Abstract
This study aimed to investigate the association of periodontitis with subclinical atherosclerosis in young adults. In total, 486 non-diabetic military personnel were included in Taiwan. Carotid intima-media thickness (cIMT) was assessed utilizing sonography for subclinical atherosclerosis. Periodontitis severity was defined based on the 2017 US/European consensus. Mean cIMT was compared by analysis of covariance (ANCOVA), and multiple logistic regression model was used to determine the association of periodontitis severity and the highest quintile of cIMT (≥ 0.8 mm) with adjustments for age, sex, metabolic risk factors and leukocyte counts. The mean cIMT increased in those with greater stages (periodontal health (N = 349): 0.65 mm, Stage I (N = 41): 0.72 mm, Stage II (N = 57): 0.74 mm and Stage III: 0.76 mm, respectively, p < 0.01). In multiple logistic regression, a dose–response association from Stage I to Stage III periodontitis for cIMT ≥ 0.8 mm was also found [ORs and 95% CIs 1.41 (0.60–3.29), 1.62 (0.79–3.31) and 3.20 (1.42–7.18)]. Leucocyte counts ≥ 7.6 × 103/µL (the highest quintile) was associated with cIMT ≥ 0.8 mm [OR 1.86 (1.11–3.12)], while no association existed for other metabolic risk factors. In conclusion, severe periodontitis and leukocyte counts are independent risk factors of increased cIMT, emphasizing the critical role of inflammation in subclinical atherosclerosis.
Similar content being viewed by others
Introduction
Atherosclerosis, the main process in atherosclerotic cardiovascular diseases, is a focal thickening of the arterial intima residing between the endothelial lining and smooth muscle layers of arterial blood vessels in response to an immune response1. Periodontitis is associated with disproportionate host inflammatory immune responses that are induced by an imbalance in the microbial ecological environment in oral cavity; this instigates microbial dysbiosis, along with failed resolution of the chronic destructive inflammation2. The European Federation of Periodontology (EFP) and the American Academy of Periodontology (AAP) joint workshop in 2012 suggests a possible link between atherosclerotic disease and periodontitis3. Two mechanisms are proposed to explain such association. First, the subgingival microbiota and their products may directly access to the circulation and provide a persistent gram-negative bacterial challenge to the host4. Low level bacteremia may initiate host responses that alter coagulability, endothelial and vessel wall integrity, and platelet function, thereby resulting in subsequent atherogenic changes and thromboembolic event in patients with periodontitis5,6,7. Second, periodontitis can indirectly alter systemic homeostasis through the release of endotoxins as well as inflammatory cytokines, and lead to endothelial dysfunction8. In addition, in epidemiology, both atherosclerosis and periodontitis may be modified by similar risk factors, e.g., age, elements of tobacco and metabolic disorders9,10. However, the findings for the association between periodontitis and atherosclerosis markers, i.e., carotid intima-media thickness (cIMT) were inconsistent among middle- and old-aged individuals in previous reports11,12. The conflicting results might be due in part to many risk factors prevalently presented in the process of atherosclerosis and periodontitis development in older individuals, and the association might be over-adjusted if these risk factors were all included as confounders. It is critical to carry out a study in a population with low vascular risk factors exposure, e.g., military subjects who are healthier and have better physical activity than the general population, and are unlikely to have atherosclerosis, to verify the association. Therefore, this study aimed to investigate the association between periodontitis severity and cIMT, a marker of subclinical atherosclerosis, in a population of military young adults in Taiwan.
Methods
Study population
This study was a cross-sectional study including participants without taking any antihypertensive medications from the cardiorespiratory fitness and health in eastern armed forces (CHIEF) atherosclerosis study in Hualien City, Taiwan in 2018–2020, which has been described in detail previously13,14,15,16. Participants who underwent both an oral examination and a carotid ultrasonography were included. Participants whose age older than 40 years and total leukocyte counts greater than 11.0 × 103/µL were excluded to avoid a presence of unrecognized infectious diseases in the body. The study protocol was approved by the Ethics Committee of the Mennonite Christian Hospital (No. 16-05-008) in the Hualien City of Taiwan and the study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Participants were informed the nature of the study, and all gave written informed consent to provide the ability to opt out without consequence.
