Abstract
The human epidermal growth factor receptor-2 (HER2) enriched subtype of breast cancer is associated with early recurrence, mostly within 5 years. However, anti-HER2 therapies have improved outcomes and their benefits persist in the long term. This study aimed to determine predictive factors for late survival in patients with HER2-positive breast cancer. We analyzed 20,672 patients with HER2-positive stage I–III breast cancer. The patients were divided into two groups based on a follow-up period of 60 months. The multivariate analysis of factors associated with poor overall survival included old age, advanced pathologic tumor size stage (pT), advanced pathologic regional lymph node stage (pN), high histological grade, presence of lymphatic and vascular invasion, and HR-negative status within 60 months. In the breast cancer-specific survival (BCSS) of the > 60 months follow-up group, the hazard ratios (HRa) based on pN-negative were 3.038, 3.722, and 4.877 in pN1 (p = 0.001), pN2 (p < 0.001), and pN3 (p < 0.001), respectively. Only pT4 level was statistically significant in the pT group (HRa, 4.528; p = 0.007). Age (HRa, 1.045, p < 0.001) and hormone receptor-positive status (HRa, 1.705, p = 0.022) were also associated to worse BCSS. Although lymphatic invasion was not significantly associated with BCSS, there was a tendency toward a relationship (p = 0.079) with worse BCSS. In HER2-positive breast cancer patients, node status had a more significant relationship with long-term prognosis than T stage. Patients with HER2-positive breast cancer who have T4 or node-positive should be considered for clinical observation and education beyond 5 years.
Similar content being viewed by others
Introduction
Breast cancer with amplification or overexpression of human epidermal growth factor receptor 2 (HER2) accounts for approximately 15% of primary invasive diseases1. HER2 positivity is associated with high recurrence and mortality2. In addition, HER2-positive breast cancers have a higher propensity to metastasize to the brain3. However, the advent and implementation of new anti-HER2 therapies have dramatically changed the treatment of patients with HER2 positivity4.
Significant progress has been made in treating patients with HER2-positive breast cancer, and studies are underway on the continuous improvement of outcomes in a subset of these patients. Nevertheless, patients treated for breast cancer remain at risk of recurrence5. There are many cases of late recurrence of hormone receptor (HR)-positive breast cancer, occurring even after 5 years6,7. Therefore, most studies have focused on HR-positive HER2-negative breast cancer. The HER2-enriched and basal subtypes are mostly associated with early recurrence within 5 years8. Recent advancements in treatment have improved the outcomes of patients with HER2-positive breast cancer. The survival rate at 4 years among women was 90.3% for HR-positive/HER2-positive breast cancer and 82.7% for HR-negative/HER2-positive breast cancer in 20189.
Breast cancer has poor prognosis to be high nuclear grade, HR-negative, and HER2-positive, have a high proliferation fraction, present with lymphovascular invasion (LVI), and be diagnosed at young age and at more advanced stage10,11,12. Clinical factors that affect the prognosis of HER2-positive breast cancer have been studied. HR-positive/HER2-positive breast cancers are associated with better survival rates than HR-negative/HER2-positive breast cancers13.
Despite improvements in both disease-free survival and overall survival (OS) in early-stage HER2-positive breast cancer, long-term follow-up results indicate that approximately 15–24% of patients still develop recurrent disease14,15. Few studies have examined the long-term prognostic factors for nonmetastatic HER2-positive breast cancer. We aimed to identify factors associated with the long-term prognosis of patients with HER2-positive breast cancer.
