Abstract
Drought tolerance is a complex trait in plants that involves different biochemical mechanisms. During two years of study (2019–2020), the responses of 64 arugula genotypes to drought stress were evaluated in a randomized complete block design with three replications under field conditions. Several metabolic traits were evaluated, i.e. relative water content, photosynthetic pigments (chlorophyll and carotenoids), proline, malondialdehyde, enzymatic antioxidants (catalase, ascorbate peroxidase, and peroxidase), total phenolic and flavonoid contents and seed yield. On average, the drought stress significantly increased the proline content (24%), catalase (42%), peroxidase (60%) and malondialdehyde activities (116%) over the two years of study. As a result of the drought stress, the seed yield (18%), relative water content (19.5%) and amount of photosynthetic pigments (chlorophyll and carotenoids) dropped significantly. However, the total phenolic and flavonoid contents showed no significant changes. Under drought stress, the highest seed yields were seen in the G50, G57, G54, G55 and G60 genotypes, while the lowest value was observed in the G16 genotype (94 g plant−1). According to the findings, when compared to the drought-sensitive genotypes, the drought-tolerant arugula genotypes were marked with higher levels of proline accumulation and antioxidant enzyme activity. Correlation analysis indicated the positive effects of peroxidase, catalase and proline on seed yield under drought conditions. These traits can be considered for the selection of drought-tolerant genotypes in breeding programs.
Similar content being viewed by others
Introduction
Arugula (Eruca sativa Mill.) (Brassicaceae) is an annual, herbaceous oil crop and has a broad range of valuable industrial and pharmaceutical applications1. According to the available literature, arugula originated in the Mediterranean region. Today, arugula is widely cultivated in different regions, such as North Africa, Eastern Asia (Pakistan, Afghanistan, and India), and the Middle East (Iraq, Iran, and Syria)2. The extracted arugula oil can be used for manufacturing biodiesel and various other industrial products3.
The seeds and leaves of the plant have been used in different medicinal and industrial applications1. Arugula is considered a source of several phytochemicals with health-promoting properties (e.g. fibers, carotenoids, flavonoids, glucosinolates and vitamin C)4. The plant reportedly has different medicinal properties. It is considered a digestive, astringent, diuretic, tonic, emollient, depurative, laxative, and stimulant agent1. Moreover, the spicy taste of the buds and leaves of this plant have broadened its use in foods and salads1,2. Nowadays, arugula is being widely cultivated around the world due to its importance in different fields, such as agriculture, medicine and various industries5.
Drought stress has been identified as one of the most critical environmental stresses, and leads to harmful effects on crop performance by restricting plant yield and productivity6. Drought-prone environments adversely affect plant yield and quality7. Certain factors including genotype, species, developmental stage, stress duration and severity can impact plant response to water limitation8. Different physiological and molecular processes are involved when plants respond to the deleterious effects of drought stress through scavenging7, such as the accumulation of various osmolytes and secondary metabolites (SMs), the increase of different enzymatic and non-enzymatic antioxidant activities, and the activation of certain mechanisms to compensate the water content loss in leaves9.
Under environmental stresses, such as the drought stress, an increasing trend has been observed in the production of certain reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide (O2·), hydroxyl (•OH) and alkoxy radical (RO·)10. To scavenge the deleterious effects of ROS, the enzymatic and non-enzymatic defense systems have evolved under environmental stresses10. Therefore, certain enzymes such as catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX) and glutathione reductase (GR) have been activated in the scavenging system to minimize the oxidative damages to plants9,11. On the other hand, the different SMs that act as non-enzymatic antioxidants, such as leaf pigments (e.g. carotenoids), and different phenolic compounds (e.g. phenolics, flavonoids and anthocyanins) have a protective role in avoiding ROS generation12. The production of SMs by a plant species depends to a great extent on the differential impact of the environmental stress and its severity on the metabolic pathways associated with their biosynthetic pathways7. Several studies showed that stressed plants created higher concentrations of SMs, compared to non-stressed plants13,14.
A deeper understanding of drought-tolerant mechanisms in plants can be used to select superior genotypes to obtain better productivity under drought stress conditions. The breeding activities that have been carried out on arugula are quite limited. The number of studies that have examined the responses of certain traits of the arugula plant to drought stress are few15,16. To the best of our knowledge, there is no information available regarding the effects of drought stress on the seed yield (SY) and the different biochemical traits of arugula.
One of the major goals of plant breeding when faced with climate change is to determine and improve drought-tolerant genotypes. This investigation is a continuation of our previous efforts, and provides further insight regarding the response of different physio-biochemical traits to drought stress in a wide range of arugula germplasm. Therefore, the present study is the first research to assess the effects of drought stress on seed yield and the changes that occur in the physiological and biochemical traits of arugula genotypes. The objectives of the present study were: (1) to investigate the physio-biochemical and antioxidant responses of arugula genotypes to drought stress and their relations with seed yield, and (2) to identify superior arugula genotypes best suited for drought tolerance improvement in arugula germplasm for future programs.
Results
The analysis of variance showed that the effects of years, genotypes, and environment (non-stress and drought stress) were significant on all traits (Table 1). The year × environment interaction was significant for proline content, SY, Chla, TChl, carotenoids, and CAT (Table 1). With the exception of TPC, TFD, RWC, Chla, and APX, a significant interaction of genotype × environment was detected in all other studied traits (Table 1). The year × genotype interaction was non-significant for Chla, APX, RWC, TPC, and TFD (Table 1). Significant year × genotype and environment × genotype interactions were seen in SY, proline, Chlb, TChl, Car, POX, CAT, MDA and H2O2 (Table 1). In addition, the year × genotype × environment interaction was significant for SY, proline, Chlb, Car, POX, CAT, and MDA (Table 1).