Assessment of anthropometric and hemodynamic variables
Waist circumference, body height and body weight were assessed in a standing position. The body mass index (BMI) was defined as body weight in kilograms divided by square of body height in meters. Abdominal obesity was defined as waist circumference ≥ 90 cm for men and ≥ 80 cm for women17. Blood samples were collected in the morning after an overnight fast for total cholesterol, low density lipoprotein (LDL-C), high density lipoprotein (HDL-C), triglycerides, fasting glucose (FPG), serum uric acid and leucocyte counts. Dyslipidemia was defined as total cholesterol ≥ 200 mg/dL, LDL-C > 100 mg/dL, or triglycerides ≥ 150 mg/dL in both men and women. HDL-C < 40 mg/dL in men and HDL-C < 50 mg/dL in women were also defined as dyslipidemia17. Hyperuricemia was defined as serum uric acid ≥ 7.0 mg/dL in men and ≥ 6.0 mg/dL in women18. Systemic inflammation was defined as elevated leucocyte counts ≥ 7.6 103/µL19. Measurement of systolic blood pressure (SBP)/diastolic blood pressure (DBP) at rest over right upper arm was performed once, using an automatic device (FT201 Parama-Tech Co., Ltd, Fukuoka, Japan). Hypertension was diagnosed as SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg17. Before the physical examination, participants were asked about their alcohol consumption and tobacco smoking status (active versus former/never)20,21, and daily tooth brushing frequency.
Assessment of periodontitis severity
The 2017 AAP/EFP joint workshop on the classification of periodontal diseases were used for the definition of periodontitis stages22. Since the study was aimed at physically fit young adults, the extent of periodontitis was merely restricted to the localized (< 30% of teeth involved)22. Stage I periodontitis was defined if either one criterion was fulfilled: (1) interdental clinical attachment loss (CAL) of 1–2 mm at side of greatest loss; (2) radiographic bone loss < 15%; (3) no tooth loss due to periodontitis; (4) maximum probing pocket depth (PD) ≤ 4 mm, or mostly horizontal bone loss. Stage II periodontitis was defined when either one criterion was fulfilled: (1) interdental CAL of 3–4 mm at side of greatest loss; (2) radiographic bone loss of 15–33%; (3) no tooth loss due to periodontitis; (4) maximum PD ≤ 5 mm, or mostly horizontal bone loss. Stage III periodontitis was defined if either one criterion was met: (1) interdental CAL ≥ 5 mm at side of greatest loss; (2) radiographic bone loss extending to mid-third of root and beyond; (3) with tooth loss because of periodontitis; (4) in addition to Stage II periodontitis severity further with PD ≥ 6 mm, or vertical bone loss ≥ 3 mm, or class II/III furcation involvement, or moderate ridge defect. No cases of Stage IV periodontitis were observed in this study. Full mouth oral examination and radiograph of each participant were performed by the same dentist. All subjects returned to the outpatient department for a re-evaluation and a treatment for the oral pathologies within one month. The inter-observer agreement (kappa coefficient) for verifying the severity of periodontitis was estimated 90.6%23,24,25,26.
Assessment of cIMT
All ultrasonographic images were acquired at end diastole (defined as the R wave of an electrocardiogram) by the same certificated technician and verified by a cardiologist. The cIMT diameter was quantitatively measured between the leading edges of the media–adventitia interface and the lumen–intima interface over the far wall of the left carotid bulb, using an ultrasound scanner equipped with a linear p robe of 4–8 MHz on the iE33 machine (Philips Medical Systems, Andover, MA, USA). Intima–media interface diameter of each participant was manually traced as a continuous line for a calculation13. To verify the cIMT, the measurement procedure was performed repeatedly by the technician, and the coefficient of variation was up to 96.8%14. In whole of the carotid sonographic examinations, no carotid plagues were found. The increased cIMT was defined as those over the highest quintile level, corresponding to the diameter ≥ 0.8 mm, which was modestly greater than the upper normal cIMT level (0.75 mm) in the general population in prior studies27.
