Abstract
Land-use conversion affects soil organic carbon (SOC) dynamics. Therefore, an in-depth study of change in SOC, SOC pool, fractions of SOC and enzymatic activities of soil microbial biomass carbon (SMBC) and dehydrogenase (DHA) with the conversion of forest land to jhum, fallow jhum and settle cultivation use has been undertaken on the hills of Arunachal Pradesh of India. Geo-referenced soil samples from eight different locations, each from different land uses were collected at three depth. One part of the soil sample had been used for the analysis of SOC and its carbon fraction. The second portion was kept in a deep freezer for determining SMBC and DHA. The third part was used for the analysis of bulk density. The result revealed that the highest loss of SOC pool was recorded in jhum land (41.8 to 13.4%), and the labile carbon was also found to decrease in jhum land. The highest SMBC was observed on the surface soil of the natural forest; the highest DHA was found in the natural forest; and the lowest DHA was recorded in jhum land. This study found that the converting natural forest to jhum reduces SOC storage, enzymatic activities and C fractions significantly whereas fallow jhum shows sign of recovery because all of these parameters improved when compared to the jhum land-use system. This study also confirms that the fallow period helps restore the initial situation.
Similar content being viewed by others
Introduction
Arunachal Pradesh is situated in the northeast Himalayan region of India, where jhum cultivation (JC) is one of the main sustenance activities of small-scale farmers and rural populations for food requirements, economic growth, and cultural activities.To carry out the jhum cultivation, farmers are going for deforestation and land use transformation, which are vital ecological issues in these regions. The transformation of natural forests into jhum significantly modifies the soil properties and their processes, and therefore, soil functioning1,2. This has also been reported in earlier studies that land use and cover alter the soil's organic carbon dynamics due to the influence of physical, chemical, and biological properties, which eventually modify the soil fertility and quality3. Further, the variability in land cover also impacts the soil microbial activities, which influence the soil organic carbon pool considerably. This is due to changes in the rate of input (e.g. addition of plant litter) and rate of output (e.g. mineralization of soil organic carbon) of soil organic matter (SOM) and land management operations2,4.
The forests influence the concentration of carbon present in the atmosphere5. As a result transformation of natural forests into other land uses such as jhum, plantations and crop lands for socioeconomic activities results in the modification of soil properties and its processes. A study in the northern part of India has reported that continuous cultivation resulted in a loss of 21–36% of total organic carbon as compared to uncultivated soils6, which is comparatively less than the values (30–60%) SOC loss reported in other agro-climatic regions of India7. When natural forests are converted to different land uses, the soil as a whole gets disrupted, leading to an enhancement in the rate of mineralization of organic matter by microbial activities and subsequently leading to SOC loss8. A wide range of SOC concentrations from 0.85 to 3.56%9 with SOC stock of 20–40 Mg/ha– in the top 0–30 cm of soil depth10 has also been reported in Arunachal Pradesh. The SOC in Banana cultivation was 1.75%11 as reported in North Eastern Himalayan Region.
Deforestation and the shift in vegetation composition are responsible to altering soil structure and physicochemical properties12,13. It is supposed that these processes also affects soil microbial and enzymatic activities and soil nutrient cycling14. This study hypothesizes that transformation in land uses alter the soil microbiological and enzyme activities, and therefore, soil carbon and associated attributes could be used as sensitive indicators of soil health. The objective of this investigation is to understand the effect of different land-use types and deforestation on soil organic carbon (SOC pool) and different fractions of carbon. Further, this study will also determine the activity of selected soil enzymes involved in the cycling of SOC, i.e. SMBC and Dehydrogenase under selected land use and cover types.
Materials and methods
Description of the study area
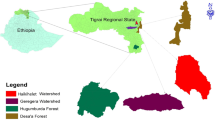
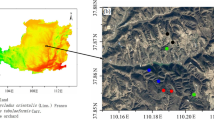
The research has been conducted at ICAR RC for NEH Region, Arunachal Pradesh Centre, Basar, in district West Siang now Leparada (Fig. 1), which is situated between longitude 93.57°E to 95.23°E and latitude 27.69°N to 29.20°N. It has a land area of 7643 square kilometersand borders Upper and East Siang in the east, China towards the north, and Upper Subansiri at West, and Assam in the South. The climatic state of the district varies from tropical to sub-tropical due to the undulating terrain. The topography of the West Siang region has an average elevation of 578 m above mean sea level, and the mean annual precipitation is approximately 2100 mm. Due to adequate rainfall the soil moisture availability for the crops generally lasts more than 270 days in a year. A detailed description of the different land uses selected is provided in Table 1.
Study site: Arunachal Pradesh Northeast Himalayan region of India, This map was prepared by the first author and co-author irmal Khatri Bapapain Makdoh and Letngam Touthang with the help of ArcGIS 10.4 (http://www.esri.com/software/arcgis) and does not require any permission from anywhere.
