Abstract
Since the beginning of the COVID-19 pandemic, new and non-invasive digital technologies such as artificial intelligence (AI) had been introduced for mortality prediction of COVID-19 patients. The prognostic performances of the machine learning (ML)-based models for predicting clinical outcomes of COVID-19 patients had been mainly evaluated using demographics, risk factors, clinical manifestations, and laboratory results. There is a lack of information about the prognostic role of imaging manifestations in combination with demographics, clinical manifestations, and laboratory predictors. The purpose of the present study is to develop an efficient ML prognostic model based on a more comprehensive dataset including chest CT severity score (CT-SS). Fifty-five primary features in six main classes were retrospectively reviewed for 6854 suspected cases. The independence test of Chi-square was used to determine the most important features in the mortality prediction of COVID-19 patients. The most relevant predictors were used to train and test ML algorithms. The predictive models were developed using eight ML algorithms including the J48 decision tree (J48), support vector machine (SVM), multi-layer perceptron (MLP), k-nearest neighbourhood (k-NN), Naïve Bayes (NB), logistic regression (LR), random forest (RF), and eXtreme gradient boosting (XGBoost). The performances of the predictive models were evaluated using accuracy, precision, sensitivity, specificity, and area under the ROC curve (AUC) metrics. After applying the exclusion criteria, a total of 815 positive RT-PCR patients were the final sample size, where 54.85% of the patients were male and the mean age of the study population was 57.22 ± 16.76 years. The RF algorithm with an accuracy of 97.2%, the sensitivity of 100%, a precision of 94.8%, specificity of 94.5%, F1-score of 97.3%, and AUC of 99.9% had the best performance. Other ML algorithms with AUC ranging from 81.2 to 93.9% had also good prediction performances in predicting COVID-19 mortality. Results showed that timely and accurate risk stratification of COVID-19 patients could be performed using ML-based predictive models fed by routine data. The proposed algorithm with the more comprehensive dataset including CT-SS could efficiently predict the mortality of COVID-19 patients. This could lead to promptly targeting high-risk patients on admission, the optimal use of hospital resources, and an increased probability of survival of patients.
Similar content being viewed by others
Introduction
In December 2019, the outbreak of the novel coronavirus disease (COVID‑19) also known as severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) was reported for the first time1. This life-threatening infection is caused by a recently originating zoonotic virus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)2.
COVID-19 is a highly contagious viral infection and continued to spread aggressively worldwide, despite all the preventive and lockdown measures. Its clinical outcomes ranged from asymptomatic to mild or moderate symptoms, and critical complications or death in some cases3. Twenty percent of COVID-19 patients must be hospitalized and, approximately, 20–30% of in-hospital COVID-19 patients need intensive care unit (ICU) care4. The pooled case fatality rates (CFR) of in-hospitalized and ICU-admitted patients were 13% (95% CI 9.0–17.0, P < 0.001, I2 = 95.6) and 37.0% (95% CI 24.0–51.0, P < 0.001, I2 = 97.8), respectively5. This complex and highly contagious virus had made a tremendous impact on the health of people all over the world and caused a significant number of deaths, particularly for the elderly, pregnant women, and infected cases with underlying comorbidities such as low immune functions, cardiopulmonary diseases, cancer, infectious diseases, hypertension, and diabetes6.
Hence, early identification of patients who are at risk of mortality is necessary to mitigate the burden on the healthcare system and to reduce deaths as much as possible. A predictive model that accurately predicts the poor outcomes for COVID-19 patients could assist in efficiently allocating limited medical resources, improve the quality of health care, and eventually optimize patient management7,8.
The disease behavior and courses are unpredictable which made the diagnosis of high-risk patients with poor prognoses a challenging problem9. Predictions made by different computational and statistical models are used to respond to this challenge10,11.