Results
Patient demographics and clinicopathological characteristics
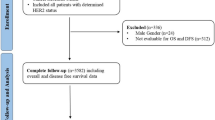
A total of 24,260 patients were identified as candidates for inclusion in the analysis. Of these patients, 3161 and 425 had in situ and distant metastases, respectively, and were excluded from this study. Finally, 20,672 patients were analyzed after excluding two patients with unknown follow-up data (Fig. 1). The ≤ 60 months and > 60 months follow-up groups had median follow-up times of 29.4 and 97.9 months, respectively. Most clinicopathological characteristics were significantly different between the groups (Table 1). The mean age was 51.4 years in the ≤ 60 months follow-up group and 49.5 years in the > 60 months follow-up group. There was no significant difference in body mass index between the two groups (23.66 and 23.57, respectively). The groups had significantly different pathologic tumor size stage (pT; p < 0.001) and pathologic regional lymph node stage (pN; p < 0.001), however, the distribution in each stage was not significantly different. Other pathological characteristics analyzed, namely, histological grade, estrogen and progesterone receptor status, ki-67 index, p53 overexpression, and the presence of lymphatic and vascular invasion, are presented in Table 1. The therapeutic characteristics were also significantly different between the two groups. In the ≤ 60 months follow-up group, the rate of breast-conserving surgery was higher than that in the > 60 months follow-up group (47.6% and 37.1%, respectively; p < 0.001), and the rate of sentinel lymph node biopsy was higher (46.5% and 10.2%, respectively; p < 0.001). In addition, the axillary lymph node dissection rates in the two groups were 51.2% and 81.4%, respectively (p < 0.001). Radiotherapy was performed more often in the ≤ 60 months follow-up group (62.8% vs. 50.4%; p < 0.001). In both groups, > 80% of patients received systemic chemotherapy (80.3% and 83.0%, respectively). No clear records were found for the 15,416 patients who underwent anti-HER2 therapy. Data on anti-HER2 therapy were missing for 6524 and 8892 patients in the ≤ 60- and > 60 months follow-up groups, respectively. A total of 539 and 157 patients in the ≤ 60- and > 60 months follow-up groups died from breast cancer, corresponding to rates of 4.8% and 1.7%, respectively.
Survival outcomes and associated factors
Univariate analysis was performed for the factors associated with breast cancer specific survival (BCSS). Old age, advanced pT and pN, elevated CEA levels, lymphatic and vascular invasion, and HR-negative status were statistically significant in both groups (Table 2). Multivariate analysis of factors associated with poor OS included old age, advanced pT, advanced pN, high histological grade, lymphatic and vascular invasion, and HR-negative status within 60 months (Table 3A). Advanced pT and pN, the presence of vascular invasion, and HR-positive status were associated with worse OS after 60 months (Table 3A). In the ≤ 60 months follow-up period of BCSS, poor outcome was associated with old age, advanced pT and pN stages, histological grade III, presence of lymphatic invasion, and HR-negative status. In the > 60 months follow-up period, the hazard ratios (HRa) based on pN0 were 3.038, 3.722, and 4.877 for pN1-3, indicating that pN stage was the most significant variable related to outcome (p = 0.001, p< 0.001, and p < 0.001, respectively). Only pT4 was significantly associated with worse BCSS than pT1 (reference level) in the > 60 months follow-up group (HRa, 4.528; p = 0.007). Old age (HRa, 1.045; 95% confidence interval (CI) 1.023–1.066; p < 0.001) and HR positivity (HRa, 1.705; 95% CI 1.079–2.963; p = 0.022) were also associated with poor outcomes. Although lymphatic invasion was not statistically significant, there was a tendency toward a p-value of 0.079 indicating a worse outcome (Table 3B). The Kaplan–Meier curve was used to analyze the pT and pN to compare the effect of OS and BCSS in the > 60 months follow-up period. The differences between the pT and pN stages were statistically significant (p < 0.001 for both; Fig. 2A,B). However, the patterns differed between the OS and BCSS. The Kaplan–Meier curve showed that pT4 was associated with significantly poorer BCSS than pT1-3. Regarding pN stage, pN0 was associated with better BCSS than pN1-3.