Soil sampling, processing, and analysis
Geo-referenced The soil samples from georeferenced locations has been collected at the depth of (0–15, 15–30, and 30–45 cm depth) from the core sampler for bulk density and from the auger sampler for other parameters. The sample is collected from 8 different locations and each location has four land uses: jhum, natural forest, fallow jhum, and Pineapple plantation, Each location is divided on a 50 × 50 m grid, and each grid divided into three parts. Thus in total, 288 composite samples (4 land uses × 8 sampling locations × 3 depths × 3 replications) were collected. A portion of the soil sample was air-dried, ground, and sieved through a 2.00-mm sieve and then used for the analysis of chemical properties such as soil pH, soil organic carbon (SOC), and carbon fraction. The soil organic carbon was determine by wet digestion method15. The second part was passed through 0.5-sieve and kept in a deep freezer for determination of biological properties: soil microbial carbon biomass (SMBC) and soil dehydrogenase activity (DHA). The third part was used for the analysis of physical properties such as bulk density.
Analysis of soil carbon fractions and pools
Soil organic carbon fractions (SOC) were determined under an increasing grade of the oxidising condition by employing three aqueous sulfuric acid concentration solutions in the ratios of 0.5:1, 1:1, and 2:1, respectively16. Thus the estimated carbon content leads to the partitioning of SOC into the following four different organic carbon fractions in decreasing order of oxidisability. Fraction I (very labile): organic carbon oxidisable under 0.5:1 H2SO4, Fraction II (labile): The difference in carbon oxidisable under 1:1 and 0.5:1 H2SO4, Fraction III (less labile): The difference in carbon oxidisable under 2:1 and 1:1 H2SO4, Fraction IV (non-labile): The difference between soil organic carbon and carbon oxidisable under 2:1 H2SO4. The active pool of organic carbon was calculated by adding fraction I and fraction IIwhile the passive pool of organic carbon is determined by the adding of fraction III and fraction IV. An active pool of organic carbon represents the amount of organic carbon present in an readily oxidisable form. While the passive pool of organic carbon represents the carbon fraction resistant to decomposition.
The soil organic carbon (SOC) pool (Mg C ha−1) was determined by the following equation:
Analysis of soil biological properties
The dehydrogenase activity was determined by reducing 2, 3, 5-triphenyl tetrazolium chloride17. A standard curve of triphenylformazan (TPF) in methanol was used to determination of the DHA activity, and it is expressed in terms of μg TPFg−1 soil h−1.For the determination of Soil Microbial biomass carbon the chloroform fumigation-extraction method18 was used and it is expressed in μg g–1 dry soil.
Statistical analysis
For statistical analysis of data regarding the different properties of soils in our study, a general linear model of SAS 9.4 (SAS Institute, 2003) was utilized. The least significant difference between LSD (p = 0.05) was computed to compare means among various land-use systems.
Results
Soil organic carbon content, bulk density and Soil pH
The result of the present study revealed that different land-use systems have an significant influence on soil pH and bulk density. Soil pH ranged from 4.8 in fallow jhum to 5.4 in natural forests. In general, they were showing an increasing trend of pH deeper soil layer i.e. with the increasing soil depth. The highest pH was recorded at 30–45 cm soil layers of respective land-use systems (Tables 2 and 3). The lowest values of pH were recorded at 0–15 cm, and the highest was recorded at 30–45 cm depth. Moreover, the soil layer of 30–45 cm does not show a significant effect of land use on soil pH.
The bulk density is significantly influenced by the land use system across all soil layers. The lowest bulk density values (1.09 Mgm–3) were found in natural forests 0–15 cm soil layers, while the highest (1.29 Mgm–3) were found in jhum land uses 0–15 and 30–45 cm soil layers. The natural forest and pineapple land uses showed an increasing trend in bulk density across the soil depth. However, in jhum and fallow jhum land, the soil layer (15–30 cm) recorded a relatively lower bulk density than the upper layer. The soil organic carbon has also shown significant changes under different land-use systems. It is gradually decreasing with increasing soil depth from 0 to 45 cm, and the highest SOC content was recorded on surface soil i.e. 0–15 cm depth irrespective of land use system (Table 3). The natural forest had the highest SOC (2.43% in 0–15 cm, 1.75 in 15–30 cm and 1.25% in 30–45 cm depth), followed by the pineapple-based land-use system (1.68% in 0–15 cm, 1.39% in 15–30 cm, and 0.99% 30–45 cm soil layer), fallow jhum (1.24% in 0–15 cm, 1.4% in 15–30 cm, and 0.98% 30–45 cm soil layer) and lowest was recorded in jhum (1.19% in 0–15 cm, 1.05% in 15–30 cm, and 0.95% 30–45 cm soil layer). When compared to the natural forest at surface soil, jhum land had the greatest depletion in SOC content at all depths. Up to 15 cm soil layer 50.8%, from 15-30 cm soil layer 39.8%, and from 30–45 cm soil layer 23.9% depletion was recorded. Continuous cultivation of 2–3 crops prior to leaving land fallow resulted in reduction and further showed signs of recovery as SOC depletion was less than that of jhum land and natural forest. A minimum loss in SOC of 30% at 0–15 cm, 30% at 15–30 cm, and 21% at 30–45 cm was observed with the cultivation of pineapples.