Since the beginning of the COVID-19 pandemic, new and non-invasive digital technologies such as artificial intelligence (AI) had been introduced for mortality prediction of COVID-19 patients. In AI problems, machines learn from past experiences and would adjust to new inputs. The machine learning (ML) approach as a subfield of AI is a complex and flexible classification modeling that leverages big datasets to reveal significant hidden relationships or patterns12. For the prediction of clinical outcomes in COVID-19 patients, ML methods have more accurate results than conventional statistics models13. The prognostic performances of the ML-based models for predicting clinical outcomes of COVID-19 patients had been mainly evaluated using demographics, risk factors, clinical manifestations, and laboratory results14,15,16,17. There is a lack of information about the prognostic role of imaging manifestations in combination with demographics, clinical manifestations, and laboratory predictors. Computed tomography (CT) scan is a valuable method routinely used in the diagnosis, monitoring, and management of COVID-19 patients. A significant correlation between the chest computed tomography severity score (CT-SS), which is determined based on the severity of pulmonary involvement on CT scans, and mortality in COVID-19 patients has been reported1,18,19. This pulmonary involvement score was proposed as an appropriate prognostic factor for mortality prediction in COVID-19 patients by recent meta-analysis studies1,19. Thus, CT-SS might improve the prognostic performances of the ML algorithms for mortality prediction of COVID-19 patients.
The purpose of the present study is to develop an efficient ML prognostic model for mortality prediction of COVID-19 patients based on a more comprehensive dataset including CT-SS, demographics, clinical manifestations, and laboratory predictors. Therefore, this study seeks to answer two questions. What are the most relevant predictors of patients’ mortality? And using the most relevant predictors of patients’ mortality, which ML model is more effective for mortality prediction of COVID-19 patients?
Methods
Dataset description
In this study, a COVID-19 hospital-based registry database was retrospectively reviewed from February 9, 2020, to December 20, 2020. This dataset included the data of the patients referred to Ayatollah Talleghani Hospital (COVID-19 referral centre), Abadan city, Iran.
A total of 6854 suspected cases had been referred to the hospital’s ambulatory and emergency departments (EDs), of whom 1853 cases were introduced as positive RT-PCR COVID-19, 2472 as negative, and 2529 as unspecified.
In the COVID-19 hospital-based registry database, seventy-two primary features in six main classes including patient’s demographics (eight features), clinical features (21 features), history of personal diseases/comorbidity (13 features), laboratory results (28 features), CT-SS (one feature), and an output variable (0: survived and 1: deceased) had been registered for COVID-19 patients. Primary features registered in the COVID-19 hospital-based registry database are listed in Table 1. Numerical parameters were quantitatively measured and nominal parameters were registered as Yes or No. In this database, demographic information of patients and their history of personal diseases/comorbidity were registered from the medical records or by asking the patient and the patient's companions. For each patient, the clinical features including cough, fever, shortness of breath, loss of smell, loss of taste, etc. were registered at the time of admission. In the first 24 h hospitalization of the patients, their blood and urine samples were analyzed and they were subjected to chest CT imaging. The laboratory results were automatically registered in their medical records.
Chest CT scores quantify the severity of pulmonary involvement in CT images. For each patient, five lung lobes were visually scored as 0 (no involvement), 1 (less than 5% involvement), 2 (5–25% involvement), 3 (25–50% involvement), 4 (50–75% involvement), and 5 (75–100% involvement). The total CT-SS is the sum of the individual lobar scores and ranges from 0 to 25. All CT images were separately reviewed by two radiologists. Any disagreements were resolved through consulting with an attending radiologist with 23 years of experience.
Data pre-processing
Data pre-processing is an imperative step to address irrelevant, redundant, and unreliable data and it could significantly resolve inconsistencies20. In this paper, data pre-processing was performed before the training of the ML models. First, records with more than 70% of missing data were excluded from the dataset. The remaining missing values of continuous and discrete variables were imputed by mean and mode values, respectively. Noisy and abnormal values, errors, and meaningless data were addressed by an expert panel including one health information management expert (HKA), two infectious diseases specialists, and two haematologists.
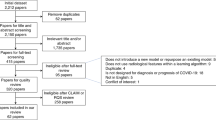
The positive RT-PCR COVID-19 cases were only entered into the study. Negative RT-PCR COVID-19 test, unknown dispositions, discharge or death from the emergency department, missing data > 70%, and age lower than 18 years old were the study exclusion criteria. Figure 1 depicted the schematic of the study inclusion and exclusion criteria. After applying the inclusion/exclusion criteria, the final sample size was 815 patients.