Additional data and subgroup analysis
The factors for BCSS related to a follow-up period of > 60 months could not be identified by analyzing only the patients who received anti-HER2 therapy. Among 5249 patients who received anti-HER2 therapy, 15 died of breast cancer within 60 months of diagnosis (Supplement 1). Subgroup analyses were performed according to staging. In stage I, age was the only factor related to BCSS after 60 months, whereas age and lymphatic invasion were associated factors in stage II. No associated factors were identified in stage III patients (Supplement 2).
Discussion
The advent of anti-HER2 therapy has dramatically improved the outcome of patients with HER2-positive breast cancer. Few studies have reported the factors associated with the long-term prognosis of patients with HER2-positive nonmetastatic breast cancer. Although it was difficult to analyze the long-term prognosis with data reflecting the rapidly changing trend of HER2-positive breast cancer therapy, it was possible to infer factors affecting breast cancer specifically in HER2-positive breast cancer, by comparing the OS with the same period. In this study, we analyzed 20,672 patients and demonstrated that closer follow-up of HER2-positive breast cancer patients might be required, even after 5 years in patients with T4 or node-positive breast cancer. Multivariate analysis and Kaplan–Meier survival curves indicated that pT4 or node positivity was also a significant factor in long-term prognosis. Additionally, in elderly individuals, HR positivity or lymphatic invasion may be associated with the prognosis of patients with HER2-positive breast cancer.
The characteristics between the two groups by follow-up period indicated similar distributions of each factor, notwithstanding a statistically significant divergence. In particular, although the pathological characteristics of the patients showed similar distribution patterns, the treatment factors adopted discrepancies between groups. The differences in treatments might be influenced by evolving trends in clinical practice, reflecting the more recent diagnosis of patients in the ≤ 60 months follow-up group. Specifically, this data showed that breast-conserving surgery was more prevalent by 10.5% in the ≤ 60 months follow-up group relative to the > 60 months follow-up group. Furthermore, the incidence of patients undergoing sentinel lymph node biopsy alone was considerably higher in the ≤ 60 months follow-up group, standing at a marked 36.3%. Radiotherapy was also more common in the ≤ 60 months group, with a 12.4% higher rate. These observed variations may be attributed to changing paradigms in breast cancer management, highlighting the gradual shift towards less aggressive and more optimized patient treatment modalities.
Lee and Kim analyzed 131,178 patients with nonmetastatic breast cancer between 1980 and 2014. The study showed that lymph node metastasis was associated with increased mortality in HER2-positive tumors compared to an increase in T stage16. This was observed in HER2-positive patients regardless of HR status. Other studies have also shown that HER2-positive breast cancer has a higher rate of lymph node metastasis than the other types17,18,19. The degree of lymphatic vessel density was significantly associated with breast cancer subtype, with the HER2 subtype showing the highest density of20. Similar results were observed in this study. Lymph node metastasis showed the most significant association with long-term prognosis, and lymphatic invasion showed a similar trend; however, the linear association with T stage was insignificant.
Another study reported that tumor size > 2 cm and positive node status, irrespective of subtype, affected breast cancer-related survival in long-term follow-up (median follow-up of 18.7 years)21. Our study only analyzed patients with HER2-positive diseases. Nodal positivity showed the same results in each analysis, but the tumor stage revealed a different result from that of BCSS. We showed that only the T4 stage was significantly associated with BCSS. T3 tended to be associated with BCSS (p = 0.056). There was a difference within 5% for T1 in the Kaplan–Meier curve, and there was no notable difference for T2. This finding indicates that tumor size is not related to poor long-term outcomes.
A distinct pattern of recurrence was observed according to HR status in HER2-positive disease for 5–10 years from NCCTG N9831 and NSABP B-3122. The benefit of adjuvant trastuzumab persisted in the long term, and the effect was similar in HR-positive and HR-negative, HER2-positive breast cancer patients22. We also found that HR status was a statistically significant factor in late prognosis. HR-negative status was related to poor survival outcomes within 60 months, whereas HR-positive status was associated with worse survival outcomes over 60 months of follow-up. The luminal type may show a recurrence pattern that can occur over a long period of time.