Effects of land use transformation on carbon pools
Land use transformation has been found a significant effect on the SOC pools across all soil depths (Table 3). The SOC storage ranged from 39.73 Mg ha−1 in natural forests to 23.13 Mg ha−1 in jhum land use. The natural forest has a significantly higher SOC pool across all soil layers, followed by the Pineapple land use, fallow jhum, and the jhum land-use system, which has the lowest. . At 0–15 and 15–30 cm depth, the jhum and fallow jhum were significantly at par with each other, while at 30–45 cm soil layer, except for the natural forest, all three land-use systems at par with each other, whereas the natural forest recorded significantly higher SOC pool. In this study, it is revealed that the conversion of natural forest land to different land-use systems depleted the SOC stock. Jhum land had highest depletion of SOC pool (41.8% in 0–15 cm, 32.4% in 15–30 cm and 13.4% 30–45 cm soil layer), followed by fallow jhum (38.9% in 0–15 cm, 28.7% in 15–30 cm and 13.7% 30–45 cm soil layer), and pineapple had the lowest depletion (26.4% in 0–15 cm, 17.3% in 15–30 cm and 16.6% 30–45 cm soil layer). The maximum depletion of soil organic carbon pool (ranging from 41.7 to 26.4%has been observed on the surface soil layer (0–15 cm), and the loss is gradually decreased with the soil depth; the lowest depletion was found on the 30–45 cm soil layer, ranging from 16.6 to 13.4%.
Carbon fractions are influenced by the land use system
A significant effect was observed in soil carbon fractions among the various land uses in all soil layers (Table 4). The natural forest had the highest very labile carbon (9.53 g Kg–1 soil) followed by the pineapple (3.68 g Kg–1 soil) and the lowest was found in jhum land use. Moreover, at the 0–15 cm soil layer, except for natural forests, all other land uses were statistically on par with each other. A similar trend was also found for labile carbon. The non-labile carbon of the pineapple land use was found to be statistically at par with that of natural forest. This suggests that when natural forest land is converted into jhum or horticulture based land-use systems, such as pineapple land use, the very labile and labile carbon start depleting, while, the non-labile carbon remain preserved. The very labile carbon, labile carbon, less labile and non-labile ranged from 2.84 to 9.64 g Kg–1 soil, 1.44 g to 3.67 g Kg–1 soil, 1.05 to 2.70 g Kg–1 soil and 5.02 to 9.06 g Kg–1 soil respectively, at 0–15 cm soil layer.
In the 15–30 cm soil layer, land use showed a significant effect on the labile and very labile carbon fractions, whereas the less labile and non-labile fractions were not significantly affected by land use. At this soil layer, very labile carbon, labile carbon, less labile, and non-labile fractions ranged from 2.0 to 5.33 g Kg–1 soil, 5.01 to 1.36 g Kg–1 soil, 1.31 to 3.16 g Kg–1 soil, and 3.98 to 6.90 g Kg–1 soil, respectively. The natural forest showed a significantly higher amount of very labile carbon (5.33 g Kg–1 soil), followed by the pineapple and fallow jhum (2.33 g Kg–1 soil), and the lowest was recorded in jhum (2.0 g Kg–1 soil). A similar trend was also found in the labile carbon, whereas, the less labile and non-labile carbon showed no significant effect. In the 30–45 cm soil layer, labile carbon was found in the highest amount (4.44 g Kg–1soil) in the natural forest followed by the pineapple followed by the jhum and fallow jhum land. However, the jhum and fallow jhum are statistically at par with each other. The less labile form of carbon showed a similar trend, while the non-labile form had shown no significant effect; however, the highest amount of non-labile carbon was found in the natural forest land use (4.78 g Kg–1 soil), and in the pineapple land-use system.
In the 30–45 cm soil layer, very labile, labile, and less labile fractions showed a significant effect on land use, whereas, the non-labile did not find a significant effect on land use. In this soil layer, very labile carbon, labile carbon, less labile carbon, and non-labile carbon ranged from 1.11 to 2.33 g Kg–1 soil, 1.37 to 4.44 g Kg–1 soil, 5.45 to 7.75 g Kg–1 soil, and 2.87 to 4.78 g Kg–1 soil, respectively.
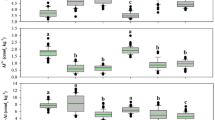
Across land uses, the active pool in soils ranged from 4.27 to 13.23 Mg ha−1 in 0–15 cm, 6.36 to 10.42 Mg ha−1 in 15–30 cm, and 3.27– 5.55 Mg ha−1 in 30–45 cm depths. Among land-use systems, the natural forest had a significantly higher amount of active pool in different soil layers, followed by pineapple (Fig. 2). The smallest amount of active pool was obtained in soils under jhum land-use systems, which were on par with fallow jhum land. Similarly, the passive pool was also significantly influenced by the land-use system in the 0–15 cm depth and 15–30 cm soil layer, however, in the 30–45 cm soil layer, it was not significantly influenced by the land-use. It ranged from 7.71 to 11.06 Mg ha−1 in 0–15 cm, 7.13 to 8.24 Mg ha−1in 15–30 cm and 4.99 to 6.98 Mg ha-1 in 30–45 cm soil layers in different land uses. Of the various land-use systems, soils under the natural forest land-use system had the highest passive pools (11.06 Mg ha−1) at 0–15 cm, whereas, the lowest passive pool was recorded in pineapple land use at 30–45 cm soil depth. Non labile and less labile carbon pool contributed the most to total carbon in Pineapple and fallow jhum land (Fig. 3), indicating that carbon is being rapidly lost from labile pool while nonlabile pool is remaining intact.