This dataset contains 707 and 108 cases in the survival and death groups, respectively. This imbalanced input would cause delivering biased results toward the dominant class.
The problem of the imbalanced dataset was dealt with using the synthetic minority over-sampling technique (SMOTE) method (https://imbalanced-learn.org/stable/). SMOTE algorithm is the most frequently employed synthetic oversampling which creates synthetic samples of the minority class using randomly selected instances of the minority class and their k nearest neighbors21. In this method, a random data instance with its k nearest neighbors are selected. Then, the second data instance would be selected from the k nearest neighbors set. The new synthetic sample is generated along the line joining the two samples as a convex combination. This procedure would be repeated until the minority class is balanced with the majority one22. Unlike the random oversampling method, the risk of overfitting was avoided in SMOTE method and this method can yield relatively better results23.
Feature selection
The feature selection process is widely used in data mining to determine the most important variables highly correlated with the output variable24. One of the main advantages of using this method is to prevent overfitting of the ML algorithms25. In this study, the most important variables for mortality prediction of COVID-19 were determined using XGBoost, random forest, and Chi-squared tests. The chi-squared test evaluates the statistical differences in the parameters between the deceased and survived groups. The importance scores of the predictors calculated using XGBoost and random forest tests are depicted in Fig. 2. In all feature selection methods, a high score was achieved for strong predictors such as CT-SS, WBC, serum creatinine, etc. But, there were significant discrepancies in the importance scores calculated using XGBoost and random forest tests for some parameters. The dialysis history of the patient has moderate importance in the XGBoost method and the random forest algorithm assigned low importance to it. There was no statistically significant difference in the dialysis history of the patient between the deceased and survived cases (P = 0.011). In another hand, phosphorus concentration in blood samples has low and moderate importance scores in XGBoost and random forest methods, respectively. A strong predictor must first have a statistically significant difference between the deceased and survived cases in order to predict the mortality of COVID-19 patients correctly. According to the observed discrepancies and to determine the predictors which have significant differences between the deceased and survived cases, the independence test of Chi-square was used to determine the most important variables in the mortality prediction of COVID-19 patients. The predictors selected by the Chi-square test had moderate to high importance scores in XGBoost and random forest methods. It is worth mentioning that predictors such as CT-SS have high importance scores in both XGBoost and random forest tests and they have a significant statistical difference between the deceased and survived cases (P < 0.001). The SPSS software (version 23) was used to calculate the Chi-square coefficients and P < 0.01 was regarded as the significant level.
Model development
In this study, the predictive models were developed using eight ML algorithms including the J48 decision tree (J48), support vector machine (SVM), multi-layer perceptron (MLP), k-nearest neighbourhood (k-NN), Naïve Bayes (NB), logistic regression (LR), random forest (RF), and eXtreme gradient boosting (XGBoost)22. These mortality prediction models were implemented using Waikato Environment for Knowledge Analysis (Weka) software (version 3.9.2, University of Waikato, New Zealand). The k-fold cross-validation method was used in the performance evaluation of the developed classifiers. The k-fold cross-validation method has a relatively low level of bias and variation, which makes it a preferred technique. The parameters of the selected ML algorithms for COVID-19 mortality prediction are described in Table 2.
The performances of the predictive models were evaluated using accuracy, precision, sensitivity, specificity, and area under the ROC curve (AUC) metrics. These performance metrics were compared for all ML algorithms to determine the best model for mortality prediction of COVID-19 patients.
Ethical considerations
The ethical committee board of Abadan University of Medical Sciences approved the study (Ethics code: IR.ABADANUMS.REC.1401.124). To protect the privacy and confidentiality of patients, the unique identification information of patients was concealed during all steps of the study. All methods of the present study were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s).
Results
A total of 6854 suspected cases had been referred to Ayatollah Talleghani Hospital, where records of 815 positive RT-PCR patients remained after applying the exclusion criteria. Overall, 54.85% of the enrolled patients were male and the mean age of the study population was 57.22 ± 16.76 years. As was mentioned, the deceased group contained only 108 records (13%) and SMOTE method has been used to balance these data. The number of records in this class was raised to 707 after balancing the dataset.