Effects of drought stress on the studied traits
The investigated traits were compared under two environmental conditions (non-stress and drought stress) (Table 2). As shown by the comparisons of the interaction effects of year × environment, the values obtained for the investigated traits in 2020 were greater than those calculated in 2019. According to the results, the drought stress had a diminishing impact on the photosynthetic pigments (TChl, Car, and Chla) and SY in both years, whereas the levels of the other traits, such as proline content, RWC, MDA, and antioxidant enzyme activity (POX, APX, and CAT) increased significantly (Table 2).
Seed yield
The means of the studied genotypes showed a significant SY reduction under drought stress conditions (Table 2). A wide range of variation was observed in SY under the non-stress (107.5–185.17 g plant−1) and drought stress (94–151.5 g plant−1) conditions (Tables 3, 4). Under non-stress conditions, the highest SY was seen in G58 (185 g plant−1) (Table 3). However, the highest SY under drought stress was recorded in G50 (151 g plant−1) (Table 4), while the lowest value was observed in G16 (94 g plant−1) (Table 4). At the end of the two-year study period, the average SY of the arugula genotypes was higher in the second year, which could be justified by the genotype × year interaction.
Relative water content
Water deficiency induced a significant decrease in RWC over the two years of study (Table 2). Under non-stress conditions, the G12 genotype had the highest amount of RWC (90.69%) (Table 3). Under drought stress conditions, the highest RWC values were seen in the G37 (76.57%) and G38 (76.35%) genotypes, while the lowest values were observed in the G10 (53.55%), G1 (53.36%), and G24 (53.02%) genotypes (Table 4).
Proline
In the two years of study, significant increases were observed under drought stress conditions in the proline concentrations of the studied genotypes (Table 2). Under non-stress conditions, the highest and lowest mean proline content values (0.22 μmolg−1 FW and 0.18 μmolg−1 FW) were recorded in the G48 and G27 genotypes, respectively (Table 3). However, under drought-stress conditions, the G58 and G32 genotypes had the highest and lowest mean proline contents of 0.28 μmolg−1 FW and 0.22 μmolg−1 FW, respectively (Table 4).
Photosynthetic pigments
The mean comparisons revealed a significant decrease in Chla, Chlb, TChl, and carotenoid contents under drought stress during the two-year study period (Table 2). Under non-stress conditions, the highest content values for both TChl (0.36 mg g−1 FW) and Chla (0.27 mg g−1 FW), Chlb (0.11 mg g−1 FW) and carotenoids (0.51 mg g−1 FW) were observed in G30, G10 and G29, respectively (Table 3). On the other hand, the highest content values of TChl (0.21 mg g−1 FW), Chla (0.14 mg g−1 FW), Chlb (0.08 mg g−1 FW) and carotenoids (0.40 mg g−1 FW) under drought stress were observed in the G37, G39 and G37 genotypes, respectively. The lowest content values of TChl (0.12 mg g−1 FW) and carotenoids (0.24 mg g−1 FW) were seen in G50 and G25, respectively (Table 4).
Enzymatic antioxidants
During the two-year study period, the APX, POX, and CAT activities of the drought-stressed samples increased significantly (Table 2). The APX activity values ranged from 3.19 units mg−1 protein in G62 to 6.48 unit mg−1 protein in G37 under non-stress conditions (Table 3). Under drought stress conditions, the highest APX activity values were recorded in G54 (8.46 units mg−1 protein) (Table 4), and the lowest APX values were observed in G23 (5.90 units mg−1 protein) and G11 (5.834 units mg−1 protein) (Table 3). Under non-stress conditions, the CAT activity values ranged from 0.09 units mg−1 protein in G64 to 0.13 units mg−1 protein in G9 (Table 3). However, these values varied from 0.17 units mg−1 protein in G32 to 0.22 units mg−1 protein in G37 under drought stress conditions (Table 4). The POX activity values ranged from 0.11 µmol min−1 mg−1 protein in G14 to 0.24 µmol min−1 mg−1 protein in G45 under non-stress conditions (Table 3). Under drought stress conditions, these values varied between 0.19 µmol min−1 mg−1 protein in G7 and 0.30 µmol min−1 mg−1 protein in G56 (Table 4). The MDA concentration of the drought-stressed samples increased significantly over the study period (Table 2). The lowest MDA concentration (0.05 nmol g−1 FW) was seen in the G27 genotype under non-stress conditions (Table 3). Under drought stress conditions, the highest (0.21 nmol g−1 FW) and lowest (0.13 nmol g−1 FW) accumulations of MDA were observed in G50 and G24, respectively (Table 4).
Total phenolic and flavonoid contents
When compared to the non-stress conditions (Table 2), a non-significant increase was observed in the TPC and TFD values of the drought-stressed samples (Table 3). Under non-stress conditions, TPC varied from 20.98 mg TAE g−1 DW in G44 to 42.12 mg TAE g−1 DW in G6 (Table 3). The TPC values of the drought-stressed samples ranged between 33.15 mg TAE g−1 DW in G23 and 51.23 mg TAE g−1 DW in G3 (Table 4). Under non-stress conditions, the TFD values varied from 1.98 mg QE g−1 DW in G14 to 6.26 and 6.23 mg QE g−1 DW in G10 and G36, respectively (Table 3). The TFD values ranged from 3.22 and 3.25 mg QE g−1 DW in G9 and G24, respectively, to 7.19 mg QE g−1 DW in G37 under drought stress conditions (Table 4).