Effects of land use management on soil microbial biomass carbon (SMBC)
The study expressed that the soil microbial biomass carbon (SMBC) and dehydrogenase activities (DHA) were significantly affected by the land use system across the different soil layers. The data divulge that SMBC and DHA were consistently decreasing deeper in the soil layer (Table 5). The surface soil layer of various land uses had the highest SMBC (497–306 μg g–1 dry soil). Significantly higher concentrations of SMBC were found in soils under natural forest, followed by pineapple land use, fallow jhum and the lowest was recorded in jhum land use in the respective soil layers. The highest depletion in SMBC (38.4% at 0–15 cm, 34.9% at 15–30 cm, and 19% at 30–45 cm soil layer) was found in the jhum, whereas the lowest depletion in SMBC (14.1% at 0–15 cm, 13.1% at 15–30 cm and 10.8% at 30–45 cm soil layer) was recorded in pineapple and the natural forest. Moreover, the fallow jhum showed a little less depletion (29.9% at 0–15 cm, 26.2% at 15–30 and 18.4% at 30–45 cm soil layer) as compared to the jhum; this indicates that the fallow jhum is in the process of regaining the lost microbial activities.
Effects of land use management on dehydrogenase activities (DHA)
The highest DHA activity in soils of all land uses was recorded, and it was significantly affected by the different land-use systems. With increasing depth, dehydroginase activity decreased. The highest dehydroginase activity was reported at 0–15 cm depth and the lowest at 30–45 cm depth in all land uses (Table 5). Among land uses, the highest dehydrogenase activities was found in the natural forest land use (61.6 µg TPFg−1 soil h−1at 0–15 cm, 54 µg TPFg−1 soil h−1 at 15–30 and 50.7 µg TPFg−1 soil h−1 at 30–45 cm soil layer) followed by the Pineapple land-use system and lowest DHA was recorded in jhum land (43.7 µg TPFg−1 soil h−1 at 0–15 cm, 36.3 µg TPFg−1 soil h−1 at 15–30 cm and 30 µg TPFg−1 soil h−1 at 30–45 cm soil layer). Moreover, the DHA of fallow jhum and jhum land were found at par at surface soil layer (0–15 cm) and 15–30 cm soil layer, while at the 30-45 cm soil layer, the natural forest is significantly higher and rest all at par.
Discussion
Soil pH, bulk density and soil organic carbon
Soils in the natural forest had significantly higher pH, which could be attributed to extensive root systems and more exudates from different vegetation in natural forests affecting soil fertility status19, addition of plant residues can increase, decrease or have little effect on soil pH, depending on initial soil pH, The input of plant residues decreases soil pH at high initial soil pH through nitrogen nitrification in the residue. However, at low initial soil pH, the activity of nitrification bacteria will be suppressed and mineralized nitrogen tends to be ammonified and thus increase soil20. whereas the jhum-land use system had the lowest pH, followed by Pineapple land use. In the deeper layer at 30–45 cm depth, the effect was less pronounced as some of the leached out bases were expected to be deposited or adsorbed in the lower soil horizons21, Due to this phenomenon, lower layers had a relatively higher pH than the surface and subsurface layers, irrespective of land use. Other researcher22,23 have also reported variation in soil pH due to different years of jhum cultivation and management practices followed in various land-use types. SOC and bulk density had a negative correlation24,25. The tendency of bulk density to increase with depth could be attributed to the mass of the overlying soil26 and the decrease in SOC27 and the fertility of the soil28. The natural forest land use showed the lowest bulk density at all the soil depths compared to the other land-use systems in our study, whereas, it was recorded highest in jhum and the reverse trend was recorded for SOC. Our findings were consistent with29 who found that bulk density and soil organic carbon were negatively correlated in soils of the western Himalayas. However, it is also a well-established fact that bulk density and soil organic carbon are oppositely proportional to each other30,31, the higher value of SOC in the soil is an indicator of a lower BD and good aeration.
Changes in land-use systems and subsequent changes in soil organic carbon have strongly affected soil physical properties: most prominently the bulk density and porosity of the soil. The deforestation of natural forests and subsequent burning for shifting cultivation resulted in a significant reduction in soil organic carbon; the reduction of SOC up to a depth of 0–30 cm was significant. The forest had the greatest content of SOC due to its higher net primary productivity, and very high litterfall that covered the soil surface. This litterfall acts like mulch contributing to increasing soil organic matter32. As a result, the SOC in the natural forest is high. When this land is converted to shifting cultivation, such as jhum, the SOC drastically reduced due to forest burning and oxidation of organic matter33. A similar report was also reported by34 in the East Siang district of Arunachal Pradesh. This study well explains why deforested land use, viz., jhum, fallow jhum, and pineapple land use, has undergone depletion of a considerable amount of SOC, and this further reduces the C input due to crop cultivation in the jhum land. The fallow jhum land-use showing signs of recovery after being fallow for two or more years. As a result, this landuse showed a higher SOC compared to the jhum land use. The pineapple land use had a considerable improved SOC over the jhum land as it was consistently under pineapple cultivation for the last 7 years (Table 1) after the 2 years of jhum.
At large, SOM is presumed to be higher in natural forests than in deforested soils like jhum and fallow jhum soils due to larger inputs and less intense decomposition of SOM. Contrary to the presented study, The highest concentration of SOM (13.2 g kg–1) was found in grassland in the topsoil layer (0–15 cm), followed by forest land (10.2 g kg–1) and cultivated (7.23 g kg–1) soils35. Similar to our study36,37 found that conversion of native vegetation to agricultural systems significant impact on SOC, lowering it by 9–25%. The magnitude of the decrease in SOC in their studies was not as large as in our study (41.8–13.4%), probably due to differences in soil type, texture, organic matter source, sampling depth, and jhumimg processes and also due to altitudinal locations which influencing SOC controlling temperature regimes, precipitation, solar radiation, relative humidity, and geologic deposition processes38. The increasing soil depth decreasing aeration, soil microbial activity, and fine root turnover might have resulted in the corresponding decrease in the concentration of SOC39. Our study showed that natural forest systems had a significantly higher concentration of SOC across soil depths, while jhum land use had the least SOC.