Feature selection
Twenty-seven features were chosen as the most important and relevant predictors using the independence test of Chi-square. These features included demographics, risk factors, clinical manifestations, laboratory results, and CT-SS data. The list of the most important variables and results of the independence test of Chi-square are demonstrated in Table 3. In this table, the mean decreases in Gini and the importance scores of these variables, calculated using XGBoost and random forest tests, were also listed. Descriptive statistics of these features are listed in Table 4. In this study, in agreement with other studies that have reported some important clinical predictors for COVID-19 patient mortality, the most relevant features included age6,11,15,16,17,26,27,28,29,30, gender6,16,17,26,29,30,31,32,33,34, dry cough6,11,14,17,27,29,32,33,35 as the clinical symptom, underlying diseases including cardiovascular disease6,15,17,27,28,34,36,37, hypertension6,15,17,27,29,30,34,36, diabetes6,15,16,17, neurological disease6,16,17, cancer6,17,26,29,37, laboratory indices such as serum creatinine6,17, RBC6, WBC6,29,35, haematocrit6, absolute lymphocyte count6,14,17,27,31,33,34, absolute neutrophil count6,14,15,17,27,28,33,35,36, calcium6,11,33, phosphor6, blood urea nitrogen6,14,33, total bilirubin6,35, serum albumin6,14,29,33,34, glucose6,17, creatinine kinase6,11,14,29,34,35, activated partial thromboplastic time6, prothrombin time6,34, hypersensitive troponin6,17,28, and CT-SS as the imaging manifestation6,28. These predictors were used as inputs to develop ML-based models for mortality prediction of COVID-19 patients.
On the other hand, smoking6,15,17,28,30, alcohol/addiction6,17,30, sore throat6,15,16,17,26,27,31,33,38, myalgia and malaise6,14,15,16,17,26,28,34, diarrhea and gastrointestinal symptoms6,14,16,17,29,30,36, headache6,11,17,26,30,31,37, platelet count6,14,28,29, and alanine aminotransferase (ALT)6,14,29,31 were the irrelevant features in predicting COVID-19 mortality. Despite the clinical importance of these parameters for treatment success and mortality prediction, many of them could be eliminated from ML analysis and mortality prediction would be performed with fewer factors and the same accuracy.
Evaluation of the developed models
In this study, COVID-19 mortality prediction models were developed using eight ML algorithms including J48, SVM, MLP, k-NN, NB, LR, RF, and XGBoost. These predictive models were built using the best feature subset determined in the previous step. The ML algorithms were trained using the same dataset. The performances of these models were evaluated using sensitivity, specificity, accuracy, precision, and AUC metrics. Results of the performance evaluation for the developed models are listed in Table 5.
Results showed that the RF algorithm yielded better performance to predict the mortality of COVID-19 patients than other ML algorithms. The sensitivity, specificity, accuracy, precision, F1-score, and AUC of the RF algorithm were 100.0%, 94.5%, 97.2%, 94.8%, 97.3%, and 99.9%, respectively. Figure 3 depicted the comparison of the area under the ROC curve for the developed ML algorithms.
Discussion
With the COVID-19 outbreak, the global health system had been faced a life-threatening infection with a wide range of symptoms and complications. For appropriate preparedness against to ongoing global pandemic, it is important to implement intelligent-based models for predicting which patients are at high risk for disease progression and poor outcomes. Timely and accurate identification of COVID‑19 patients with poor outcomes can guide physicians in selecting appropriate treatment and allocating limited hospital resources. AI has created remarkable opportunities to determine the best models for diagnosis, risk analysis, screening, and prediction in response to the challenges ahead of the healthcare system.
AI-based classification of chest scanning images for the automatic diagnosis of COVID-19 was evaluated by Jyoti et al. and Goel et al.39,40. The accuracy of MCA-inspired TQWT-based classification of chest X-ray images to the automatic diagnosis of COVID-19 was 98.82% and 94.64% for small and large datasets, respectively39. The AI system achieved an AUC of 0.92 to screen and detect COVID-19. Its diagnostic sensitivity is equal to a senior thoracic radiologist and for the patients with positive RT-PCR results and normal CT scans, the developed AI model improved the diagnosis of patients while the radiologist had reported them as COVID-19 negative41.
