Abstract
By 2030, the World Health Organization wants to decrease viral hepatitis incidence and mortality by 90% and 65%, respectively. One of the agents responsible for the increased burden of viral hepatitis is the hepatitis E virus (HEV). This emerging pathogen is prevalent worldwide causing both acute and chronic infection. The rising risk profile of HEV has become a source of increased global public health concern. Despite this challenge, South-Eastern Asia (SEA), where many at-risk people are found, lacks uniform HEV prevalence data. Therefore, a meta-analysis was conducted to assess the overall prevalence of hepatitis E in SEA. Using R statistical software, a random effect model was used to estimate the logit-transformed prevalence. Moderator analyses were used to investigate the potential sources of variation. Thirty-two studies comprising 29,944 with 6806 anti-HEV antibody-positive individuals were evaluated. The overall HEV seroprevalence in SEA was 21% (95% confidence interval [CI]: 17–27) with high heterogeneity. At the country level, Laos has the highest prevalence estimate of 39% (CI: 16–69). Also, the studied population, year of publication, duration of sampling, and diagnostic method are significant HEV prevalence predictors accounting for 22.61% of the observed heterogeneity. The high HEV prevalence found in this study necessitates coordinated national and regional efforts to combat this emerging disease.
Similar content being viewed by others
Introduction
Globally, millions of people are infected with viral hepatitis yearly; a significant public health concern. The primary known aetiological agents of viral hepatitis are hepatitis A, B, C, D, and E viruses1. Some of these agents cause infections that may result in severe complications such as hepatocellular carcinoma or even death2. According to estimates from the World Health Organisation (WHO), viral hepatitis is responsible for the death of approximately 1.45 million people annually1,2. About 5–10% of those fatalities are attributed to hepatitis A and E, while the remaining are caused by hepatitis B and C1,2. Thus, hepatitis E virus (HEV) is now considered a significant contributor to the rising global burden of viral hepatitis.
Furthermore, HEV genotypes (HEV-1 to HEV-8) are being identified from diverse hosts3. Five of these genotypes (HEV-1 to HEV-4 and HEV-7) are known to be of human clinical importance. According to seroprevalence studies, a third of the world’s population is at risk of HEV infection3. Equally, annual new infections, including symptomatic cases, are increasing worldwide. Some infections also result in fatalities, with HEV accounting for 3.3% of all viral hepatitis mortalities globally3. Higher seroprevalence rates are recorded in developing countries than the developed nations4. However, epidemics are restricted to only developing regions, particularly in Africa and Asia4. The increasing global hepatitis E burden can be attributed to the multiple transmission routes of HEV. These transmission routes include water, food, and blood-borne as well as vertical and zoonotic4. Although most infections by HEV are usually mild and self-limiting4, certain groups are at risk of severe and or chronic disease. Acute HEV infection in pregnant women, mostly in developing countries, may present with fulminant hepatic failure with high mortality rates4,5. At the same time, chronic disease is seen in immunocompromised populations, mainly in developed regions4. Thus, HEV seriously threatens global health in developed and developing nations.
So, with the renewed effort by WHO to reduce the global burden of viral hepatitis1, providing a comprehensive information on the epidemiology of hepatitis E is critical. The need for this critical data is even more pertinent in the South-Eastern Asia (SEA) sub-region. This objective can be achieved efficiently through conducting a systematic review and meta-analysis. Thus, this study was undertaken as a follow-up to the previous systematic review (SR)6 by pooling the estimated effect of the seroprevalence of HEV from the included studies. Also, to investigate possible sources of heterogeneity through moderator analysis.
Methods
Study design
This study is an addition to an SR that was previously published6. The earlier review was conducted according to the PRISMA (S1 File) guidelines7. The review was preceded by developing an a priori protocol based on the PRISMA-P checklist6. Identified citations were screened based on some prespecified eligibility criteria (S2 File). Additionally, only studies adjudged to be of high quality after quality assessment were included in the previous review. Thus, all included articles in this study are of high quality.
Outcomes:
-
To determine the overall seroprevalence of hepatitis E in south-Eastern Asia through meta-analysis.
-
To determine factors that influence hepatitis E virus seroprevalence using moderator analyses.