Soil organic carbon pools and fractions
Soil carbon pools present in the soil are a product of the equilibrium between carbon addition and carbon depletion6. In the present study, the SOC pool was the highest under natural forests, followed by pineapple, whereas, the lowest was found under the jhum land-use system. Conversion of carbon-rich natural forests to croplands results in a rapid loss of carbon pool40 and threatens the ecosystem’s normal functioning41.
The Arunachal Pradesh region of India (the study site) has a cooler climate and receives very high annual rainfall (> 3000 mm per annum). It is expected to have a low rate of mineralization in the soils of these regions owing to high rainfall and a cooler climate compared to the northern Indo-Gangetic plain of India. The active carbon (AC) and passive carbon (PC) pools were significantly affected by the land use system across all depths, except in the 30–45 cm soil layer, where only passive pool land use showed a significant effect with land use. Similar to our study42 also reported that land-use changes have significantly impacted the AC and PC pools in soil. The AC pool in the soil acts serves as a source of energy for soil microorganisms and as a predictor of soil quality16. Whereas, the PC reserve is not swiftly affected by soil management practices and soil microbial activities43. The natural forest had the highest AC and PC pools in 0–15 cm, 15–30 and 15–30 cm depths and jhum land use had the lowest AC & PC poll in all three soil layers. In this study, the higher amount of passive C pools in soils under natural forest and Pineapple land-use systems might be due to the continuous addition of C inputs through different types of fine roots, root exudation, forest litters, nominal soil interruption and reduced soil erosion44. Similar to our study, which discovered that soil pH influenced the quantitative distribution of soil organic carbon between fractions, more acidic soil is less transformed into an active pool45. Statistically similar values of AC pools among the natural forest soil and pineapple land-use systems in 0–15 cm soil depth are mainly attributed due to variation in soil bulk density. The magnitude of variation in active and passive pools is less in deeper soil as compared to the upper soil layer among the different land-use systems.
The amount of carbon reserve depend on the crop biomass productivity that augments the AC fragment42,46. The addition of readily decomposable leaf litter throughout the year in the natural forest and pineapple system might have produced a high AC pool. Among the land uses studied, the jhum and fallow jhum land-use systems had the largest reduction in the AC pool. The jhum had deleterious activities while preparing the land for cultivation, which might have increased losses of the AC pool from the soil. Similar variation was also reported by47 the variation in AC and PC pools across land uses could be attributed to variations in vegetation type, the addition of litter and the intensity of soil distribution26. SignificantlyThe higher very labile carbon fractions in natural forest and pineapple land-use systems compared to fallow land are attributed to the high input of plant litters and protection of added carbon, which increased the microbial activity and thus the very labile carbon fraction. Besides this, the natural forest area was surrounded by Alnus nepalensis, which is a non-leguminous N-fixing plant with a higher rate of litterfall. Leaves with better N content have a greater capacity to increase the labile carbon in soil48,49. A higher amount of very labile carbon in forest soil was also reported by50.
The higher amount of Non-labile carbon (NLC) fractions in soils under natural forest and Pineapple land-use systems in this study could be attributed to minimal soil disturbance, a slow rate of decomposition, and composition of the plant, underlying grasses, and shrubs51. Generally, forest litters are rich in tannin and wax, which are resistant to decomposition and may contribute toward greater non-Labile carbon under forest soil than the deforested (jhum and fallow jhum) land as these materials might be lost during the burning process. However, the higher relative proportion of NLC (32.5 to 37.5%) to SOC in all the depths under deforested land than in natural forest and pineapple land-use systems suggested that losses of the very labile carbon fraction were higher under deforested land due to jhuming and soil disturbance as compared to natural forest and pineapple land-use systems. The non-labile carbon fraction is resistant to soil microorganisms decomposition and soil management practises due to sorption on fine particles43,52.
Soil microbial biomass carbon and dehydrogenase activities
In the current study, significant influences of land use on microbial carbon biomass and dehydroginase activities were observed. The natural forest recorded the highest microbial carbon biomass (497 µ g g–1dry soil dry soil) followed by pineapple land use, whereas the lowest was reported in the jhum and fallow jhum on surface soil. The highest microbial carbon biomass was recorded on surface soil and gradually decreases with the inceasing the soil depth. This could be due to forest fire being burn while shifting cultivation. Similar to our findings, reduction in microbial biomass after the fire has been reported in many studies53,54. This indicated that the burning killed soil microorganisms, which manifested in the reduction of microbial biomass and microbial activities. Furthermore, burning could also change the nutrient supply and could also be a reason for the reduction in microbial biomass after a forest fire55,56. Compared with the land under native forest, the deforested land particularly jhum reduces 38.4% reduction in microbial biomass. The soil microbial biomass content decreased with deforestation, agreeing with the results reported by57, who also reported that the soil of degraded land had less microbial biomass than land under native vegetation (undisturbed). The decrease in soil microbial biomass content found in the deforested lands is a consequence of the loss of vegetation cover and SOC from the soil surface. The vegetation covering the soil surface has fundamentally influenced the properties of soils58 because it contributes to the input of organic matter to the soil and protects the soil from erosion. Furthermore, the absence of vegetation cover exposes the soil microbial biomass at the soil surface to the direct effects of high temperatures and rain. A similar result was also observed by59,60 for African tropical soils and Indian soils, respectively.