Statistical analysis
Periodontitis severity was classified as health, Stage I, Stage II and Stage III. Group differences among the four groups were evaluated using the analysis of variance (ANOVA) for continuous variables and chi-square test for categorical variables. Analysis of covariance (ANCOVA) with adjustments for age, sex, BMI, SBP, DBP, tooth brushing frequency, alcohol intake and tobacco smoking was used to examine if a difference in the mean of cIMT existed among the four groups. We utilized univariate and stepwise multiple linear regression models to assess the association of periodontitis severity with cIMT. In model 1, age, sex, SBP, DBP, waist circumference, lipid profiles, FPG, serum uric acid, tooth brushing frequency, alcohol intake and smoking status and leukocyte counts were adjusted. In model 2, BMI was further adjusted. Univariate and multiple logistic regression models were also utilized to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of periodontitis severity for increased cIMT ≥ 0.8 mm. In Model 1, age, sex, teeth brush frequency, alcohol intake, tobacco smoking, metabolic syndrome risk factors, e.g., dyslipidemia and hypertension, and elevated leukocyte counts within normal limits were adjusted. In model 2, BMI was additionally adjusted. In addition, participants were divided into four groups, based on the presence( +) or absence(−) of elevated leukocyte counts and severer periodontitis (Stage II or Stage III) to investigate the moderation effect of elevated leukocyte counts in the association between severer periodontitis and increased cIMT ≥ 0.8 mm in the univariate and multiple logistic regression models. Since the sample of the male participants in this study were much more than the female participants, sensitivity analyses were performed for men only with regard to their clinical characteristics and the association of periodontitis severity with increased cIMT ≥ 0.8 mm. The statistical analysis was carried out using SPSS software (Version 26.0. Armonk, New York: International Business Machines Corporation), and a value of p < 0.05 was considered to be statistically significance.
Results
Demographic and clinical characteristics
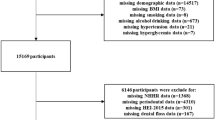
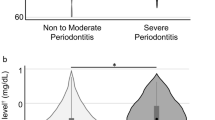
Of those who underwent a carotid ultrasonography for cIMT measurement (N = 1,822), participants who received an oral examination were included (N = 501). Those who were older than 40 years of age (N = 5) and those with total leucocyte counts > 11.0 × 103/µL (N = 10) were excluded, leaving a sample of 486 subjects for analysis (Fig. 1). Table 1 shows that the prevalence of periodontitis (any grades) was 28.19% (N = 137), and the others (N = 349) were periodontal health (71.81%). Of those with periodontitis, there were 41 cases (6.62%) classified to Stage I, 57 cases (12.18%) classified to Stage II and 39 cases (8.33%) classified to Stage III. Of the military subjects, about 80% had the behavior of tooth brushing > 1 time per day. There were statistically differences in age, waist circumference, BMI, SBP, DBP, total cholesterol, LDL-C, serum uric acid, FPG and triglycerides. Participants with Stage III periodontitis were found with the highest BMI, waist circumference, SBP, DBP and total cholesterol in the four groups. The results of ANCOVA were shown in Fig. 2. The highest cIMT level was found in those with Stage III periodontitis (0.76 ± 0.17 mm), followed by those with Stage II periodontitis (0.74 ± 0.05 mm), and then those with Stage I periodontitis (0.72 ± 0.08 mm) and lowest in those with periodontal health (0.65 ± 0.12 mm) with adjustments for the confounders (all p-values < 0.05). The clinical characteristics of the men are shown in supplemental Table 1, which are similar to that of the whole population of both men and women.
The cIMT was highest in those with Stage III periodontitis (0.76 ± 0.17 mm), followed by those with Stage II periodontitis (0.74 ± 0.05 mm), then those with Stage I periodontitis (0.72 ± 0.08 mm) and lowest in those with periodontal health (0.65 ± 0.12 mm) which was the reference group with adjustments for potential confounders (all p-values < 0.05).