In Asteris et al. study42, AI approaches were used for early prediction of COVID-19 outcomes. They predicted intensive care unit (ICU) hospitalization of COVID-19 patients using artificial neural networks (ANN). Laboratory parameters of the adult patients were used to develop this predictive model. The accuracy, precision, sensitivity, and F1-score of the ANN for the validation cohort were 95.97%, 90.63%, 93.55%, and 92.06%, respectively. Their study showed that an AI-based predictive model could accurately predict ICU hospitalization using only 5 laboratory indices at the time of admission. These studies showed that AI could solve several issues affecting the diagnosis and prediction of COVID-19.
In the present study, we retrospectively analysed the data from a hospital-based registry database to develop and evaluate ML models capable of predicting the risk of COVID-19 mortality. First, demographic information, risk factors, clinical manifestations, laboratory results, and imaging findings were examined to identify the most relevant predictors for mortality prediction of COVID-19 patients. The selected set of the most relevant predictors was used to train and test ML algorithms. In our study, eight ML algorithms including the J48 decision tree, k-NN, MLP, SVM, XGBoost, NB, RF, and LR were used to develop the prediction models based on a dataset of laboratory-confirmed COVID-19 hospitalized patients. The results showed that RF with an accuracy of 97.2%, sensitivity of 100%, precision of 94.8%, specificity of 94.5%, F1-score of 97.3%, and AUC of 99.9% had the best performance among the other ML approaches. Decision tree, XGBoost, k-NN, and MLP models with AUC ≥ 93.9 showed good prediction performances in predicting COVID-19 mortality. Although other ML algorithms are categorized in the last ranks in terms of performance; they had also an acceptable performance (AUC ranged from 81.2 to 88.9%). The SVM model had the weakest performance among ML models (AUC = 81.2%).
The prognostic performances of ML techniques for the mortality prediction of COVID-19 patients have been evaluated in different studies. In Gao et al. study14, the mortality prediction of 2520 COVID-19 hospitalized patients was evaluated using LR, SVM, gradient boosted decision tree (GBDT), and neural network (NN) algorithms. For predicting COVID-19 patients’ physiological deterioration and death up to 20 days, the neural network-based prediction model with an AUC of 97.60% had a better performance than LR, SVM, and GBDT algorithms.
In Zakariaee et al. study6, the prognostic significance of chest CT severity score in mortality prediction of COVID-19 patients was evaluated using k-NN, MLP, SVM, and J48 decision tree ML approaches. The retrospective analysis of the data of 815 COVID-19 hospitalized patients showed that the prognostic performances of the ML algorithms would improve by the integration of CT-SS data with demographics, risk factors, clinical manifestations, and laboratory parameters. SVM was the weakest method in predicting mortality and the k-NN model with an accuracy of 94.1%, sensitivity of 100. 0%, precision of 89.5%, specificity of 88.3%, and AUC of around 97.2% had better performance than MLP, SVM, and J48 decision tree algorithms.
The prognostic performances of decision tree (J48), MLP, k-NN, random forest (RF), and SVM data mining models were also evaluated by Moulaei et al.16. The ML algorithms were developed using demographics, risk factors, and clinical manifestations of 850 COVID-19 hospitalized patients. Although all ML algorithms had good prognostic performances for mortality prediction of COVID-19 patients (AUCs > 96%), the RF model yields the best prognostic results and SVM was the weakest method.
In another study by Moulaei et al.17, the mortality prediction for 1500 COVID-19 hospitalized patients was performed using the decision tree (J48), RF, k-NN, MLP, Naïve Bayes (NB), eXtreme gradient boosting (XGBoost), and logistic regression (LR) algorithms. The results demonstrated that the RF model with an accuracy of 95.03%, sensitivity of 90.70%, precision of 94.23%, specificity of 95.10%, and AUC of 99.02 had the best performance. The results of these studies were in close agreement with our findings. A summary of these studies is presented in Table 6. In this table, developed ML models, datasets, and prognostic performances of ML models to predict mortality in COVID-19 patients are listed.