Correlation analysis
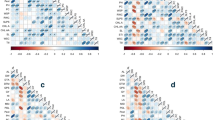
The simple correlation coefficients were calculated for all traits under both non-stress and stress conditions (Fig. 1). Under non-stress conditions (Fig. 1a), the highest correlation coefficients were observed between TChl and Chla (0.98**), followed by TChl and Chlb (86**). The seed yield had a significant positive correlation with POX (0.18*), but a significant negative correlation with TPC (− 0.32**) and TFD (− 0.32**). Under drought-stress conditions, the highest significant positive correlation (0.89**) was observed between Chlb and TChl (Fig. 1b), followed by Chla and Car (0.82**). Seed yield had the highest significant and positive correlations with POX (0.73**), CAT (0.53**), proline (0.57**) and MDA (0.47**).
Principal component analysis
Principal component analysis (PCA) was applied to distinguish the arugula genotypes with regard to their physio-biochemical and biochemical traits under the two drought stress and non-stress conditions. Accordingly, under normal conditions, the first principal component (PC1) explained 27.17% of the total variance in the dataset, while the second principal component (PC2) showed 18.53% of the variance (Fig. 2a). Under non-stress conditions, the PCA biplot identified three predominant groups mainly discriminated by their physio-biochemical traits. The first group (red group) consisted of the G9, G10, G7, G34, G42, G64, G17, G46, G62, G15, G20, G60, G29, G63, G51, G44, G30, G56, G27 and G42 genotypes. The red group genotypes in the PCA biplot had medium SY and high contents of chlorophyll, carotenoids and TFD. The second group (green group) consisted of the G1, G2, G3, G4, G5, G6, G8, G11, G12, G14, G25, G26, G28, G32, G33, G35, G36, G37, G38, G37, G53, and G55 genotypes. The green group genotypes in the PCA biplot had low SY, low chlorophyll and carotenoid contents, but high contents of TPC, CAT, RWC, MDA, APX and proline. The genotypes of the third group (black group) in the PCA biplot were distinguished by their high seed yield and low to moderate POX content (Fig. 2a).
(a) Biplot drawn based on the first and second components obtained from principal component analysis using the seed yield and physiological traits of 64 arugula genotypes under non-stress conditions averaged over the two study years (2019 and 2020). (b) Biplot drawn based on the first and second components obtained from principal component analysis using the seed yield and physiological traits of 64 arugula genotypes under drought stress conditions averaged over the two study years (2019 and 2020). SY: Seed yield; Chla: Chlorophyll a; Chlb: Chlorophyll b; TChl: Total chlorophyll; Car: Carotenoids; MDA: Malondialdehyde; APX: Ascorbate peroxidase; CAT: Catalase; POX: Peroxidase; RWC: Relative water content; TPC: Total phenolics content; TFD: Total flavonoids.
The results of the second biplot explained the relationship of the traits and genotypes under drought stress. The findings showed that the first two PCs (PC1 and PC2) explained 52.52% of the total variance (Fig. 2b). The first PC (PC1) showed 37.76% of the total variation and had a positive correlation with all studied traits (Fig. 2b), while PC2 explained 14.76% of the total variance. With the exception of Chla, TChl, Car and MDA, PC2 showed a high positive contribution by all other studied traits (Fig. 1). The biplot identified three main groups discriminated by the traits under study. Due to having the highest TChl and Chlb content values, the G39 genotype was separated from the other groups. The G5, G47, G63, G54, G55, G64, G48, G54, G62 and G46 genotypes were allocated to the first group (green group), and had high accumulations of photosynthetic pigments (chlorophyll and carotenoids). The second group (red group) consisted of the G55, G64, G46, G41, G53, G60, G36, G49, G56, G49, G42, G51, G33, G58, G38, G59, G57, G44, G34 and G35 genotypes. These genotypes were characterized by their higher values of seed yield, RWC, TFD, TPC, POX, APX and MDA. The samples of the third group (black group) had lower values of seed yield and all other traits. The genotypes in this group had significantly lower TFD, TPC and RWC. Nevertheless, the G50, G57, G56, G58, G45, G38, G44 and G49 genotypes were identified as superior arugula genotypes that could produce the highest SY under both normal and drought stress conditions. According to the PCA results under non-stressed conditions, TChl, Chla, Chlb and carotenoids were the important components of PC1, while the main components of PC2 were proline, TPC, RWC and TFD (Table 5). However, under drought stress conditions, the important components of PC1 were carotenoids, CAT, proline and SY, while MDA and proline were the main components of PC2 (Table 5).
Hierarchical cluster analysis (HCA)
To evaluate the relationships among the studied arugula genotypes, a cluster analysis was performed based on the studied traits under non-stress and drought stress conditions (Fig. 3a,b). Based on this analysis, the non-stressed arugula genotypes were classified into three distinct groups (Fig. 1a). The first group consisted of 20 genotypes: G36, G64, G51, G42, G34, G44, G20, G29, G21, G63, G60, G46, G62, G15, G61, G64, G56, G30, G10, G9, and G7 (red color). The G48, G47, G45, G16, G43, G39, G40, G50, G41, G54, G49, G59, G33, G52, G13, G56, and G18 genotypes comprised the second group (green color). The remaining 27 genotypes were allocated to the third group (blue color) (Fig. 3a).
Under drought stress conditions, the G39 genotype was separated from the other genotypes of the first group (Fig. 3b). The first group (blue color) consisted of 10 genotypes: G63, G60, G48, G47, G49, G62, G46, G5, G37, and G43. The G38, G36, G35, G34, G41, G50, G54, G55, G56, G57, G51, G58, G59, G52, G61, G40, G44, G45, G64, G42 and G53 genotypes comprised the second group (purple color). The remaining 32 genotypes were allocated to the third group (red color) (Fig. 3b). Based on the hierarchical cluster analysis results, the G39 genotype was separated from the other genotypes of the first group due to its exceptionally high levels of Chlb and TChl under drought stress conditions. The first group had high values of chlorophyll pigments and carotenoid content, but a moderate seed yield value. The arugula genotypes of the second group (purple color) showed high values of CAT, APX, TFD, TPC, RWC and a higher value of seed yield. The remaining genotypes (red color) had low values of SY and all other studied traits (Fig. 3b, Table 4).