Associations of periodontitis stages and lipid profiles with cIMT levels
The results of the univariate and multiple linear regression analyses for the association of periodontitis severity with cIMT were revealed in Table 2. In the univariate linear analysis, Stage I periodontitis, Stage II periodontitis and Stage III periodontitis, waist circumference, SBP, DBP, triglycerides, total cholesterol, LDL-C, age and BMI were positively and significantly associated with cIMT. In the multiple linear analysis Model 1, Stage I, Stage II and Stage III periodontitis were positively and significantly associated with cIMT levels, and the strength of association increased with greater periodontitis Stage (β = 0.066 (0.025, 0.107), 0.085 (0.050, 0.120) and 0.107 (0.065, 0.149), all p-values < 0.01, respectively). Of the metabolic variables, only total cholesterol and LDL-C were associated with cIMT levels ((β = 0.001 (0.000, 0.003) and − 0.001 (− 0.002, 0.000), respectively, both p-values < 0.05), while there were no associations for waist circumference, SBP and DBP. The main results were not changed in Model 2.
The independent risk factors of increased cIMT
The results of the univariate and multiple logistic regression analyses models of periodontitis severity, systemic inflammation and metabolic syndrome risk factors for increased cIMT are revealed in Table 3. In the univariate logistic regression analysis, participants with Stage III periodontitis, abdominal obesity, hypertension, hyperlipidemia and systemic inflammation were more likely to have cIMT ≥ 0.8 mm. In the multiple logistic regression analysis Model 1, the strength of the association for cIMT ≥ 0.8 mm increased from Stage I, Stage II and Stage III periodontitis [ORs 1.41 (0.60, 3.30), 1.62 (0.79, 3.29), and 3.19 (1.42, 7.14), respectively; p-value for trend = 0.001]. Of the other risk factors, systemic inflammation reflected by total leucocyte counts ≥ 7.6 103/µL was the only risk factor associated with cIMT ≥ 0.8 mm [OR 1.86 (1.11, 3.10)]. Notably, there were no associations for the metabolic syndrome risk factors, e.g., abdominal obesity and hypertension. The main results were not changed in Model 2. With regard to the sensitivity analysis for men only (supplemental Table 2), the main results were consistent to that for the whole population of men and women.
Associations of the severer periodontitis and systemic inflammation status for increased cIMT
The moderation effects of systemic inflammation on the association of severer periodontitis for increased cIMT are shown in Table 4. In the univariate analysis, the association for cIMT ≥ 0.8 mm was significant and most remarkably in those with severer periodontitis( +)/higher leukocyte counts( +) [OR: 7.83 (2.89, 21.2)] and then in those with severer periodontitis( +)/higher leukocyte counts(−) [OR: 1.96 (1.09, 3.50)], while compared to those with severer periodontitis(-)/higher leukocyte counts(−). However, the association for cIMT ≥ 0.8 mm in those with severer periodontitis( +)/higher leukocyte counts(−) reduces with adjustments for covariates in Model 1 and Model 2 (ORs 1.92 (1.05, 3.50) and 1.82 (0.98, 3.36), respectively), where the association was not significant in Model 2, indicating the moderation effect of systemic inflammation.
Discussion
The principal findings of this study were that among young adults who have a low vascular risk burden, periodontitis severity was positively associated with cIMT levels. In addition, there was a dose–response association for clinically increased cIMT with greater periodontitis stages. Systemic inflammation rather than metabolic risk factors and blood pressure was found to be associated with clinically increased cIMT, and systemic inflammation might be a moderator between periodontitis and subclinical atherosclerosis during young ages.