These ML algorithms were mostly developed using demographics, risk factors, clinical manifestations, and laboratory parameters. Their dataset has no clinical imaging data. Chest CT is one of the most common methods used to evaluate and diagnose patients with suspected SARS-CoV-2 infection41. The systematic review and meta-analysis of chest CT manifestations in COVID-19 patients indicated that vascular enlargement, ground-glass opacities (GGOs), subpleural bands, and interlobular septal thickening were typical CT features of COVID-19 patients. These common patients are less likely to have radiographic abnormalities with over two lobes involved compared to severe patients. For severe patients, vascular enlargement, GGOs, interlobular septal thickening, air bronchogram, consolidation, subpleural bands, crazy-paving pattern, and traction bronchiectasis were the predominant CT features; and traction bronchiectasis, consolidation, interlobular septal thickening, crazy-paving pattern, reticulation, pleural effusion, and lymphadenopathy were related parameters to the severity of the disease43. The severity of pulmonary involvement on CT scans is significantly associated with mortality of COVID-19 patients (OR = 7.124 (95% CI 5.307–9.563)19 and it could predict the patient mortality with a sensitivity of 0.67 [95%CI (0.59–0.75)] and specificity of 0.79 [95%CI (0.74–0.84)]1.
In our study, the importance and efficiency of CT-SS to predict COVID-19 mortality were evaluated using three feature selection methods including XGBoost, random forest, and Chi-squared tests. Our results showed that CT-SS is one of the most important and relevant parameters to predict mortality risk in COVID-19 patients. A high importance score was observed for CT-SS in both XGBoost and random forest tests. In this study, similar to previous studies, deceased patients had higher CT-SSs and there was a significant statistical difference between the deceased and survived cases (P < 0.001). These findings indicate that CT-SS is a strong predictor to predict mortality risk in COVID-19 patients. Thus, the integration of this predictor with demographics, risk factors, clinical manifestations, and laboratory parameters, would improve prognostic performances of the ML algorithms for mortality prediction of COVID-19 patients.
These observations showed ML models are a valuable tool for making reliable clinical decisions and achieving evidence-based patient management to improve patient outcomes and the quality of medical care. The RF predictive models with the more comprehensive dataset including CT-SS could efficiently predict the mortality of COVID-19 patients. This could lead to the optimal use of hospital resources and an increased probability of survival of patients.
Limitations
This study had some limitations. First, the predictive performances of the ML models to predict the mortality of COVID-19 patients were not evaluated in a prospective cohort due to the retrospective nature of the study. Second, this is a single-centre study and patients included were primarily local residents from Abadan, Iran. External validation of the proposed model merits future investigations on bigger and multi-centre databases.
Conclusions
In this study, we compared the prognostic performances of the J48 decision tree, k-NN, MLP, SVM, XGBoost, NB, RF, and LR algorithms for mortality prediction of COVID-19 patients using a more comprehensive collection of features including CTSS data, demographics, risk factors, clinical manifestations, and laboratory findings. Results showed that timely and accurate risk stratification of COVID-19 patients could be performed using ML-based predictive models fed by routine data. The RF predictive model with a comprehensive collection of predictors could lead to promptly targeting high-risk patients on admission and therefore it would improve patient survival probability.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Zakariaee, S. S., Naderi, N. & Rezaee, D. Prognostic accuracy of visual lung damage computed tomography score for mortality prediction in patients with COVID-19 pneumonia: A systematic review and meta-analysis. Egypt J. Radiol. Nucl. Med. 53(1), 1–9 (2022).
Mohanty, S. K. et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19)–anatomic pathology perspective on current knowledge. Diagn. Pathol. 15(1), 1–17 (2020).
Rokni, M., Ghasemi, V. & Tavakoli, Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev. Med. Virol. 30(3), 1–6 (2020).
Smith, E. M. et al. COVID-19 and post-intensive care syndrome: Community-based care for ICU survivors. Home Health Care Manag. Pract. 33(2), 117–124 (2021).