Neoadjuvant chemotherapy has become standard clinical practice and has increased the proportion of patients who receive neoadjuvant chemotherapy in recent years. In May 2022, the NCCN guidelines were updated to state that neoadjuvant systemic therapy can be considered for cT1c, cN0 HER2-positive disease23. A strong relationship between the pathological response and prognosis after neoadjuvant therapy has been reported24,25. Patients who achieved pathological complete response (pCR) had excellent long-term prognosis after neoadjuvant therapy26. In particular, the number of HER2-positive breast cancer patients who received neoadjuvant chemotherapy has increased because of the increased pCR rate resulting from trastuzumab plus pertuzumab27. Neoadjuvant chemotherapy with trastuzumab and pertuzumab increase the pCR rate by approximately 50–70%27,28,29. In addition, recurrence in patients with residual disease after neoadjuvant therapy improves with advanced adjuvant treatment30. Considering these current trends, when the data of patients treated with current advanced treatment were analyzed with respect to factors related to long-term prognosis, there is a possibility that these results would not agree with those based on data from patients treated earlier. Several studies have attempted to predict the prognosis of HER2-positive breast cancer patients after neoadjuvant therapy. The integration of tumor-infiltrating lymphocytes, circulating tumor cells, or circulating tumor DNA may enhance the prediction; however, for intuitive use in clinical practice, a more accessible factor is needed31,32,33. Therefore, it is necessary to identify clinical factors associated with long-term prognosis. Data from this study showed that systemic chemotherapy had no significant effect on the prognosis in the 60 months group. However, it should be taken into consideration that data were included from 2000, when systemic treatments, including anti-HER2 therapies, were different from current treatments.
There were several limitations in this study. The clinicopathological characteristics of patients with a follow-up time shorter than 60 months had a little representative and might cause bias in the results because of including the following situations. First, the patients had reached the clinical endpoint (death). Second, the follow-up time of some patients was short. The durations of follow-up were 0 to 60 months. Third, the rate of lost-to-follow-up was not analyzed in this data. However, the distribution of patients was inferred, and the flow of differences in the treatment was shown.
In addition, the important limitation was that data on death were recorded until 2014. Therefore, analyses of breast cancer mortality over 60 months of follow-up were more distributed in the 2000s, which is different from the current treatment. Considering the trends in systemic therapy, including the recent widespread use of anti-HER2 agents, the prognosis of these patients cannot be directly compared with that of patients currently being treated in 2022.
There are many missing data on anti-HER2 therapy. According to the National Health Insurance Service in Korea, considering the treatment policy, it is possible to assume that anti-HER2 therapy had been applied to most patients. Therefore, we attempted to re-analyze the patient data after 2008, assuming that they received trastuzumab treatment. However, data that did not provide information on anti-HER2 therapy were analyzed by treating them as missing data. There were several reasons for this: too much missing data for anti-HER2 treatment, survival data up to 2014, and T1a–b stage patients who were not treated with anti-HER2 therapy. In advanced breast cancer, anti-HER2 agents have doubled the median OS to > 50 months and have more than tripled the 5 years survival rate34. Therefore, anti-HER2 therapy, including trastuzumab, was predicted to be one of the most significant factors affecting long-term prognosis; however, this was excluded from the analysis. To complement this limitation, we compared OS and BCSS and identified that the higher the N stage in BCSS compared to OS, the more associated the long-term prognosis.
Another limitation was that missing Ki-67 data were found in 8376 (40.5%) cases, and available data were mostly included within the 5 years follow-up group (8731; 71% of the available Ki-67 data). Ki-67 has been shown to be a 10 years prognostic factor in HER2-positive or triple-negative breast cancer groups35. Therefore, the study was limited to analyzing long-term prognosis, and the Ki-67 index was excluded from the multivariate analysis. Neoadjuvant systemic therapy was not actively administered to the patients who received treatment between 2000 and 2014.