Epidemiological evidence suggested that periodontitis may be associated with a greater risk of incident cardiovascular diseases in adults, and the association was found stronger in younger adults than in older ones28, indicating that age was a moderator for the association between periodontitis and atherosclerotic cardiovascular diseases. In addition, the severity of periodontitis may increase risk of atherosclerosis in some animal studies29,30. With regard to cIMT, Cairo et al. also revealed that severe periodontitis was associated with cIMT ≥ 0.82 mm in young healthy adults, which was in line with the present study finding31. On the contrary, López-Jornet et al. revealed that periodontitis was associated with carotid atheroma plaques while not cIMT in middle- or old-aged individuals11. Range et al. also found periodontal pathogens and neutrophil activation within atherosclerotic carotid plaques, supporting a link between severe periodontitis and carotid atheroma plaques formation in older individuals32. This study was the first report using physically fit young adults to maximally reduce the effect of age and clarify the association between periodontitis and cIMT.
Periodontitis was considered as a promoter of many systemic diseases, but the signaling pathways for the interconnection remain elusive3. In previous studies, various mechanisms linking periodontal disease to cardiovascular disease, e.g., increased cytokines due to inflammation, bloodstream infection, dental plaque accumulation and alveolar bone loss have been proposed in middle- and old- aged individuals33,34,35,36,37. In this study, the mechanisms for the association between periodontitis and subclinical atherosclerosis among young adults were mainly reasoned by systemic inflammation assessed by elevated total leukocyte counts. In patients with periodontitis, cytotoxic neutrophil proteases and histones are responsible to ulcer formations and discontinuity on the sulcular epithelium33. Daily oral hygiene practices, e.g., tooth brushing and flossing might access the pathogens and its lipopolysaccharide from periodontal pocket to the peripheral blood via the sulcular epithelium, which foster bacteremia and endoxemia34. In addition, chronic infection status, e.g., low-grade endoxemia and inflammatory cascade, in which the circulating cytokines bind to the signaling receptors and ligands to generate an inflammatory circle35.
Metabolic disorders were common non-communicable diseases and previous studies have shown a bidirectional adverse association with periodontitis38. In addition, a large body of evidence from epidemiologic studies also supported an association between metabolic disorders and atherosclerosis9. Periodontitis contributes to the progress of atherosclerosis, through the subgingival biofilm, either directly modifying lipid structure by bacteria or indirectly stimulation of inflammatory pathways to affect lipid synthesis and atheroma development34. Ylo¨stalo et al. reported an association between periodontal infection and cIMT in middle-aged adults with a low HDL-C level39. Metabolic disorders, however, the present study did not show any association with cIMT, possibly because of merely young fit adults included. Age might play a crucial role in this relevance. Atherosclerosis is a process involving inflammation and lipids interaction that is required decades of time to develop clinically significant diseases. In contrast, this study showed periodontitis and inflammatory markers were independent risk factors of clinically greater cIMT, indicating inflammation status is critical for the development of atherosclerosis in young adults.
Although oral hygiene was emphasized and obliged in military in Taiwan that all our study participants were found at least once daily tooth brush, some oral unhealth behaviors, e.g., smoking and alcohol intake were prevalent and might increase the risk of periodontitis. In addition, the oral hygiene status in military was found to be associated with rank40 which is highly related to age of military personnel. In this regard, the effects of oral health behaviors on the association between periodontitis and cIMT in military require further investigation.
Some limitations should be considered while interpreting the study findings. First, evidence of the causality of periodontitis for atherosclerosis could not be obtained for the cross-sectional design. Besides, although leucocyte count was a reliable biomarker of inflammation, other inflammatory mediators such as C-reactive protein and interleukin-6 were not available in this study. Moreover, the findings in the military cohort in the present study might not be applicable to the general population of young adults. Finally, more than 90% of the study subjects were men, and thus whether the results were reliable for women may require further study. However, several advantages were present in this study. First, military subjects were characterized by highly homogeneity with unified life style and no regional differences related to access to the dental care, which could reduce unmeasured confounders. Second, there were no participants with diabetes, which has been recognized as an important risk factor for both periodontitis and atherosclerosis.