Discussion
In the near future, changes in climatic conditions will result in the common occurrence of droughts, thereby posing a serious threat to food security8. Drought has an adverse effect on the metabolic process and seed yield of plants as it impacts their water relation, photosynthesis, and nutrient uptake6. The incidence of genotype × environment interaction poses difficulties for the improvement of new drought-tolerant genotypes in crops. Therefore, the selection of drought-tolerant genotypes, and the elucidation of the underlying mechanisms by plant-breeding scientists are both essential to the increase of agricultural production in arid and semi-arid regions. The findings of this study showed a highly diversified array of genotypic responses to both non-stress and drought stress conditions with regard to all studied traits. The genotype comparisons under drought stress and non-stress conditions showed that certain genotypes had a cross-over genotype × environment interaction (G × E). This means that some genotypes are appropriate for typical environments, but not suitable for water-deficit conditions. For example, the SY values were 162.33 g−1 plant and 178 g−1 plant in the G14 and G18 genotypes under non-stress conditions, respectively (Table 3), but their SY under stress conditions were 99.3 g−1 plant and 97.1 g−1 plant, respectively (Table 4). The data for SY suggested that the G50 and G57 genotypes were the most drought-tolerant (Table 4). Therefore, drought-tolerant genotypes can be considered as superior and seem to be suitable candidates for breeding programs in drought-stressed environments. The decrease in SY under drought stress can be related to the reduction of photosynthetic activities, due to the stomatal closure and limited carbon dioxide uptake17.
Moreover, lipid peroxidation was induced by apoplastic ROS, making MDA a reliable marker for membrane damage, particularly under drought conditions9. Therefore, under environmental stresses, MDA may have certain damaging impacts on plants, such as the interruption of photosynthetic pigments, inhibition of enzyme activity, protein denaturation, and programmed cell death18. Similarly, a number of previous studies reported enhanced levels of MDA in other plant species under drought stress conditions19,20. According to the findings of these studies, drought-induced oxidative stress was moderate to severe at the flowering stage of these genotypes.
Under drought stress, the RWC serves as a marker for the leaf water status. The RWC may also indicate the balance between the water absorbed by the plant and the water consumed through transpiration. In this study, a considerable decrease in RWC was observed in all genotypes. However, the amount of RWC loss varied among different genotypes. The results of this research showed a non-significant correlation between SY and RWC (0.08) under drought stress, which were different from the findings of other studies conducted on other species, such as safflower21 and wheat22. These dissimilarities could be due to the differences in stress duration, drought intensity, genotype, and species23. This may be because, rather than conserving water in their leaves, the arugula genotypes apply other defensive mechanisms to tolerate drought. Therefore, RWC cannot be considered as a good selective biochemical marker for drought-tolerance in these arugula genotypes. The reduction of RWC under drought was greater in G24 (53.02%), which showed the water status in this genotype to be more susceptible to drought than the other genotypes. On the other hand, G37 and G38 were considered the best genotypes for conserving water content under drought stress conditions.
Photosynthetic pigments (such as carotenoids and chlorophylls) play a crucial role in photosynthesis, as they protect the photosynthetic apparatus from the harmful impacts of ROS under drought stress24. Carotenoids have a significant role in hindering oxidative damage by quenching chlorophyll triplets and singlet oxygen25. The decrease in photosynthetic pigments in the drought-stressed arugula genotypes may be due to certain drought-induced events. For example, the rapid decomposition of chlorophyll could subsequently decrease the carbon dioxide exchange rate and damage the chloroplast structures24,26. The chlorophyll and carotenoid content decrease in these genotypes was similar to those reported in safflower19,21, fennel17, canola27, and Amaranthus tricolor28. The reduction in photosynthetic pigments can directly limit photosynthetic potential, and consequently reduce seed production17. This confirms the positive correlation of SY with Chla (r = 0.29**), TChl (0.31**) and carotenoids (r = 0.41**) under drought stress (Fig. 1b). In this regard, our data highlighted a noteworthy photosynthetic performance of the G24 genotype under drought stress.
Proline plays a vital role in stabilizing sub-cellular structures and increasing drought stress tolerance through osmotic pressure adjustment29,30. Therefore, greater accumulations of proline are linked to increased stress tolerance. As a molecular chaperone, proline acts as an antioxidative defense molecule. Proline scavenges reactive oxygen species (ROS) to activate specific gene functions that are crucial for the plant’s recovery from stresses30. In certain industrial crops, such as safflower19,21, fennel17, cotton31, and maize32, an enhanced level of proline (as an osmolyte metabolite) has been reported to be a marked response to drought stress. In cases as such, the increase in proline content under drought could be due to the induction of proline biosynthesis, as well as the inhibition of its oxidation under drought stress30. In this study, the significant correlation between proline and SY (0.56**) demonstrated the important adjusting role of this osmolyte under drought stress in the arugula plant. Also, the positive correlation of proline with POX (0.47**), APX (0.42**) and CAT (0.59**) suggested the effective role of proline in maintaining cell turgidity under drought, and the subsequent enhancement of antioxidant enzyme activity (POX, APX and CAT)30,32. Under drought stress, the correlation between the phenolic traits (TFD and TPC) and proline was non-significant. This can be due to the independent biosynthetic pathways of the phenolic compounds (shikimate/phenylpropanoid pathway)33 and proline (glutamate pathway) in this genus.