In conclusion, node status has a more significant relationship with long-term prognosis than T stage in patients with HER2-positive breast cancer. This finding indicates that tumor size itself is not related to poor outcomes in terms of long-term prognosis, and aggravation of nodal stage is associated with poor outcomes after 5 years. Additionally, close follow-up is required in elderly individuals and patients with lymphatic invasion or HR positivity. Although the evidence level is low, associated indications or guidelines for patients who require long-term observation and education may need to be established in the future.
Material and methods
Study population
This study used nationwide data from the Korean Breast Cancer Registry (KBCR; http://registry.kbcs.or.kr/ecrf). Since 1996, The Korean Breast Cancer Society has been prospectively collecting data from patients with breast cancer. The database provides demographic characteristics, patient history, clinicopathological characteristics, treatment modality information, and follow-up data. It was estimated that enrollment in 2013 included more than 65% of all newly diagnosed breast cancer patients in Korea36. The Korean Central Cancer Registry, Ministry of Health and Welfare, Korea, provided dates and causes of death on December 31, 2014. The KBCR database does not contain sufficient data regarding recurrence.
This study collected data from patients who underwent surgery for HER2-positive primary breast cancer between January 1, 2000, and December 31, 2014, in South Korea. Among the c-erbB2 ++ results, the patients who did not undergo in situ hybridization testing were excluded. The included patients were women aged > 18 years with pathological stage I–III disease. Patients were enrolled regardless of whether targeted therapy was administered. Patients who had received neoadjuvant systemic therapy or had a history of other cancers were also included. The pT was categorized as 1, 2, 3, and 4. The pN was divided into 0–3, and the micrometastatic lymph nodes were included in pN1. Institutions recorded HR status data as assessed by their analysis and cutoff values. HR positivity was defined as estrogen or progesterone receptor positivity. The cut-off value for the Ki-67 labeling index was set at 20%37. HER2-positive status was defined as an immunohistochemistry score of 3+ cell surface protein expression, or equivocal cases followed by a positive fluorescent or silver in situ hybridization test result according to the American Society of Clinical Oncology/College of American Pathologists HER2 testing guidelines (2007). Patients with no recorded HER2 status or equivocal status without in situ hybridization results were excluded. This study was approved by the Institutional Review Board of Incheon St. Mary’s Hospital (IRB number: OC22ZASI0020) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was not obtained from any of the participants.
Patients were categorized according to the follow-up period. They were grouped into early and late prognosis groups, defined as having follow-up periods ≤ 60 and > 60 months, respectively. The chi-square test and Fisher’s exact test were used for categorical variables. Continuous variables were assessed using a t-test. This study aimed to determine predictive factors for late mortality in patients with HER2-positive breast cancer. Patients who died within 60 months were censored when analyzing the late mortality. The two groups were analyzed for OS and breast cancer-specific survival (BCSS). OS was defined as the interval from surgery to the date of death or last follow-up. BCSS was defined as survival until death due to breast cancer and censored by death from other causes. The Cox proportional hazard regression model was used for the univariate and multivariate survival analyses. Adjusted hazard ratios (HRa) with 95% confidence intervals (CIs) are reported. Statistical significance was set at p < 0.05. All statistical analyses were performed using Statistical Package for the Social Sciences, version 26.0 (IBM Corporation, Armonk, NY, USA).
Ethical approval and consent to participate
The need of informed consent was waived by the Catholic University of Korea, Incheon St. Mary's Hospital Institutional Review Board (IRB no. OC22ZASI0020) of the Ethics Committee.
Data availability
Data files are available from the Korean Breast Cancer Registry (KBCR; http://registry.kbcs.or.kr/ecrf). The datasets generated and/or analysed during the current study are not publicly available because the data is owned by the Korea Breast Cancer Society and is only available to those with permission among the society members; doctors associated with breast oncology. The data are available from the corresponding author on reasonable request.