The activity of DHA gradually decreases from natural forest to Jhum land use (Table 5) in the order of natural forest > pineapple > fallow Jhum > Jhum. Conversion of natural forest (61.7 µg TPFg−1 soil h−1) to jhum (43.7 µg TPFg−1 soil h−1) accounts for a 29.1% loss of DHA. The increase in activity of this enzyme reflects metabolic ability of the soil and its activity is varied with presence of microbial biomass The lower DHA levels imply that the loss of vegetation cover reduced the activity of these enzymes. Similar to aur study it was discovered by61 that as vegetation cover decreased, so did the values of enzyme activities in the soils Deforestation decreases the amount of organic matter in the soil that suppresses microbial biomass, thus decreasing the rate of enzymatic reactions62. However, the results for soil microbial biomass at the fallow jhum land also suggest that the restoration of jhum by leaving it for growing vegetation can promote the recovery of soil microbial biomass and DHA. The dynamics responsible for such retrieval could be allowed to regenerate the vegetation that vitalizes the growth of soil microbial biomass by increasing the input of plant roots and plant litter63.
Conclusions
The hypothesis of this study was that transformation of natural forests to deforestation-based land-use systems decreases SOC storage, C fraction pools, and enzymatic activities. While deforested land allows for the regeneration of vegetation and the recovery of the status of SOC, C fraction pools, and enzymatic activities. This study indicates that from natural forest to jhum there was a drastic reduction in the SOC storage, enzymatic activities and C fractions, whereas, from jhum to fallow jhum showed the sign of recovery as all these parameters had improved compared to the jhum land-use system. The fallow phase is a response well to regain the initial soil conditions before the transformation of natural forest to the jhum land. The average fallow period allows for the return to initial soil conditions. However, pineapple, the horticulture-based land-use system has further improved all these parameters compared to both deforested land jhum and fallow jhum. One more important piece of information the non-labile carbon of the pineapple land use was statistically at par with the natural forest. This showedthat when natural forest land is converted into jhum or horticulture-based land-use systems, such as pineapple land use, the labile carbon deplete first while the non-labile carbon remain intact.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Celik, I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey. Soil Tillage Res. 83(2), 270–277 (2005).
Dawson, J. J. & Smith, P. Carbon losses from the soil and its consequences for land-use management. Sci. Total Environ. 382(2–3), 165–190 (2007).
Zhao, D. et al. The influence of different types of urban land use on soil microbial biomass and functional diversity in Beijing, China. Soil Use Manag. 29(2), 230–239 (2013).
Poeplau, C. & Don, A. Sensitivity of soil organic carbon stocks and fractions to different land-use changes across Europe. Geoderma 192, 189–201 (2013).
Wei, X., Shao, M., Gale, W. J., Zhang, X. & Li, L. Dynamics of aggregate-associated organic carbon following conversion of forest to cropland. Soil Biol. Biochem. 57, 876–883 (2013).
Benbi, D. K., Brar, K., Toor, A. S. & Singh, P. Total and labile pools of soil organic carbon in cultivated and undisturbed soils in northern India. Geoderma 237, 149–158 (2015).
Lal, R. Soil carbon sequestration impacts global climate change and food security. Science 304(5677), 1623–1627 (2004).
Golchin, A. & Asgari, H. Land use effects on soil quality indicators in north-eastern Iran. Soil Res. 46(1), 27–36 (2008).
Bhattacharyya, T. et al. Soil carbon storage capacity as a tool to prioritize areas for carbon sequestration. Curr. Sci. 95, 482–494 (2008).
Kumar, J. et al. Impact of application of fertilizer and lime on yield of banana (Grand Naine) and soil parameters in acidic soil of Arunachal Pradesh. Int. J. Plant Soil Sci. https://doi.org/10.9734/ijpss/2020/v32i430264 (2020).
Choudhury, B. U. et al. Spatial variability in distribution of organic carbon stocks in the soils of North East India. Curr. Sci 104, 604–614 (2013).
Pickett, S. T. et al. Urban ecological systems: Linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Evol. Syst. 32(1), 127–157 (2001).
Yan, B. et al. Urban-development-induced changes in the diversity and composition of the soil bacterial community in Beijing. Sci. Rep. 6(1), 1–9 (2016).
Rai, P. K., Rai, A. & Singh, S. Change in soil microbial biomass along a rural-urban gradient in Varanasi (UP, India). Geol. Ecol. Landsc. 2(1), 15–21 (2018).
Walkley, A. & Black, I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37(1), 29–38 (1934).
Chan, K. Y., Bowman, A. & Oates, A. Oxidizible organic carbon fractions and soil quality changes in an oxicpaleustalf under different pasture leys. Soil Sci. 166(1), 61–67 (2001).
Casida, L. E. Jr., Klein, D. A. & Santoro, T. Soil dehydrogenase activity. Soil Sci. 98(6), 371–376 (1964).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19(6), 703–707 (1987).