Analysis
Meta-analysis
The R studio desktop programme (version 2020.02.3 + 492) through the R software (version 4.1.2-2021-11-01) environment8 was used to conduct the meta-analysis. The pooled estimate prevalence (logit transformed) was obtained by adopting the random effect (RM) model. The generalised linear mixed-effect model (GLMM) method9,10,11 was used to pool the effect estimates. The confidence interval around the pooled effect was calculated using the Knapp–Hartung adjustment.
Assessment of heterogeneity
Heterogeneity was estimated by X2, Cochrane Q test, I2, and T2 statistics using restriction maximum likelihood (REML) estimation to calculate heterogeneity variance. The Cochrane Handbook of Systematic Reviews of Intervention12 guideline served as a reference for interpreting the heterogeneity threshold. Thus, an I2 value of 0–40% was considered not important; 30–60% represented moderate heterogeneity; 50–90% was regarded as substantial heterogeneity; 75–100% was deemed considerable heterogeneity. While a p-value of < 0.05 was interpreted as indicating significant heterogeneity. A 95% prediction interval (PI) was also estimated to determine the effect size variation across studies13.
Sensitivity analysis
Outlier and influence analyses were conducted to determine the robustness of pooled estimate and assess each study's influence on the summary proportion. Studies with extreme effect sizes were identified as outliers14 through the “find.outlier” function of “dmetar” package of R (version 4.1.2-2021-11-01) software. Influential studies that may push the effect estimate into one direction were investigated by conducting influence diagnostics using the “influential analyses” function of “dmetar” and “metafor” packages of R (version 4.1.2-2021-11-01) software. The influence diagnostics include the Baujat plot15, Viechtbauer–Cheung influence plot14, graphic display heterogeneity (GOSH) plot16, and leave-one-out meta-analysis.
Moderator analyses
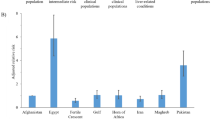
Subgroup and meta-regression analyses were conducted to investigate the possible sources of the observed heterogeneity in the meta-analysis. Variables considered for evaluation as potential moderators were based on the reports from previous studies17. The variables used for the analyses include country of study, location setting (rural or urban setting), diagnostic method, assay type, and studied population. Others are gender, age, year of publication, sample size, and duration of sampling (in years). The age variable was determined for each of the included studies by the reported mean age. While gender was captured based on the predominant gender of the study population as reported in the study. The studied population variable was categorised as healthy, clinical, or mixed depending on whether a healthy general population, hospital-based (patients) or both respectively were sampled. For meta-regression, each of the covariates was assessed in univariate analysis. Only predictors with p < 0.25 were included in the multivariate meta-regression analysis model.
Publication bias
Potential publication bias was assessed by constructing and observing the funnel plot of the logit-transformed prevalence against standard error18. Egger’s regression test was further used to investigate the significance of the observed asymmetry19.
Results
Characteristics of included studies
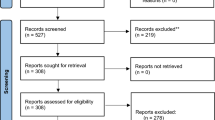
All included studies are strictly seroprevalence studies evaluated from the previous review as high-quality studies6. Thirty-two studies from seven SEA states are included in the meta-analysis out of the 35 studies screened for eligibility (Fig. 1). The included studies with the quality assessment scores and the excluded studies are outlined in the S2 File. These studies comprised 29,944 individuals with 6806 positive for HEV antibodies. Out of the 32 included studies, 18 sampled the general (healthy) population, ten had clinical samples, and the remaining had a mixed population. The sampled general population has an overall sample size of 19,040 and 3622 positive events. Other relevant study characteristics are outlined in Table 1.
Meta-analysis
The estimated HEV infection seroprevalence in the SEA region was 21% (95% confidence interval [CI]: 17–27; tau2 = 0.7341; tau = 0.8568; I2 = 98.7% [CI: 98.5%; 98.8%] Fig. 2). The estimated 95% PI shows that all comparable populations’ seroprevalence ranges from 4 to 64% (Supplementary Fig. 1).
Sensitivity analyses
Outliers’ identification
The detected outliers were 19 studies20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38, and meta-analysis was recalculated using the remaining 13 studies21,39,40,41,42,43,44,45,46,47,48,49,50. The I2 has reduced from 98.7% to 88.9% from the rerun analysis, and the PI became narrower (S3 File: Item 2).