References
Noone, A. M. et al. Cancer incidence and survival trends by subtype using data from the surveillance epidemiology and end results program, 1992–2013. Cancer Epidemiol. Biomark. Prev. 26, 632–641. https://doi.org/10.1158/1055-9965.EPI-16-0520 (2017).
O’Shaughnessy, J., Gradishar, W., O’Regan, R. & Gadi, V. Risk of recurrence in patients with HER2+ early-stage breast cancer: Literature analysis of patient and disease characteristics. Clin. Breast Cancer 23, 350–362. https://doi.org/10.1016/j.clbc.2023.03.007 (2023).
Garcia-Alvarez, A., Papakonstantinou, A. & Oliveira, M. Brain metastases in HER2-positive breast cancer: Current and novel treatment strategies. Cancers (Basel) https://doi.org/10.3390/cancers13122927 (2021).
Patel, A., Unni, N. & Peng, Y. The changing paradigm for the treatment of HER2-positive breast cancer. Cancers (Basel) https://doi.org/10.3390/cancers12082081 (2020).
Colzani, E. et al. Time-dependent risk of developing distant metastasis in breast cancer patients according to treatment, age and tumour characteristics. Br. J. Cancer 110, 1378–1384. https://doi.org/10.1038/bjc.2014.5 (2014).
an overview of the randomised trials. Early breast cancer trialists’ collaborative, G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival. Lancet 365, 1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0 (2005).
Berry, D. A. et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295, 1658–1667. https://doi.org/10.1001/jama.295.14.1658 (2006).
Lin, N. U. & Winer, E. P. Advances in adjuvant endocrine therapy for postmenopausal women. J. Clin. Oncol. 26, 798–805. https://doi.org/10.1200/JCO.2007.15.0946 (2008).
Howlader, N., Cronin, K. A., Kurian, A. W. & Andridge, R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 27, 619–626. https://doi.org/10.1158/1055-9965.EPI-17-0627 (2018).
Fredholm, H. et al. Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Res. Treat 160, 131–143. https://doi.org/10.1007/s10549-016-3983-9 (2016).
Nishimura, R. et al. An evaluation of lymphovascular invasion in relation to biology and prognosis according to subtypes in invasive breast cancer. Oncol. Lett. 24, 245. https://doi.org/10.3892/ol.2022.13366 (2022).
Garutti, M. et al. Definition of high-risk early hormone-positive HER2-negative breast cancer: A consensus review. Cancers (Basel) https://doi.org/10.3390/cancers14081898 (2022).
Untch, M. et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann. Oncol. 19, 1090–1096. https://doi.org/10.1093/annonc/mdn005 (2008).
Cameron, D. et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389, 1195–1205. https://doi.org/10.1016/S0140-6736(16)32616-2 (2017).
Perez, E. A. et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 32, 3744–3752. https://doi.org/10.1200/JCO.2014.55.5730 (2014).
Lee, S. B. et al. Dynamic and subtype-specific interactions between tumour burden and prognosis in breast cancer. Sci. Rep. 10, 15445. https://doi.org/10.1038/s41598-020-72033-3 (2020).
Van Calster, B. et al. Axillary lymph node status of operable breast cancers by combined steroid receptor and HER-2 status: Triple positive tumours are more likely lymph node positive. Breast Cancer Res Treat 113, 181–187. https://doi.org/10.1007/s10549-008-9914-7 (2009).
Reyal, F. et al. The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PLoS ONE 6, e20297. https://doi.org/10.1371/journal.pone.0020297 (2011).
Bartlett, J. M. et al. Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor (ER) negative, but with grade in those with ER-positive early-stage breast cancer suitable for cytotoxic chemotherapy. J. Clin. Oncol. 25, 4423–4430. https://doi.org/10.1200/JCO.2007.11.0973 (2007).