Conclusion
This study suggests that periodontitis and increased leukocyte counts within normal limits are independent risk factors of clinically increased cIMT, whereas no associations were present between other metabolic risk factors and clinically increased cIMT in young adults, highlighting the critical role of inflammation status in subclinical atherosclerosis. In clinical practice, an oral examination for the presence of severer periodontitis incorporating a blood test for the presence of increased leukocyte counts within normal limits could be treated as the signs of increased cIMT in young adults which an early prevention of cardiovascular diseases could be taken by physicians. Future study should be aimed to lower the inflammatory status, i.e., periodontitis therapy, in young adults to prevent the following clinical atherosclerotic cardiovascular diseases development.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CHIEF:
-
The Cardiorespiratory Fitness and Health in Eastern Armed Forces study
- cIMT:
-
Carotid intima-media thickness
References
Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Meuric, V. et al. Signature of microbial dysbiosis in periodontitis. Appl. Environ. Microbiol. 83, e00642-17 (2017).
Tonetti, M. S. & Van Dyke, T. E., working group 1 of the joint EFPAAPw. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the joint efp/aapworkshop on periodontitis and systemic diseases. J. Periodontol. 84 Suppl 4S, S24–S29 (2013).
Offenbacher, S., Barros, S. P. & Beck, J. D. Rethinking periodontal inflammation. J. Periodontol. 79, 1577–1584 (2008).
De Nardin, E. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann. Periodontol. 6, 30–40 (2001).
Herzberg, M. C. Coagulation and thrombosis in cardiovascular disease: Plausible contributions of infectious agents. Ann. Periodontol. 6, 16–19 (2001).
Ridker, P. M. & Silvertown, J. D. Inflammation, c-reactive protein, and atherothrombosis. J. Periodontol. 79, 1544–1551 (2008).
Roth, G. A. et al. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J. Thromb. Haemost. 4, 2256–2261 (2006).
Song, P. et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet. Glob. Health. 8, e721–e729 (2020).
Tsai, K. Z. et al. Associations between metabolic biomarkers and localized stage ii/iii periodontitis in young adults: The chief oral health study. J. Clin. Periodontol. 48, 1549–1558 (2021).
Lopez-Jornet, P. et al. Measurement of atherosclerosis markers in patients with periodontitis: A case-control study. J. Periodontol. 83, 690–698 (2012).
Nitya, K. N. et al. Assessment of periodontal status based on carotid artery intima media thickness. Oral. Health. Prev. Dent. 18, 511–519 (2020).
Lin, G. M. et al. Cardiorespiratory fitness and carotid intima-media thickness in physically active young adults: CHIEF atherosclerosis study. J. Clin. Med. 11, 3653 (2022).
Lin, G. M. et al. Muscular strength and carotid intima-media thickness in physically fit young adults: The CHIEF atherosclerosis study. J. Clin. Med. 11, 5462 (2022).
Lin, Y. P. et al. Electrocardiographic and echocardiographic predictors of greater carotid intima-media thickness in tactical athletes: The CHIEF atherosclerosis study. Ann. Noninvasive. Electrocardiol. 28, e13045 (2023).
Lin, G. M. et al. Rationale and design of the cardiorespiratory fitness and hospitalization events in armed forces study in Eastern Taiwan. World. J. Cardiol. 8, 464–471 (2016).
Grundy, S. M. et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752 (2005).
Lin, J. W. et al. Sex-specific association between serum uric acid and elevated alanine aminotransferase in a military cohort: The CHIEF study. Endocr. Metab. Immune. Disord. Drug. Targets. 19, 333–340 (2019).
Chung, P. S., Tsai, K. Z., Lin, Y. P., Lin, Y. K. & Lin, G. M. Association between leukocyte counts and physical fitness in male military members: The CHIEF study. Sci. Rep. 10, 6082 (2020).
Tsai, K. Z. et al. Comparisons of various anthropometric indexes with localized stage ii/iii periodontitis in young adults: The chief oral health study. J. Periodontol. 92, 958–967 (2021).
Tsai, K. Z., Su, F. Y., Cheng, W. C., Lin, Y. P. & Lin, G. M. Association of hepatic and systemic inflammation with localized stage ii/iii periodontitis in young males: The chief oral health study. J. Clin. Periodontol. 49, 458–466 (2022).