Influence analyses
Baujat plot
The Baujat plot displays how each study contributed to the overall heterogeneity and how the studies affected the pooled effect. The studies20,34,38 in the upper right corner of the plot have a huge impact on both the heterogeneity and pooled effect, thus, are regarded as the most influential studies in the meta-analysis (Fig. 3). At the same time, the study31 in the lower right corner contributed heavily to the heterogeneity but not to the pooled effect.
Viechtbauer–Cheung Influence analysis
From the spikes observed in the plot, the study determined to be influential is the Corwin (1995) study (Supplementary Fig. 2). This study was also identified in the two previous analyses.
GOSH diagnostics
The plot revealed distinct effect size–heterogeneity clustering patterns of (1) high effect size–high heterogeneity (Supplementary Fig. 3) and (2) low effect size–high heterogeneity (Supplementary Fig. 4). This clustering pattern indicates more than one effect size population. Using the three clustering algorithms (S3 File: Item 4), the studies clustering in each of the clusters are outlined as follows:
-
K-means clustering: Study 10, Study 1, and Study 1320,24,27.
-
Connectivity (DBSCAN) clustering: Study 25, Study 1, Study 8, Study 10, Study 18, Study 23, Study 19, and Study 2820,24,31,34,39,43,48,49.
-
Gaussian Mixture Model (GMM) clustering: Study 25, Study 1, Study 8, Study 10, Study 18, Study 23, Study 19, and Study 2820,24,31,34,39,43,48,49.
So, nine studies in total were identified by the GOSH diagnostics and the analysis was recalculated after removing the studies to determine their influence. After the removal of the studies, the I2 was 97.7%, and the Q statistic was still significant (S3 File: Item 5).
Leave-one-out analysis
Sensitivity analysis was also done using the leave-one-out analysis to determine the studies that impact the robustness of the meta-analysis. Each included study was removed one after another, and the result was presented in a plot sorted by proportion (Fig. 4).
However, after conducting all the sensitivity analyses, the heterogeneity remained substantial, indicating that the identified studies could not effectively account for the observed heterogeneity. Therefore, moderator analyses must further explore the data's source(s) of heterogeneity.
Moderator analyses
Subgroup analysis
Subgroup analysis based on the country of study revealed that among the seven evaluated countries in SEA, Laos People’s Democratic Republic (PDR) had the highest seroprevalence of 39% (CI: 16–69, tau2 = 0.2677; tau = 0.5174; I2 = 97.6%. Other countries' seroprevalences and their respective statistics are presented in the subgroup analysis forest plot (Fig. 5). A graphic presentation of the respective seroprevalence rates is shown in the SEA map (Fig. 6). Details of the subgroup analyses for the other evaluated variables are presented in Table 2.
Meta-regression analysis
Univariate analysis
Univariable meta-regression was used to ascertain the impact of study-level covariates on the pooled seroprevalence. The analysis revealed that the studied population, year of publication, duration of sampling, and diagnostic method are significant predictors. Table 3 outlines the respective proportions of the effect of the evaluated covariates.
Multivariate analysis
The three significant moderators in the univariate analysis were included in the multivariable analysis, accounting for 22.61% of the observed heterogeneity (Table 3).
Publication bias
There is an observed asymmetry of the funnel plot, which illustrates potential publication bias (Fig. 7). Further quantitative evaluation of the observed asymmetry shows that Eggers' test does not indicate the presence of funnel plot asymmetry (Table 4).
Discussion
A comprehensive evaluation of HEV seroprevalence in SEA will assist in reducing the global burden of viral hepatitis as envisioned by the global health strategy on viral hepatitis1. The evaluation of HEV seroprevalence will provide the necessary information for informed decision-making concerning policymakers, public health practitioners and physicians. An informed decision based on a proper understanding of the disease epidemiology will help prevent and control hepatitis E. Therefore, this study provides data on evaluating the overall seroprevalence of hepatitis E and its associated predictors in SEA.