Plant enzymes such as APX, POX and CAT are able to scavenge H2O2 with different mechanisms and suppress its toxic effects34. The scavenging of H2O2 production under drought stress conditions is a common phenomenon, which is done by both the enzymatic (POX, CAT, and APX) and non-enzymatic antioxidants35. In this study, the drought stress caused an increase in enzymatic activity (CAT, POX and APX) (Table 2), which was in agreement with the results of other reports13,21,36,37. Another study reported the efficient role of APX in scavenging H2O2 in the ascorbate–glutathione cycle of plant cells under drought stress conditions34. The significant positive correlation of the studied enzymatic antioxidants (APX, POX and CAT) with SY, and the positive correlation of MDA with CAT (0.52**), POX (0.29*) and APX (0.44**) under drought stress, may be due to the adequate effects of these antioxidants, as they can scavenge the ROS species and alleviate the harmful effects of the drought stress. These results are similar to the findings of previous reports on other species21,28,38. Therefore, these enzymatic antioxidants (POX, APX and CAT) can be used as efficient and economical biochemical indices to either screen or enrich arugula germplasm for drought tolerance in the early growth stages. Catalase is considered as a key ancillary component of photosynthesis in green leaves by preventing ROS accumulation39. When plants are subjected to drought stress, the maintenance and subsequent increase of CAT activity in the plant leaves can result in the removal of the produced photo respiratory H2O2. This confirms the significant increase of CAT activity under drought stress in the current study. The significant increase of CAT activity appears to be linked to lower oxidative damage, which was previously detected in plants with increased CAT activity.
Moreover, CAT activity had a positive correlation with RWC (0.34**), which was similar to reports by Alizadeh Yeloojeh et al.21 on safflower and Merwad et al.40 on cowpea (Vigna unguiculata). An association between increased CAT activity and greater water retention in the arugula leaves was observed. Therefore, genotypes that maintain increased CAT activity under drought stress in their leaves may show a greater water retention ability and higher stress tolerance.
Phenolic compounds have antioxidant characteristics and scavenge the free radicals generated under drought stress41. The positive correlations of RWC with TFD (0.62*) and TPC (0.68**) under drought stress and non-stress conditions (Fig. 1) demonstrate the positive effects of TFC and TPC as secondary metabolites in maintaining the chloroplast content and turgor pressure of both non-stressed and stressed arugula leaves41. When compared to non-stressed conditions, the non-significant changes in TPC and TFD under drought stress may be due to the involving effects of other factors, such as the degradation of photosynthetic pigments, which play an important role in their synthesis42. Therefore, it may be concluded that polyphenolics act as subtracts for the synthesis of enzymes in the antioxidant defense network43. The second hypothesis suggests that plants, instead of using the phenolic compounds, primarily attempt to reduce their ROS levels with antioxidative enzymes43.
The arugula genotypes mostly showed a similar stress response pattern, which included the accumulation of proline, oxidative damage to membrane lipids, elevation in hydrogen peroxide content, increase of the POX, APX, and CAT antioxidant activities and proline content, and the reduction of photosynthetic pigments (carotenoids and chlorophyll). Distinct regulation mechanisms were observed under drought-induced oxidative stress by the various trends of these enzymatic and non-enzymatic antioxidants.
The results of the clustering analysis were moderately consistent with the identifications of the three groups in PCA. Under normal irrigation conditions, the cluster analysis and PCA allocated 49% of the genotypes to the same groups. However, under drought stress conditions, the cluster analysis and PCA placed approximately 86% of the genotypes in the same groups. The hybridization between the first and third group genotypes that had the greatest distance under stress conditions, could lead to the production of hybrids with increased secondary metabolites and heightened enzyme activities, which is highly beneficial to the development of arugula in dry areas.
Conclusion
According to our observations on a global collection of arugula genotypes, the drought stress caused a broad range of variation in seed yield and various other physio-biochemical traits, such as the phenolic compounds, enzymatic antioxidants, RWC, and MDA content. Based on the current results, the highly active enzymatic antioxidants APX, CAT, and POX were responsible for the higher drought-tolerance in arugula genotypes. Based on the principal component analysis, the G50, G57, G54, G55 and G60 genotypes could produce higher seed yields under drought stress conditions. This new information may be used to breed drought-tolerant genotypes by selecting superior genotypes of arugula. Subsequent researches should be conducted in different geographical regions and under different environmental stress conditions to confirm the superiority of genotypes that contain valuable genes.
Materials and methods
Ethics statement
The plant seeds were collected and handled in accordance with all relevant guidelines.
Plant materials and Experimental design
The seeds of the non-Iranian accessions were collected from the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Germany. After considering the descriptions of each genotype (Table S1), the genotypes were planted based on a completely randomized block design with three replications under both non-stress and drought conditions from October to June of 2018–2019 and 2019–2020 at Isfahan University of Technology, Iran (32° 32′ N, 51° 23′ E, 1630 asl). Each plot, as an experimental unit, consisted of two rows that were 2 m in length and 70 cm apart. The plants were spaced 10 cm apart within the rows. The soil was characterized as silty clay loam with a bulk density of 1.3 g cm−3 and a pH range of 7.4–7.9. The monthly temperature (mean, max, and min) and rainfall values are shown in Fig. S1a,b. The reported mean annual temperatures and precipitations were, respectively, 19.4 °C and 98.6 mm in 2019, and 19.41 °C and 159 mm in 2020.