Niemiec, J. A. et al. Prognostic role of lymphatic vessel density and lymphovascular invasion in chemotherapy-naive and chemotherapy-treated patients with invasive breast cancer. Am. J. Transl. Res. 9, 1435–1447 (2017).
Zanardi, E. et al. Insights from a long-term follow-up evaluation of early breast cancer outcomes by tumor subtype. Oncol. Res. Treat. 43, 362–371. https://doi.org/10.1159/000507736 (2020).
Chumsri, S. et al. Incidence of late relapses in patients with HER2-positive breast cancer receiving adjuvant trastuzumab: Combined analysis of NCCTG N9831 (Alliance) and NRG oncology/NSABP B-31. J. Clin. Oncol. 37, 3425–3435. https://doi.org/10.1200/JCO.19.00443 (2019).
National Comprehensive Cancer Network. Breast Cancer (Version 3, 2022) (2022).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384, 164–172. https://doi.org/10.1016/S0140-6736(13)62422-8 (2014).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804. https://doi.org/10.1200/JCO.2011.38.8595 (2012).
Symmans, W. F. et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol. 35, 1049–1060. https://doi.org/10.1200/JCO.2015.63.1010 (2017).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13, 25–32. https://doi.org/10.1016/S1470-2045(11)70336-9 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24, 2278–2284. https://doi.org/10.1093/annonc/mdt182 (2013).
Loibl, S. et al. Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: A subanalysis of data from the randomized phase III GeparSepto trial. Ann. Oncol. 28, 497–504. https://doi.org/10.1093/annonc/mdw610 (2017).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628. https://doi.org/10.1056/NEJMoa1814017 (2019).
Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 19, 40–50. https://doi.org/10.1016/S1470-2045(17)30904-X (2018).
Bidard, F. C. et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: A meta-analysis. J. Natl. Cancer Inst. 110, 560–567. https://doi.org/10.1093/jnci/djy018 (2018).
Rothe, F. et al. Circulating tumor DNA in HER2-amplified breast cancer: A translational research substudy of the NeoALTTO phase III trial. Clin. Cancer Res. 25, 3581–3588. https://doi.org/10.1158/1078-0432.CCR-18-2521 (2019).
Roth, J. et al. Survival gains from advances in first-line systemic therapy for HER2 overexpressing metastatic breast cancer in the U.S., 1995–2015. Ann. Oncol. 28, 85 (2017).
Nishimiya, H. et al. Prognostic significance of Ki-67 in chemotherapy-naive breast cancer patients with 10-year follow-up. Anticancer Res. 34, 259–268 (2014).
Kang, S. Y. et al. Basic findings regarding breast cancer in Korea in 2015: Data from a breast cancer registry. J. Breast Cancer 21, 1–10. https://doi.org/10.4048/jbc.2018.21.1.1 (2018).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. 24, 2206–2223. https://doi.org/10.1093/annonc/mdt303 (2013).
Acknowledgements
This article was supported by the Korean Breast Cancer Society.
Author information
Authors and Affiliations
Contributions
Study design and conception: Y.J.K., Y.S.K.; Data collection: Y.J.K., S.J.O., E.K.K., Y.J.L., E.H.P., J.J., H.K.P., and Y.S.K.; Analysis and interpretation of results: Y.J.K., S.Y.B., Y.S.K., Draft manuscript: Y.J.K., S.Y.B., E.K.K., Y.J.L., E.H.P., J.J., H.K.P., and Y.S.K., Revision and approval of final manuscript: Y.J.K., S.J.O., S.Y.B., E.K.K., Y.J.L., E.H.P., J.J., H.K.P., and Y.S.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, YJ., Oh, S.J., Bae, S.Y. et al. Predictive biological factors for late survival in patients with HER2-positive breast cancer. Sci Rep 13, 11008 (2023). https://doi.org/10.1038/s41598-023-38200-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38200-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.