It has been established in an earlier review that hepatitis E is endemic in SEA, with evidence of its existence dating back more than 25 years6. Thus, it is unsurprising that the average prevalence of hepatitis E in this sub-region is high. The estimated seroprevalence in this study is higher than the global average of 12.5%51. This study's prevalence estimate is higher than the findings from some regions. Hepatitis E seroprevalence was estimated at 7.7% and 9.31% in the Americas and Europe, respectively51,52. The prevalence was 11.81% in the Middle East and 12.17% in the Eastern Mediterranean53. However, the finding of this study is similar to the average prevalence of 21.76% obtained in Africa51. In addition, this survey has also shown the possibility of even higher rates of up to 64% in future studies, as evidenced by the estimated prediction interval.
Different factors could be responsible for the observed high prevalence in SEA and the disparity from other regions. Apart from Singapore, a high-income country, the remaining countries with studies included in this meta-analysis are either low, lower-middle or upper-middle income54. Thus, the prevailing challenges of poor hygiene and environmental conditions associated with developing countries could have contributed to the high seropositivity rate. Numerous studies have already established that factors such as insufficient access to safe drinking water, floods, and inadequate health education are risk factors for HEV infection4. Additionally, contact with domestic and wild animals is not uncommon in this sub-region4,51,52. There is also the risk associated with consuming raw or undercooked animal products such as pork liver4,52. The unprecedented expansion in the livestock sector witnessed in this sub-region in recent years, and its attendant negative consequences may have aggravated these risk factors55. Some of these adverse effects include livestock-associated water and soil contamination and the threat of zoonotic disease surges55. Also, the SEA is a culturally and ethnically diverse region and experiencing a rapid increase in international tourism in recent years56,57. These two factors could also play a role in the high rate of HEV seroprevalence in the region. Another possible explanation for the high prevalence rate may be that some of the studies included in this meta-analysis used total antibodies for diagnosis. This method contrasts several other analyses that included studies that used only IgG diagnostic method.
Further, the high seroprevalence estimate was accompanied by substantial in-between study heterogeneity. It was discovered in this study that seroprevalence varied between countries. The seropositivity rates are high in all the countries, with Laos PDR identified as the country with the highest prevalence.
However, it should be noted that Laos PDR had only two studies included in the meta-analysis. Yet still, environmental and hygiene challenges peculiar to developing countries might be contributing factors. Others include increased contact with animals due to expansion in livestock farming. Livestock farming in Laos PDR is still largely traditional, with more of small-scale (backyard) local production system58,59. This farming system promotes more animal contact and the adverse effects associated with livestock farming that may serve as risk factors for HEV infection. There is equally the cultural dietary preference for consuming raw or undercooked animal products practised in many countries in SEA60.
Living in rural areas has been established in numerous studies as a risk factor for HEV infection. Thus, expectedly, higher hepatitis E prevalence were discovered in the rural than the urban areas. Other associated factors might have contributed to the observed outcome besides the earlier enumerated risk factors. The populace may have lower educational and economic status in rural areas than the urban settlers61. However, studies that combined urban and rural populations produced the highest prevalence. This phenomenon can also be explained by the increase in rural–urban migration and the possibility of sampling high-density urban areas61.
Similarly, this study shows that reported seroprevalence rates were impacted by the employed diagnostic method and assay type. Studies using total HEV antibodies as diagnostic methods showed higher prevalence than those using only IgG. Also, there was observed variation in HEV prevalence among the evaluated assays. The performance of assay types is often influenced by their specificity and sensitivity levels. HEV assay performance comparative studies have shown varied seroprevalence rates among the assays evaluated62. Thus, assays have predictive and modifier effects on HEV seroprevalence17. Therefore, the findings of this meta-analysis agree with several primary studies and meta-analyses17,51,63. The result did not show any specific pattern in sample sizes, probably due to the relatively small number of studies used in most groupings.
Furthermore, this current study also showed that the HEV seropositivity rate is lower in the healthy population compared to the clinical and mixed populations. The result further established that those with underlying diseases and the immunocompromised have an increased risk of HEV infection compared to the healthy population. Differences were also observed between genders, ages, years of publication, and sampling duration. The prevalence was higher in males than females, as previously established. Studies have reported higher HEV seroprevalence in males than non-pregnant females4. The most suggested reason for the propensity is the increased behavioural exposure in men compared to women4. This study also revealed a pattern that suggests an association of anti-HEV antibody positivity with increasing age. Prevalence was lowest in the age group of 20–25 years and peaked at 44–49 years age group. The observed pattern conforms with many primary and secondary studies4,52. Some explanations for this pattern include lifetime cumulative exposure and the impact of ageing on immunological function52. Others are genomic instability and other ageing processes; thus, prevalence tends to increase with age52.