Alimohamadi, Y., Tola, H. H., Abbasi-Ghahramanloo, A., Janani, M. & Sepandi, M. Case fatality rate of COVID-19: A systematic review and meta-analysis. J. Prev. Med. Hyg. 62(2), 311–320 (2021).
Zakariaee, S. S., Abdi, A. I., Naderi, N. & Babashahi, M. Prognostic significance of chest CT severity score in mortality prediction of COVID-19 patients, a machine learning study. Egypt J. Radiol. Nucl. Med. 54(73), 1–9 (2023).
Mamandipoor, B. et al. COVID-19 machine learning model predicts outcomes in older patients from various European countries, between pandemic waves, and in a cohort of Asian, African, and American patients. PLoS Digit. Health 1(11), 1–20 (2022).
Bertsimas, D. et al. COVID-19 mortality risk assessment: An international multi-center study. PLoS ONE 15(12), 1–13 (2020).
Li, X., Liao, H. & Wen, Z. A consensus model to manage the non-cooperative behaviors of individuals in uncertain group decision making problems during the COVID-19 outbreak. Appl. Soft Comput. 99, 1–14 (2021).
Wynants, L. et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 369, 1–22 (2020).
Wu, G. et al. Development of a clinical decision support system for severity risk prediction and triage of COVID-19 patients at hospital admission: An international multicentre study. Eur. Respir. J. 56(2), 1–11 (2020).
Wong, Z. S., Zhou, J. & Zhang, Q. Artificial intelligence for infectious disease big data analytics. Infect. Dis. Health 24(1), 44–48 (2019).
Afrash, M. R., Kazemi-Arpanahi, H., Shanbehzadeh, M., Nopour, R. & Mirbagheri, E. Predicting hospital readmission risk in patients with COVID-19: A machine learning approach. Inform. Med. Unlocked 30, 1–9 (2022).
Gao, Y. et al. Machine learning based early warning system enables accurate mortality risk prediction for COVID-19. Nat. Commun. 11(1), 1–10 (2020).
Yadaw, A. S. et al. Clinical features of COVID-19 mortality: Development and validation of a clinical prediction model. Lancet Digit. Health 2(10), 516–525 (2020).
Moulaei, K., Ghasemian, F., Bahaadinbeigy, K., Sarbi, R. E. & Taghiabad, Z. M. Predicting mortality of COVID-19 patients based on data mining techniques. J. Biomed. Phys. Eng. 11(5), 653–662 (2021).
Moulaei, K., Shanbehzadeh, M., Mohammadi-Taghiabad, Z. & Kazemi-Arpanahi, H. Comparing machine learning algorithms for predicting COVID-19 mortality. BMC Med. Inform. Decis. Mak. 22(1), 1–12 (2022).
Lei, Q. et al. Correlation between CT findings and outcomes in 46 patients with coronavirus disease 2019. Sci. Rep. 11(1), 1–6 (2021).
Zakariaee, S. S., Salmanipour, H., Naderi, N., Kazemi-Arpanahi, H. & Shanbehzadeh, M. Association of chest CT severity score with mortality of COVID-19 patients: A systematic review and meta-analysis. Clin. Transl. Imaging. 10(6), 663–676 (2022).
García, S., Luengo, J. & Herrera, F. Data Preprocessing in Data Mining Vol. 72 (Springer, 2015).
Gnip, P., Vokorokos, L. & Drotár, P. Selective oversampling approach for strongly imbalanced data. PeerJ Comput. Sci. 7, 1–17 (2021).
Dorn, M. et al. Comparison of machine learning techniques to handle imbalanced COVID-19 CBC datasets. PeerJ Comput. Sci. 7, 1–34 (2021).
Erol, G., Uzbaş, B., Yücelbaş, C. & Yücelbaş, Ş. Analyzing the effect of data preprocessing techniques using machine learning algorithms on the diagnosis of COVID-19. Concurr. Comput. 34(28), 1–16 (2022).
Li, J. et al. Feature selection: A data perspective. ACM Comput. Surv. 50(6), 1–45 (2017).