Drought stress assay
The drought stress treatments were applied as described by Nikzad et al.44. The irrigation depth was calculated using the following formula: I = [(FC − θ)/100] D × B); where I is irrigation depth (cm), FC (− 0.03 MPa) is soil gravimetric moisture percentage at field capacity (22%), θ (− 1.5 MPa) is soil gravimetric moisture percentage at irrigation time (10%), D is root-zone depth (50 cm), and B is soil bulk density at the root zone (1.3 g cm−3)45. Based on the plant’s water requirements, the non-stress and drought stress treatments were irrigated uniformly and simultaneously from the beginning of the trial until the initial stages of flowering (10%). Later, the non-stress and drought stress treatments were irrigated after 50% and 80% of water drainage, respectively. A pressurized irrigation system using polyethylene drip-irrigation tapes (16 mm diameter) was applied beside each planting row. In each plot, the mature and young apical leaves of the plants (from the middle of each row) were selected at the maturity stage to measure the biochemical traits. The leaf samples were then frozen and stored at − 80 °C for further analysis. Sampling was done using 10 randomly selected plants from each treatment. Finally, the plants were harvested after edge effect removal and the grain yields were determined accordingly.
RWC measurement (%)
The following equation was used for determining the relative water content (RWC): RWC (%) = [(FW–DW)/(TW–DW)] × 10017. Turgid weight (TW) was determined after the imbibition of the leaves in distilled water for 4 h. Dry weight (DW) was determined after the incubation of the leaves in an oven at 80 °C for two days.
Proline assay
To perform the proline assay46, 3 ml of sulfosalicylic acid (3% w/v) was added to the leaf samples (0.2 g). The mixture was then centrifuged at 18,000× g for 15 min. The supernatant (2 ml) was then put into a new tube and 2 ml of glacial acetic acid and 2 ml of the ninhydrin reagent were added to the test tubes. The tubes were immersed in a 100 °C hot water bath for an hour, after which the solution was cooled immediately on ice. Toluene (4 ml) was added into the mixture to stop the reaction. The toluene phase absorbance was measured at 520 nm using a spectrophotometer.
Chlorophyll and carotenoids assay
The chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (TChl), and carotenoid (Car) contents were assayed according to methods described by Lichtenthaler47. The first step was to squash fresh leaf samples (0.2 g) in a mortar by adding 10 ml of acetone 80% (Merck, Com.) until they were completely colorless. The solutions were then centrifuged at 6000× g for 10 min. A spectrophotometer was used to record the absorption value of the leaf extract solution at 662 nm for Chla, at 645 nm for Chlb, and at 470 nm for carotenoids using a UV–Vis spectrophotometer (UV-1800, Shimadzu). The results were expressed as mg of pigment per gram of leaf fresh weight.
Malondialdehyde assay
To measure the malondialdehyde (MDA) content19, powdered leaf samples (0.5 g) were mixed with 5 ml of potassium phosphate buffer (50 mM, pH = 7). After centrifuging the homogenate at 15,000× g for 10 min at 4 °C, 1.5 ml of thiobarbituric acid (TBA) 0.5% in trichloroacetic acid (TCA) 20% (w/v) was added to 0.5 ml of the supernatant. The mixture was first heated at 95 °C for 30 min and then kept in ice for 30 min to stop the reaction. Finally, the absorption value was measured at 532 nm and 600 nm for the correction of nonspecific turbidity. The MDA content was expressed as nanomol g−1 FW.
Extract preparation for antioxidant enzymes assay
Fresh leaf samples (0.2 g) of each treatment were ground using a mortar and pestle. Afterwards, a 2 ml sodium-phosphate buffer (10 mM, pH = 7) that included 50 mM potassium phosphate, 1 mM ethylene diamine tetra acetic acid (EDTA), 2% polyvinyl pyrrolidone, 2 mM α-dithiothreitol (DTT), 50 mM Tris HCl, 0.2% triton x-100 was added to the samples. The mixture was centrifuged at 12,000× g for 30 min. The supernatant was collected for antioxidant enzyme evaluation.
Catalase assay
Hydrogen peroxide (H2O2) consumption was utilized to measure the catalase activity (CAT) spectrophotometrically at 240 nm48. The assay buffer included 50 ml of enzyme extract, 15 mM of H2O2, and 50 mM of potassium phosphate buffer (pH 7.0).
Ascorbate peroxidase assay
The ascorbate peroxidase (APX) assay buffer contained H2O2 (0.5 mM), enzyme extract (50 ml), potassium phosphate buffer (50 mM) (pH 7.0), and ascorbate (5 mM)49. Based on the reduction in absorbance, the APX activity was measured spectrophotometrically at 265 nm.
Peroxidase assay
After making minor modifications, the peroxidase (POX) activity was measured specifically with guaiacol49. The increase in absorbance at 470 nm was recorded in a mixture of 0.1 ml of enzyme extract with 3 ml of phosphate buffer (50 mM; pH = 7) containing 0.05 ml guaiacol and 0.03 ml H2O2. Enzyme activity was expressed as units enzyme activity per mg of protein.
Total phenolic and flavonoid content
To prepare a methanolic extract of the arugula genotypes, 250 mg of powdered plant was extracted with 10 ml of 80% methanol by slow shaking. The obtained solution was filtered and maintained for further analysis.
To obtain the total phenolic content (TPC), 2.5 ml of the Folin–Ciocalteu reagent (1:10 diluted with distilled water) and 2 ml of 7.5% sodium carbonate solution were added to 0.5 ml of the methanolic extract according to Priyanthi and Sivakanesan50. The mixture was incubated at 45 °C for 15 min. Finally, the absorbance of the solution was read at 765 nm using a spectrophotometer. The TPC value was expressed as mg of tannic acid equivalent (TAE) per gram of each extract on a dry basis.
To estimate the total flavonoid content (TFD), the diluted extract (125 µl) was mixed with 300 µl of 5% NaNO2 solution and incubated for 5 min19. The mixture was then blended with 600 µl AlCl3 (10% w/v). In the end, the blend was mixed with 2000 µl of NaOH (1 M) and 2000 µl distilled water and used to reach the final volume of the solution. The observations were recorded at 510 nm. The TFD content was expressed as mg of quercetin equivalents (QE) per gram of each extract on a dry basis.