Evaluation based on the year of publication showed almost a two-fold increase in HEV seroprevalence rate in 2019 from the 1999 rate. Likewise, it was observed that the longer the duration of sampling, the higher the prevalence. Studies with longer sampling duration had older samples indicating that studies with more recent samples had lower prevalence. This increase could be attributed to a substantial rise in HEV infection, increased awareness and research, or the use of more sensitive diagnostic assays. Although there are concerns that hepatitis E is still underestimated, recent discoveries about the epidemiology of the disease might have contributed to the observed rise in its prevalence as detected. These discoveries have led to a series of epidemiologic and pathological studies aimed at understanding the nature of the disease. For example, studies have shown constant expansion of HEV’s host range with an increased probability of cross-species infections64. This phenomenon may result in more human infection due to frequent contact with these animal hosts of the pathogen. Also, in recent years, there seems to be an increased awareness among researchers about HEV being a critical causative agent of viral hepatitis.
Additionally, most recent assay types have shown improved efficacy in diagnosing the disease over the old4,65. The use of more effective diagnostic assays will be more accurate compared to the less sensitive techniques. These factors, individually or in combination with the contribution of others, might have been responsible for the observed rate rise reported in this study. However, with increased awareness, clinical evaluation, and research, the prevalence might increase, as indicated by the prediction interval estimate.
On a more technical note, it was observed that substantial variation persisted in each of the assessed groups after subgroup analyses. Although heterogeneity is expected in the pooled effect size, it is assumed that the variation should be reduced significantly in close groups. Thus, its persistence in homogeneous subgroups calls for deeper exploration. So, further assessment using meta-regression analysis showed four factors (individually and in combination) that are significant moderators of the effect size. The quantifiable factors are studied population, diagnostic method, year of publication, and sampling duration.
Nevertheless, the predictors could only account for a fraction of the variation. Other factors that can potentially bias effect size estimates, such as file drawer effect, p-hacking strategies and publication bias, may equally affect the heterogeneity estimate66. However, the assessment showed that publication bias was not present in this survey. Though, as already established, heterogeneity could be due to quantifiable, hidden, or random moderators66. Other hidden factors that were not evaluated such as HEV genotype variation, might be responsible for the residual heterogeneity. Some others might even be entirely unknowable thus, cannot be specified or controlled66. So, pooled studies in meta-analyses can vary due to reasons that will likely never be fully identified66.
Consequently, primary prevalence studies must be designed as similarly as feasible to reduce between-study variation. The deduced recommendation from the findings of this study is for future seroprevalence studies to sample homogenous populations. Subsequent surveys at either national or regional levels can adopt a unified diagnostic method for hepatitis E investigation. In addition, as recommended by different researchers, further primary studies should consider regional and national surveys using similar study designs and analytical methods66,67.
As the first meta-analysis of hepatitis E seroprevalence in SEA, this study has pooled many studies, leading to a relatively large sample size with enhanced statistical power. Also, this experiment was conducted using meticulous methods, and moderators were thoroughly investigated. Therefore, a comprehensive sub-regional as well as national data on the seroprevalence of HEV is provided. The provided information will thus assist further research and informed decision-making for designing HEV preventive and control measures in the sub-region. However, there are a few limitations of this study. Included studies are only from seven countries due to the non-availability of studies from the remaining four countries. This constraint may affect the generalisation of the result to represent the sub-region. Likewise, fewer studies were available in some of the groups during subgroup analyses which may affect the estimates in the respective groups. Thirdly, only HEV seroprevalence was considered in this analysis; sporadic HEV infection and epidemics that equally contributes to the disease burden were not estimated.
Conclusion
Even though there are no reports from some countries in the sub-region, SEA has high HEV seroprevalence. As expected, the prevalence is higher in some countries than others, and the variation is attributable to detectable, concealed, and random factors. Therefore, there is a need for concerted efforts towards preventing and controlling this emerging disease at national and regional levels. Increased research, surveillance, and purposeful screening of at-risk groups and blood donors will assist in prevention and control.