Saeys, Y., Inza, I. & Larranaga, P. A review of feature selection techniques in bioinformatics. Bioinformatics 23(19), 2507–2517 (2007).
Hu, H., Yao, N. & Qiu, Y. Comparing rapid scoring systems in mortality prediction of critically ill patients with novel coronavirus disease. Acad. Emerg. Med. 27(6), 461–468 (2020).
Das, A. K., Mishra, S. & Gopalan, S. S. Predicting CoVID-19 community mortality risk using machine learning and development of an online prognostic tool. PeerJ 8, 1–17 (2020).
Allenbach, Y. et al. Development of a multivariate prediction model of intensive care unit transfer or death: A French prospective cohort study of hospitalized COVID-19 patients. PLoS ONE 15(10), 1–12 (2020).
Assaf, D. et al. Utilization of machine-learning models to accurately predict the risk for critical COVID-19. Intern. Emerg. Med. 15(8), 1435–1443 (2020).
Zhou, Y. et al. Exploiting an early warning Nomogram for predicting the risk of ICU admission in patients with COVID-19: A multi-center study in China. Scand. J. Trauma Resusc. Emerg. Med. 28(1), 1–13 (2020).
Zhao, Z. et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS ONE 15(7), 1–14 (2020).
Yan, L. et al. An interpretable mortality prediction model for COVID-19 patients. Nat. Mach. Intell. 2(5), 283–288 (2020).
Booth, A. L., Abels, E. & McCaffrey, P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod. Pathol. 34(3), 522–531 (2021).
Pan, P. et al. Prognostic assessment of COVID-19 in the intensive care unit by machine learning methods: model development and validation. J. Med. Internet Res. 22(11), 1–16 (2020).
Ryan, L. et al. Mortality prediction model for the triage of COVID-19, pneumonia, and mechanically ventilated ICU patients: A retrospective study. Ann. Med. Surg. 59, 207–216 (2020).
Zhang, Y. et al. Empirical study of seven data mining algorithms on different characteristics of datasets for biomedical classification applications. Biomed. Eng. Online 16(1), 1–15 (2017).
Chin, V. et al. A case study in model failure? COVID-19 daily deaths and ICU bed utilisation predictions in New York state. Eur. J. Epidemiol. 35(8), 733–742 (2020).
Jin, C. et al. Development and evaluation of an artificial intelligence system for COVID-19 diagnosis. Nat. Commun. 11(1), 1–14 (2020).
Jyoti, K. et al. Automatic diagnosis of COVID-19 with MCA-inspired TQWT-based classification of chest X-ray images. Comput. Biol. Med. 152, 1–11 (2023).
Goel, K., Sindhgatta, R., Kalra, S., Goel, R. & Mutreja, P. The effect of machine learning explanations on user trust for automated diagnosis of COVID-19. Comput. Biol. Med. 146, 1–11 (2022).
Mei, X. et al. Artificial intelligence–enabled rapid diagnosis of patients with COVID-19. Nat. Med. 26(8), 1224–1228 (2020).
Asteris, P. G. et al. Early prediction of COVID-19 outcome using artificial intelligence techniques and only five laboratory indices. Clin. Immunol. 246, 1–8 (2023).
Zheng, Y., Wang, L. & Ben, S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J. Med. Virol. 93(1), 241–249 (2021).
Acknowledgements
We thank the research deputy of the Abadan University of Medical Sciences for financially supporting this project (Ethics code: IR.ABADANUMS.REC.1401.124).
Author information
Authors and Affiliations
Contributions
S.S.Z., H.K.A.: conceptualization; data curation; formal analysis; investigation; software; roles/writing—original draft. H.K.A., M.E.: funding acquisition; methodology; project administration; resources; supervision; writing—review and editing. H.K.A., N.N., S.S.Z.: conceptualization; investigation; methodology; validation; writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zakariaee, S.S., Naderi, N., Ebrahimi, M. et al. Comparing machine learning algorithms to predict COVID‑19 mortality using a dataset including chest computed tomography severity score data. Sci Rep 13, 11343 (2023). https://doi.org/10.1038/s41598-023-38133-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38133-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.