Chen, C., Liu, W., Jiang, X. & Wu, J. Effects of rubber-based agroforestry systems on soil aggregation and associated soil organic carbon: Implications for land use. Geoderma 299, 13–24 (2017).
Rukshana, F., Butterly, C. R., Xu, J. M., Baldock, J. A. & Tang, C. Organic anion-to-acid ratio influences pH change of soils differing in initial pH. J. Soils Sediments 14, 407–414 (2014).
Meena, V. S. et al. Land use changes: Strategies to improve soil carbon and nitrogen storage pattern in the mid-Himalaya ecosystem, India. Geoderma 321, 69–78 (2018).
Orgill, S. E. et al. Removing grazing pressure from a native pasture decreases soil organic carbon in southern New South Wales, Australia. Land Degrad. Dev. 29(2), 274–283 (2018).
Parras-Alcántara, L., Lozano-García, B., Keesstra, S., Cerdà, A. & Brevik, E. C. Long-term effects of soil management on ecosystem services and soil loss estimation in olive grove top soils. Sci. Total Environ. 571, 498–506 (2016).
Hati, K. M., Swarup, A., Dwivedi, A. K., Misra, A. K. & Bandyopadhyay, K. K. Changes in soil physical properties and organic carbon status at the topsoil horizon of a vertisol of central India after 28 years of continuous cropping, fertilization and manuring. Agric. Ecosyst. Environ. 119(1–2), 127–134 (2007).
Wen-JIE, W. et al. Changes in soil organic carbon, nitrogen, pH and bulk density with the development of larch (Larixgmelinii) plantations in China. Glob. Change Biol. 17(8), 2657–2676 (2011).
Yadav, G. S. et al. Impact of no-till and mulching on soil carbon sequestration under rice (Oryzasativa L.)-rapeseed (Brassica campestris L. var. rapeseed) cropping system in hilly agro-ecosystem of the Eastern Himalayas, India. Agric. Ecosyst. Environ. 275, 81–92 (2019).
Abad, J. R. S., Khosravi, H. & Alamdarlou, E. H. Assessment of the effects of land-use changes on soil physicochemical properties in Jafarabad of Golestan province, Iran. Bull. Environ. Pharmacol. 3(3), 296–300 (2014).
Kakaire, J., Makokha, G. L., Mwanjalolo, M., Mensah, A. K. & Menya, E. Effects of mulching on soil hydro-physical properties in Kibaale Sub-catchment, South Central Uganda. Appl. Ecol. Environ. Sci. 3(5), 127–135 (2015).
Sharma, C. M., Gairola, S., Ghildiyal, S. K. & Suyal, S. Physical properties of soils in relation to forest composition in moist temperate valley slopes of the Central Western Himalaya. J. For. Environ. Sci. 26(2), 117–129 (2010).
Pulido-Fernández, M., Schnabel, S., Lavado-Contador, J. F., Mellado, I. M. & Pérez, R. O. Soil organic matter of Iberian open woodland rangelands as influenced by vegetation cover and land management. Catena 109, 13–24 (2013).
Post, W. M. & Kwon, K. C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Change Biol. 6(3), 317–327 (2000).
Kumar, J. et al. Effect of mulching on growth and quality of tissue culture banana (var. Grand Naine) and soil properties in mid hill Jhum lands of Arunachal Pradesh. Indian J. Agrofor. 22(2), 86–89 (2020).
Kumar, J. et al. Effect of different processes of Jhuming on soil properties in mid hills of Arunachal Pradesh. Environ. Ecol. 35(2A), 999–1003 (2017).
Bhuyan, S. I., Tripathi, O. P. & Khan, M. L. Soil nutrients status in prominent agro-ecosystems of East Siang district, Arunachal Pradesh. Int. J. Environ. Sci. 3(6), 1957–1968 (2013).
Saha, D., Kukal, S. S. & Bawa, S. S. Soil organic carbon stock and fractions in relation to land use and soil depth in the degraded Shiwaliks hills of lower Himalayas. Land Degrad. Dev. 25, 407–416 (2014).
Smith, P. et al. Global change pressures on soils from land use and management. Glob. Change Biol. 22(3), 1008–1028 (2016).
Villarino, S. H. et al. Deforestation impacts on soil organic carbon stocks in the Semiarid Chaco Region, Argentina. Sci. Total Environ. 575, 1056–1065 (2017).
Tsui, C. C., Chen, Z. S. & Hsieh, C. F. Relationships between soil properties and slope position in a lowland rain forest of southern Taiwan. Geoderma 123(1–2), 131–142 (2004).
Materechera, S. A. & Mkhabela, T. S. Microbial biomass in agricultural topsoils after 6 years of bare fallow. Biol. Fertil. Soils 19, 129–134 (1995).
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Isbell, F. et al. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202 (2011).
Sahoo, U. K., Singh, S. L., Gogoi, A., Kenye, A. & Sahoo, S. S. Active and passive soil organic carbon pools as affected by different land use types in Mizoram, Northeast India. PloS One 14(7), e0219969 (2019).
Sherrod, L. A., Peterson, G. A., Westfall, D. G. & Ahuja, L. R. Soil organic carbon pools after 12 years in no-till drylandagroecosystems. Soil Sci. Soc. Am. J. 69(5), 1600–1608 (2005).