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis (World Health Organization, 2016).
Wiktor, S. Z. & Hutin, Y. J. The global burden of viral hepatitis: Better estimates to guide hepatitis elimination efforts. Lancet 388(10049), 1030 (2016).
Primadharsini, P. P., Nagashima, S. & Okamoto, H. Genetic variability and evolution of hepatitis E virus. Viruses 11(5), 456 (2019).
Raji, Y. E. et al. Hepatitis E virus: An emerging enigmatic and underestimated pathogen. Saudi J. Biol. Sci. 29(1), 499–512 (2022).
Bergløv, A., Hallager, S. & Weis, N. Hepatitis E during pregnancy: Maternal and foetal case-fatality rates and adverse outcomes—A systematic review. J. Viral Hepat. 26(11), 1240–1248 (2019).
Raji, Y. E. et al. A systematic review of the epidemiology of hepatitis E virus infection in South-Eastern Asia. Virulence 12(1), 114–129 (2021).
Page, M. J. et al. The PRISMA statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372 (2020).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (R Foundation for Statistical Computing, 2021).
Almalik, O. & van den Heuvel, E. R. Testing homogeneity of effect sizes in pooling 2×2 contingency tables from multiple studies: A comparison of methods. Cogent Math. Stat. 5(1), 1478698 (2018).
Lipsey, M. W. & Wilson, D. B. Practical Meta-analysis (SAGE Publications, 2001).
Schwarzer, G., Chemaitelly, H., Abu-Raddad Gerta, L. J. & Rücker, G. Seriously misleading results using inverse of Freeman–Tukey double arcsine transformation in meta-analysis of single proportions. Res. Synth. Methods 10(3), 476–483 (2019).
Higgins, J. P. et al. Cochrane Handbook for Systematic Reviews of Interventions (Wiley, Paris, 2019).
Borenstein, M. Research note: In a meta-analysis, the I 2 index does not tell us how much the effect size varies across studies. J. Physiother. 66(2), 135–139 (2020).
Viechtbauer, W. M. W. L. C. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1(2), 112–125 (2010).
Baujat, B., Mahé, C., Pignon, J.-P. & Hill, C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 21(18), 2641–2652 (2002).
Olkin, I., Dahabreh, I. J. & Trikalinos, T. A. GOSH—A graphical display of study heterogeneity. Res. Synth. Methods 3(3), 214–223 (2012).
Wilhelm, B. et al. Systematic review and meta-analysis of the seroprevalence of hepatitis E virus in the general population across non-endemic countries. PLoS One 14(6), e0216826 (2019).
Mavridis, G. & Salanti, G. How to assess publication bias: Funnel plot, trim-and-fill method and selection models. Evid. Based Mental Health 17(1), 30 (2014).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Nouhin, J. et al. Declining hepatitis E virus antibody prevalence in Phnom Penh, Cambodia during 1996–2017. Epidemiol. Infect. 147, e26 (2019).
Wibawa, I. D. N. et al. Prevalence of antibodies to hepatitis E virus among apparently healthy humans and pigs in Bali, Indonesia: Identification of a pig infected with a genotype 4 hepatitis E virus. J. Med. Virol. 73(1), 38–44 (2004).
Utsumi, T. et al. Prevalence of hepatitis E virus among swine and humans in two different ethnic communities in Indonesia. Adv. Virol. 156(4), 689–693 (2011).
Wibawa, I. D. N. et al. Identification of genotype 4 hepatitis E virus strains from a patient with acute hepatitis E and farm pigs in Bali, Indonesia. J. Med. Virol. 79(8), 1138–1146 (2007).
Corwin, A. et al. Two years’ investigation of epidemic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Trans. R. Soc. Trop. Med. Hyg. 89(3), 262–265 (1995).
Widasari, D. I. et al. Hepatitis E virus infection in two different regions of Indonesia with identification of swine HEV genotype 3. Microbiol. Immunol. 57(10), 692–703 (2013).
Achwan, W. A. et al. Epidemiology of hepatitis B, C, and E viruses and human immunodeficiency virus infections in Tahuna, Sangihe-Talaud Archipelago, Indonesia. Intervirology 50(6), 408–411 (2007).