Tonetti, M. S., Greenwell, H. & Kornman, K. S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 45(Suppl 20), S149–S161 (2018).
Tsai, K. Z. et al. Dental caries and periodontitis and the risk of myopia in young adults: Chief oral health study. BMC Oral Health 22, 384 (2022).
Tsai, K. Z. et al. Localized periodontitis and kidney function for the risk of proteinuria in young adults in the chief oral health study. Sci. Rep. 12, 19006 (2022).
Tsai, K. Z. et al. Associations of decayed and filled teeth with localized stage ii/iii periodontitis in young adults: The chief oral health study. J. Dent. Sci. 17, 1018–1023 (2022).
Tsai, K. Z. et al. Associations of decayed teeth and localized periodontitis with mental stress in young adults: Chief oral health study. Sci. Rep. 12, 19139 (2022).
Lu, Y. W. et al. Gender difference in the association between TyG index and subclinical atherosclerosis: Results from the I-Lan Longitudinal Aging Study. Cardiovasc. Diabetol. 20, 206 (2021).
Lamprecht, R. et al. Cross-sectional analysis of the association of periodontitis with carotid intima media thickness and atherosclerotic plaque in the hamburg city health study. J. Periodontal. Res. 57, 824–834 (2022).
Dietrich, T., Sharma, P., Walter, C., Weston, P. & Beck, J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Clin. Periodontol. 40(Suppl 14), S70-84 (2013).
Yamada, S. et al. Regular dental visits, periodontitis, tooth loss, and atherosclerosis: The ohasama study. J. Periodontal. Res. 57, 615–622 (2022).
Cairo, F. et al. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J. Clin. Periodontol. 35, 465–472 (2008).
Range, H. et al. Periodontal bacteria in human carotid atherothrombosis as a potential trigger for neutrophil activation. Atherosclerosis 236, 448–455 (2014).
Vitkov, L. et al. Connection between periodontitis-induced low-grade endotoxemia and systemic diseases: Neutrophils as protagonists and targets. Int. J. Mol. Sci. 22, 4647 (2021).
Schenkein, H. A., Papapanou, P. N., Genco, R. & Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. 2000(83), 90–106 (2020).
Zou, Y. et al. Periodontopathic microbiota and atherosclerosis: Roles of tlr-mediated inflammation response. Oxid. Med. Cell. Longev. 2022, 9611362 (2022).
Petrenya, N., Hopstock, L. A., Holde, G. E., Oscarson, N. & Jönsson, B. Relationship between periodontitis and risk of cardiovascular disease: Insights from the Tromsø Study. J. periodontol. 93, 1353–1365 (2022).
Choi, H. et al. Role of Periodontal Infection, Inflammation and Immunity in Atherosclerosis. Curr. Probl. Cardiol. 46, 100638 (2021).
Jepsen, S., Suvan, J. & Deschner, J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol. 2000(83), 125–153 (2020).
Ylostalo, P. et al. Periodontal infection and subclinical atherosclerosis: The role of high-density lipoprotein as a modifying factor. J. Clin. Periodontol. 37, 617–624 (2010).
van Klink, N. Oral health disparities in the royal netherlands armed forces. Mil. med. 188, 1054–1059 (2023).
Funding
This study was supported by the grants from the Hualien Armed Forces General Hospital (HAFGH-D-112004) and the Medical Affairs Bureau, Ministry of National Defense (MND-MAB-D-112182).
Author information
Authors and Affiliations
Contributions
K-Z.T. analyzed the data and wrote the manuscript; W-C.H., Y.K., X.S. and C.J.L. raised critical comments and edited the manuscript; Y-C.C. collected and interpreted the data; G-M.L. was the principal investigator for the administration of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsai, KZ., Huang, WC., Chang, YC. et al. Localized periodontitis severity associated with carotid intima-media thickness in young adults: CHIEF atherosclerosis study. Sci Rep 13, 10523 (2023). https://doi.org/10.1038/s41598-023-37840-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37840-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.