Kurmi, B., Nath, A. J., Lal, R. & Das, A. K. Water stable aggregates and the associated active and recalcitrant carbon in soil under rubber plantation. Sci. Total Environ. 703, 135498 (2020).
Tonon, G. et al. Effect of soil pH on the chemical composition of organic matter in physically separated soil fractions in two broadleaf woodland sites at Rothamsted, UK. Eur. J. Soil Sci. 61(6), 970–979 (2010).
Reyna-Bowen, L., Fernandez-Rebollo, P., Fernández-Habas, J. & Gómez, J. A. The influence of tree and soil management on soil organic carbon stock and pools in dehesa systems. Catena 190, 104511 (2020).
Yao, M. K. et al. Effects of land use types on soil organic carbon and nitrogen dynamics in Mid-West Cote d’Ivoire. Eur. J. Sci. Res. 40(2), 211–222 (2010).
Campbell, C. A. et al. Seasonal trends in selected soil biochemical attributes: Effects of crop rotation in the semiarid prairie. Can. J. Soil Sci. 79(1), 73–84 (1999).
Sharma, G., Sharma, R. & Sharma, E. Traditional knowledge systems in large cardamom farming: Biophysical and management diversity in Indian mountainous regions. Indian J. Tradit. Knowl. 8(1), 17–22 (2009).
Benbi, D. K., Boparai, A. K. & Brar, K. The decomposition of particulate organic matter is more sensitive to temperature than the mineral associated organic matter. Soil Biol. Biochem. 70, 183–192 (2014).
Nath, A. J., Brahma, B., Sileshi, G. W. & Das, A. K. Impact of land use changes on the storage of soil organic carbon in active and recalcitrant pools in a humid tropical region of India. Sci. Total Environ. 624, 908–917 (2018).
Sainepo, B. M., Gachene, C. K. & Karuma, A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 13(1), 1–9 (2018).
Girona-García, A. et al. Effects of prescribed fire for pasture management on soil organic matter and biological properties: A 1-year study case in the Central Pyrenees. Sci. Total Environ. 618, 1079–1087 (2018).
Holden, S. R. & Treseder, K. K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front. Microbiol. 4, 163 (2013).
Mabuhay, J. A., Nakagoshi, N. & Horikoshi, T. Microbial biomass and abundance after forest fire in pine forests in Japan. Ecol. Res. 18(4), 431–441 (2003).
Smith, N. R., Kishchuk, B. E. & Mohn, W. W. Effects of wildfire and harvest disturbances on forest soil bacterial communities. Appl. Environ. Microbiol. 74(1), 216–224 (2008).
Zhang, P., Li, L., Pan, G. & Ren, J. Soil quality changes in land degradation as indicated by soil chemical, biochemical and microbiological properties in a karst area of southwest Guizhou, China. Environ. Geol. 51(4), 609–619 (2006).
Rutigliano, F. A., D’ascoli, R. & De Santo, A. V. Soil microbial metabolism and nutrient status in a Mediterranean area as affected by plant cover. Soil Biol. Biochem. 36(11), 1719–1729 (2004).
Kirchmann, H. & Eklund, M. Microbial biomass in a savanna-woodland and an adjacent arable soil profile in Zimbabwe. Soil Biol. Biochem. 26(9), 1281–1283 (1994).
Sahani, U. & Behera, N. Impact of deforestation on soil physicochemical characteristics, microbial biomass and microbial activity of tropical soil. Land Degrad. Dev. 12(2), 93–105 (2001).
Garcia, C., Hernandez, T., Roldan, A. & Martin, A. Effect of plant cover decline on chemical and microbiological parameters under Mediterranean climate. Soil Biol. Biochem. 34(5), 635–642 (2002).
Klose, S. & Tabatabai, M. A. Urease activity of microbial biomass in soils as affected by cropping systems. Biol. Fertil. Soils 31(3), 191–199 (2000).
Grayston, S. J., Vaughan, D. & Jones, D. Rhizosphere carbon flow in trees, in comparison with annual plants: The importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 5(1), 29–56 (1997).
Acknowledgements
The authors are thankful to the Director, ICAR Research Complex for North Eastern Hill Region, Umiam, Meghalaya, India, for providing the necessary facilities for conducting the study under National Mission for a sustainable Himalayan Ecosystem. The author acknowledges the RA and SRF of the NICRA project for helping in the analysis of soil biological parameters and carbon fractions.
Author information
Authors and Affiliations
Contributions
J.K.: conceptualization of the experiment, soil sampling, Writing review and editing. H.k.: Prepared initial draft of tables, reviewed & editing. W.R., N.K.B.M.: sample collection, and soil analysis in laboratory conducted data assimilation, data compilation, R.A.A.: data assessment writing, review and editing. T.A.: preparation and finalisation of graphs and diagrams. data analysis. A.P.S. and D.J.: data analysis validation graph preparation, N.K.S., R.S.C. and D.K. has done language editing, and J.K. and L.T. have prepare a study area map.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, J., Kalita, H., Rekhung, W. et al. Dynamics of soil organic carbon of jhum agriculture land-use system in the heterogeneous hill of Arunachal Pradesh, India. Sci Rep 13, 12156 (2023). https://doi.org/10.1038/s41598-023-38421-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38421-1
This article is cited by
-
Physical–chemical characterization of Amazonian dark earth as influenced by tropical fruticulture in western Brazil’s Legal Amazon
Journal of Soils and Sediments (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.