Statistical analyses
The combined data from the two years of study were subjected to analysis of variance (ANOVA) using the SAS statistical software (ver. 9.4, SAS Institute Inc., Cary, NC, USA). Mean comparisons were conducted using Fisher’s least significant difference (LSD) test at P < 0.05. Correlation analysis and principle component analysis (PCA) were done using the R-software (ver. 3.4.3). Cluster analysis was conducted using Ward’s method based on linkage distances with Stat graphics Centurion ver. 18.1.12.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Garg, G. & Sharma, V. Assessment of fatty acid content and genetic diversity in Eruca sativa (L.) (Taramira) using ISSR markers. Biomass Bioenergy 76, 118–129. https://doi.org/10.1016/j.biombioe.2015.03.010 (2015).
Pagnotta, E., Ugolini, L., Matteo, R. & Righetti, L. Bioactive compounds from Eruca sativa seeds. Encyclopedia 2, 1866–1879. https://doi.org/10.3390/encyclopedia2040129 (2022).
Golkar, P. & Abdollahi Bakhtiari, M. Evaluation of genetic diversity in the world collection of Eruca sativa L. using oil content, fatty acids and molecular markers. Ind. Crops Prod. 148, 112280. https://doi.org/10.1016/j.indcrop.2020.112280 (2020).
Yang, T., Samarakoon, U., Altland, J. & Ling, P. Photosynthesis, biomass production, nutritional quality, and flavor-related phytochemical properties of hydroponic-grown arugula (Eruca sativa Mill.) standard’ under different electrical conductivities of nutrient solution. Agronomy 11, 1340. https://doi.org/10.3390/agronomy11071340 (2021).
Jia, C. Z., Wang, J. J., Chen, D. L. & Hu, X. W. Seed germination and seed bank dynamics of Eruca sativa (Brassicaceae): A weed on the northeastern edge of Tibetan Plateau. Front. Plant Sci. 13, 820925. https://doi.org/10.3389/fpls.2022.820925 (2022).
Takahashi, F., Kuromori, T., Urano, K., Yamaguchi-Shinozaki, K. & Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 11, 556972. https://doi.org/10.3389/fpls.2020.556972 (2020).
Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 52, 39. https://doi.org/10.1186/s40659-019-0246-3 (2019).
Wahab, A. et al. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants 11, 1620. https://doi.org/10.3390/plants11131620 (2022).
Laxa, M., Liebthal, M., Telman, W. & Dietz, J. The role of the plant antioxidant system in drought tolerance. Antioxidants. 8, 94. https://doi.org/10.3390/antiox8040094 (2019).
Mansoor, S. et al. Reactive oxygen species in plants: from source to sink. Antioxidants 11, 225. https://doi.org/10.3390/antiox11020225 (2022).
Rashid, K. et al. Response of wheat plant to drought stress: A review. Plant Cell Biotechnol. Mol. Biol. 22, 263–271 (2021).
Chen, Y. et al. Effects of drought stress on the growth, physiology and secondary metabolite production in pinellia ternata thunb. Pak. J. Bot. 53, 833–840. https://doi.org/10.1016/j.scienta.2019.108795 (2021).
Zhu, Z. B., Liang, Z. S. & Han, R. L. Saikosaponin accumulation and antioxidative protection in drought-stressed Bupleurum chinense DC. Plants. Environ. Exp. Bot. 66, 326–333. https://doi.org/10.1016/j.envexpbot.2009.03.017 (2009).
Liu, H., Wang, X., Wang, D., Zou, Z. & Liang, Z. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 33, 84–88. https://doi.org/10.1016/j.indcrop.2010.09.006 (2011).
Esteban, R. et al. Enhancement of zeaxanthin in two-steps by environmental stress induction in rocket and spinach. Food Res. Int. 65, 207–214. https://doi.org/10.1016/j.foodres.2014.05.044 (2014).
Huang, B. L. et al. Transcriptomic analysis of Eruca vesicaria subs. sativa lines with contrasting tolerance to polyethylene glycol-simulated drought stress. BMC Plant Biol. 19, 419. https://doi.org/10.1186/s12870-019-1997-2 (2019).
Askari, E. & Ehsanzadeh, P. Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol. Plant. 37, 4–14. https://doi.org/10.1007/s11738-014-1762-y (2015).
Islam, M. J. et al. Exogenous putrescine attenuates the negative impact of drought stress by modulating physio-biochemical traits and gene expression in sugar beet (Beta vulgaris L.). PLoS ONE 17, e0262099. https://doi.org/10.1371/journal.pone.0262099 (2022).
Golkar, P., Hamzeh, E., Mirmohammadi Maibody, S. A. & Taghizadeh, M. Safflower’s (Carthamus tinctorius L.) physio-biochemical mechanisms to improve its drought tolerance. Acta Physiol. Plant. 43, 82. https://doi.org/10.1007/s11738-021-03254-w (2021).
Mihaljevic, I. et al. Comparative study of drought stress effects on traditional and modern apple cultivars. Plants 10, 561. https://doi.org/10.3390/plants10030561 (2021).
Alizadeh Yeloojeh, K., Saeidi, G. & Ehsanzadeh, P. Effectiveness of physiological traits in adopting safflower (Carthamus tinctorius L.) genotypes to water deficit condition. Int. J. Plant Prod. 14, 155–164. https://doi.org/10.47176/jcpp.10.3.21733 (2020).
Sheoran, S. et al. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl. Biochem. Biotechnol. 177, 1282–1298. https://doi.org/10.1007/s12010-015-1813-x (2015).