Tritz, S. E. et al. Evidence of increased Hepatitis E virus exposure in Lao villagers with contact to ruminants. Zoonoses Public Health 65(6), 690–701 (2018).
Sa-nguanmoo, P. et al. Swine is a possible source of hepatitis E virus infection by comparative study of hepatitis A and E seroprevalence in Thailand. PLoS One 10(4), 0126184 (2015).
Pilakasiri, C. et al. Hepatitis antibody profile of Royal Thai Army nursing students. Trop. Med. Int. Health 14(6), 609–611 (2009).
Poovorawan, Y. et al. Prevalence of hepatitis E virus infection in Thailand. Ann. Trop. Med. Parasitol. 90(2), 189–196 (1996).
Gonwong, S. et al. Pork consumption and seroprevalence of hepatitis E virus, Thailand, 2007–2008. Emerg. Infect. Dis. 20(9), 1531–1534 (2014).
Siripanyaphinyo, U. et al. Occurrence of hepatitis E virus infection in acute hepatitis in Thailand. J. Med. Virol. 86(10), 1730–1735 (2014).
Hoan, N. X. et al. High hepatitis E virus (HEV) positivity among domestic pigs and risk of HEV infection of individuals occupationally exposed to pigs and pork meat in Hanoi, Vietnam. In Open Forum Infectious Diseases (Oxford University Press, 2019).
Hoan, N. X. et al. Hepatitis E virus superinfection and clinical progression in hepatitis B patients. EBioMedicine 2(12), 2080–2086 (2015).
Berto, A. et al. Hepatitis E in southern Vietnam: Seroepidemiology in humans and molecular epidemiology in pigs. Zoonoses Public Health 65(1), 43–50 (2018).
Hau, C. H. et al. Prevalence of enteric hepatitis A and E viruses in the Mekong River delta region of Vietnam. Am. J. Trop. Med. Hyg. 60(2), 277–280 (1999).
Tran, H.T.-T. et al. Prevalence of hepatitis virus types B through E and genotypic distribution of HBV and HCV in Ho Chi Minh City, Vietnam. Hepatol. Res. 26(4), 275–280 (2003).
Corwin, A. et al. Acute viral hepatitis in Hanoi, Viet Nam. Trans. R. Soc. Trop. Med. Hyg. 90(6), 647–648 (1996).
Jupattanasin, S. et al. A nationwide survey of the seroprevalence of hepatitis E virus infections among blood donors in Thailand. Viral Immunol. 32(7), 302–307 (2019).
Nouhin, J. et al. Low frequency of acute hepatitis E virus (HEV) infections but high past HEV exposure in subjects from Cambodia with mild liver enzyme elevations, unexplained fever or immunodeficiency due to HIV-1 infection. J. Clin. Virol. 71, 22–27 (2015).
Nouhin, J. et al. Hepatitis E virus antibody prevalence, RNA frequency, and genotype among blood donors in Cambodia (Southeast Asia). Transfusion 56(10), 2597–2601 (2016).
Yamada, H. et al. Hepatitis E virus in Cambodia: Prevalence among the general population and complete genome sequence of genotype 4. PLoS One 10(8), e0136903 (2015).
Surya, I. G. P. et al. Serological markers of hepatitis B, C, and E viruses and human immunodeficiency virus type-1 infections in pregnant women in Bali, Indonesia. J. Med. Virol. 75(4), 499–503 (2005).
Khounvisith, V. et al. High circulation of Hepatitis E virus in pigs and professionals exposed to pigs in Laos. Zoonoses Public Health 65(8), 1020–1026 (2018).
Hudu, S. A. et al. Hepatitis E virus isolated from chronic hepatitis B patients in Malaysia: Sequences analysis and genetic diversity suggest zoonotic origin. Alex. J. Med. 54(4), 487–494 (2018).
Seow, H. F. et al. Seroprevalence of antibodies to hepatitis E virus in the normal blood donor population and two aboriginal communities in Malaysia. J. Med. Virol. 59(2), 164–168 (1999).
Ng, K. et al. A seroprevalence study of viral hepatitis E infection in human immunodeficiency virus type 1 infected subjects in Malaysia. Med. J. Malaysia 55(1), 58–64 (2000).
Wong, C. C. et al. Seroepidemiology and genotyping of hepatitis E virus in Singapore reveal rise in number of cases and similarity of human strains to those detected in pig livers. Zoonoses Public Health 66(7), 773–782 (2019).
