 17, 152–158 (2008).
Pisacreta, E. & Mannella, P. Molecular and endocrine mechanisms involved in preterm birth. Gynecol. Endocrinol. 38, 368–378 (2022).
How, H. Y., Khoury, J. C. & Sibai, B. M. Cervical dilatation on presentation for preterm labor and subsequent preterm birth. Am. J. Perinatol. 26, 1–6 (2009).
Osterman, M. J. K., Hamilton, B. E., Martin, J. A., Driscoll, A. K. & Valenzuela, C. P. Births: Final data for 2021. Natl. Vital Stat. Rep. 72, 1–53 (2023).
McPheeters, M. L. et al. The epidemiology of threatened preterm labor: A prospective cohort study. Am. J. Obstet. Gynecol. 192, 1325–1329 (2005) (discussion 1329–1330).
Murata, T., Aoyagi, Y., Okimura, H. & Ueda, A. Report from “International Workshop for Junior Fellows 2: Tocolytic treatment for prevention of preterm birth” at the 73rd Annual Congress of the Japan Society of Obstetrics and Gynecology. J. Obstet. Gynaecol. Res. 47, 3758–3760 (2021).
Acknowledgements
The authors are grateful to all participants of the study. The members of the JECS Group as of 2022 are as follows: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Centre for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Funding
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment, Japan.
Author information
Authors and Affiliations
Consortia
Contributions
T.M. conceptualized and designed the study. T.M., H.I., K.I., T.F., H.K., S.Y., A.Y., K.H., H.N., and K.F. contributed to the study design. K.S., A.S., and Y.O. collected the data. T.M. analyzed the data and wrote the manuscript. M.H., S.Y., K.H., K.S., A.S., Y.O., H.N., K.F., and the JECS group reviewed the manuscript and provided critical advice. All authors approved the final manuscript. T.M. had full access to all the data used in the study and takes responsibility for the integrity of the data and the accuracy of its analysis. Written informed consent was obtained from all participants.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murata, T., Isogami, H., Imaizumi, K. et al. Association between gestational age at threatened preterm birth diagnosis and incidence of preterm birth: the Japan Environment and Children’s Study. Sci Rep 13, 12839 (2023). https://doi.org/10.1038/s41598-023-38524-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38524-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.