Bouchemal, K., Bouldjadj, R., Belbekri, M. N., Ykhlef, N. & Djekoun, A. Differences in antioxidant enzyme activities and oxidative markers in ten wheat (Triticum durum Desf.) genotypes in response to drought, heat and paraquat stress. Arch. Agron. Soil Sci. 63, 710–722 (2017).
Zhang, Y. B. et al. Drought-induced alterations in photosynthetic, ultrastructural and biochemical traits of contrasting sugarcane genotypes. PLoS ONE 15, e0235845. https://doi.org/10.1371/journal.pone.0235845 (2020).
Sierra, J., McQuinn, R. P. & Leon, P. The role of carotenoids as a source of retrograde signals: Impact on plant development and stress responses. J. Exp. Bot. 73, 7139–7154. https://doi.org/10.1093/jxb/erac292 (2022).
Fang, Y. & Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 72, 673–689. https://doi.org/10.1007/s00018-014-1767-0 (2015).
Akram, N. A. et al. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola under drought stress. Protoplasma 255, 163–174. https://doi.org/10.1007/s00709-017-1140-x (2018).
Sarker, U. & Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 1, 16496. https://doi.org/10.1038/s41598-018-34944-0 (2018).
Liang, X., Zhang, L., Natarajan, S. K. & Becker, D. F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011 (2013).
Ghosh, U. K., Islam, M. N., Siddiqui, M. N., Cao, X. & Khan, M. A. R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 24, 227–239. https://doi.org/10.1111/plb.13363 (2022).
Yi, X. P. et al. Rapid recovery of photosynthetic rate following soil water deficit and re-watering in cotton plants (Gossypium herbaceum L.) is related to the stability of the photosystems. J. Plant Physiol. 194, 23–34. https://doi.org/10.1016/j.jplph.2016.01.016 (2016).
Maswada, H. F., Abd El-Razek, U. A., El-Shshtawy, A. N. A. & Elzaawely, A. A. Morpho-physiological and yield responses to exogenous moringa leaf extract and salicylic acid in maize (Zea mays L.) under water stress. Arch. Agron. Soil Sci. 64, 994–1010. https://doi.org/10.1080/03650340.2017.1406079 (2018).
Martinez, V. et al. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 7, 838. https://doi.org/10.3389/fpls.2016.00838 (2016).
Sofo, A., Scopa, A., Nuzzaci, M. & Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 16, 13561–13578. https://doi.org/10.3390/ijms160613561 (2015).
Ahmad, S. et al. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 11, 359. https://doi.org/10.3390/antiox11020359 (2022).
Chen, Z., Wang, Z., Yang, Y., Li, M. & Xu, B. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 228, 1–9. https://doi.org/10.1016/j.scienta.2017.10.004 (2018).
Cheng, L. et al. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria biacalensis Georgi under drought stress. Ind. Crops Prod. 122, 473–482. https://doi.org/10.1016/j.indcrop.2018.06.030 (2018).
Sheikh-Mohammadi, M. H. et al. Antioxidant defense system and physiological responses of Iranian crested wheatgrass (Agropyron cristatum L.) to drought and salinity stress. Acta Physiol. Plant. 39, 245. https://doi.org/10.1007/s11738-017-2543-1 (2017).
Voss, I., Suni, B., Scheibe, R. & Raghavendra, S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 15, 713–722. https://doi.org/10.1111/j.1438-8677.2012.00710.x (2013).
Merwad, A. M. A., Desoky, E. S. M. & Rady, M. M. Response of Water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 228, 132–144. https://doi.org/10.1016/j.scienta.2017.10.008 (2018).
Bautista, I. et al. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 38, 9. https://doi.org/10.1007/s11738-015-2025-2 (2016).
Hura, T., Hura, K. & Grzesiak, S. Contents of total phenolics and ferulic acid, and pal activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J. Agron. Crop. Sci. 194, 104–112. https://doi.org/10.1111/j.1439-037X.2008.00297.x (2008).
Gao, S. et al. Effects of drought stress on growth, physiology and secondary metabolites of two Adonis species in Northeast China. Sci. Hortic. 259, e108795. https://doi.org/10.1080/10549811.2022.2048263 (2020).
Nikzad, S., Mirmohammady Maibody, S. A. M., Ehtemam, M. H. & Golkar, P. Evaluation of drought tolerance in a world collection of Eruca sativa L. based on selection indices and some agronomic traits. Arch. Agron. Soil Sci. 69, 2154–2169. https://doi.org/10.1080/03650340.2022.2142569 (2023).
Carter, M. R. & Gregorich, E. G. Soil Sampling and Methods of Analysis. CRC Handbook of Digital System Design 2nd edn. (CRC Press, Boca Raton, 2007). https://doi.org/10.1201/9781420005271.
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. https://doi.org/10.1007/BF00018060 (1973).
Lichtenthaler, H. K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382. https://doi.org/10.1016/0076-6879(87)48036-1 (1987).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3 (1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbat specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232 (1980).
Priyanthi, C. & Sivakanesan, R. The total antioxidant capacity and the total phenolic content of rice using water as a solvent. Int. J. Food Sci. 3, 1–6. https://doi.org/10.1155/2021/5268584 (2021).
Author information
Authors and Affiliations
Contributions
SH. N. performed the experiments, analyzed the data and wrote the initial draft of the article. S. A. M. M. M designed the research, provided the materials and edited the manuscript. P. G. guided the experiment, data analysis, manuscript writing and editing the manuscript. M. H. E guided the experiment. S. A. M. guided the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nikzad, S., Mirmohammady Maibody, S.A.M., Ehtemam, M.H. et al. Response of seed yield and biochemical traits of Eruca sativa Mill. to drought stress in a collection study. Sci Rep 13, 11157 (2023). https://doi.org/10.1038/s41598-023-38028-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38028-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.