Chow, W. et al. Hepatitis E in Singapore—A seroprevalence study. Singapore Med. J. 37, 579–582 (1996).
Hinjoy, S. et al. A cross-sectional study of hepatitis E virus infection in healthy people directly exposed and unexposed to pigs in a rural community in northern Thailand. Zoonoses Public Health 60(8), 555–562 (2013).
Li, P. et al. The global epidemiology of hepatitis E virus infection: A systematic review and meta-analysis. Liver Int. 40(7), 1516–1528 (2020).
Fernández Villalobos, N. V. et al. Seroprevalence of hepatitis E virus infection in the Americas: Estimates from a systematic review and meta-analysis. PLoS One 17(6), e0269253 (2022).
Niya, M. H. K. et al. Hepatitis E virus seroprevalence rate among Eastern Mediterranean and middle eastern countries; A systematic review and pooled analysis. Microb. Pathog. 110, 252–256 (2017).
U.N.S. Division. Methodology—Standard Country or Area Codes for Statistical Use (M49) (2019).
Otte, J., Pica-Ciamarra, U. & Morzaria, S. A comparative overview of the livestock-environment interactions in Asia and Sub-Saharan Africa. Front. Vet. Sci. 6, 37 (2019).
Chheang, V. Tourism and Regional Integration in Southeast Asia (Institute of Developing Economies, Japan External Trade Organization, 2013).
Dolezal, C. & Trupp, A. Tourism and development in Southeast Asia. Aust. J. South-East Asian Stud. 8(2), 117–124 (2015).
Napasirth, P. & Napasirth, V. Current situation and future prospects for beef production in Lao People’s Democratic Republic—A review. Asian Australas. J. Anim. Sci. 31(7), 961 (2018).
Xayalath, S. et al. A review on the trend of livestock breeds in Laos. Acta Agraria Debreceniensis 1, 227–237 (2021).
Zhou, M. et al. Culture, eating behavior, and infectious disease control and prevention. J. Ethnic Foods 7(1), 40 (2020).
Houcine, N. et al. Seroprevalence of hepatitis E virus infection in rural and urban populations, Tunisia. Clin. Microbiol. Infect. 18(5), E119–E121 (2012).
Norder, H. et al. Diagnostic performance of five assays for anti-hepatitis E virus IgG and IgM in a large cohort study. J. Clin. Microbiol. 54(3), 549–555 (2016).
Wenzel, J. J. et al. Test performance characteristics of Anti-HEV IgG assays strongly influence hepatitis E seroprevalence estimates. J. Infect. Dis. 207(3), 497–500 (2013).
Meng, X.-J. Expanding host range and cross-species infection of hepatitis E virus. PLoS Pathog. 12(8), e1005695 (2016).
Talapko, J. et al. Towards the improved accuracy of hepatitis e diagnosis in vulnerable and target groups: A global perspective on the current state of knowledge and the implications for practice. In Healthcare (MDPI, 2021).
Kenny, D. A. & Judd, C. M. The unappreciated heterogeneity of effect sizes: Implications for power, precision, planning of research, and replication. Psychol. Methods 24(5), 578 (2019).
Hartl, J. et al. Hepatitis E seroprevalence in Europe: A meta-analysis. Viruses 8(8), 211 (2016).
Acknowledgements
The authors would like to acknowledge and thank Pn Norkhasfazilla A. Rahim for the effective administrative support towards ensuring a seamless interface between the team and the research monitoring committee.
Funding
This study was sponsored by the Universiti Putra Malaysia (UPM)IPS grant (No. UPM.RMC.800/2/2/4-IPS).
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.S.; Data extraction, Y.R., Z.S., O.T., and N.T.; Statistical analysis, Y.R.; Writing—original draft manuscript, Y.R.; Editing manuscript, Z.S.; Supervision, Z.S., O.T.; and N.T.; Project administration, Z.S.; Funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raji, Y.E., Toung, O.P., Taib, N.M. et al. Meta-analysis and moderator analysis of the seroprevalence of hepatitis E in South-Eastern Asia. Sci Rep 13, 11880 (2023). https://doi.org/10.1038/s41598-023-37941-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37941-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.